Summary
We analyzed a group of nine neonates diagnosed with adrenal gland hemorrhage in the years 2007–2011, to evaluate diagnostic methods. We assessed risk factors and factors predisposing to hemorrhage. Severe and moderate perinatal hypoxia was found in 5 cases, while sepsis in 4 cases. Three patients had bilateral adrenal hemorrhage. All patients underwent ultrasound examination and color Doppler US. Their levels of vanillyl-mandelic acid in 24-h urine collection were normal. A complete regression of changes without evidence of adrenal hemorrhage or vascular flow on color Doppler US was found in the period from the 20th to the 165th day of life.
Conclusions: 1. Color Doppler ultrasound findings, observation of the evolution of changes and a lack of vascular flow in adrenal glands, are suggestive of bleeding. Color Doppler ultrasound seems to be the most important diagnostic method. 2. The level of urinary catecholamine metabolites (vanillyl-mandelic acid) does not fulfill the role of screening test for neuroblastoma. However, increased levels of these metabolites may suggest a diagnosis other than the adrenal hemorrhage.
Keywords: neonate, adrenal gland hemorrhage, diagnostic methods, differential diagnosis
Background
Adrenal gland hemorrhage in neonates is relatively common. Its incidence reaches approx. 0.2% of neonates [1–3] and correlates with neonatal immaturity, labor duration, appearing complications and perinatal injuries, hypoxia, sepsis, and coagulation disorders [1,4,5]. In a part of cases, the etiology of bleeding cannot be established. Adrenal gland hemorrhages may also occur prenatally, in the uterus [5]. In about 70% of cases, they involve the right adrenal gland, and in 10% – both adrenal glands [1,4–6]. Clinical manifestations of bleeding are usually absent. Sometimes there is an intense jaundice, ischemia, palpable abdominal mass, or, rarely and in extreme cases, symptoms of adrenal insufficiency [1,6,7].
Differential diagnosis of the hemorrhage should include first of all neuroblastoma, which is the most common malignancy in newborns. Nearly as much as 50% of cases include children aged under 2 years of life [1,5,6,8].
Aim of the work
The aim of the work was to evaluate the diagnostic methods used in adrenal gland hemorrhage in neonates.
Material and Methods
A group of 9 neonates hospitalized at the Department of Neonatal Intensive Care and Pathology of L. Rydygier Regional Hospital in Toruń, diagnosed with adrenal gland hemorrhage in the years 2007–2011, was analyzed retrospectively. Their mean birth mass was 3198 g. The group included 7 full-term newborns, and 2 pre-term neonates, born at 33 and 35 week. We analyzed the presence of risk factors and comorbidities, as well as clinical manifestations of hemorrhage. Color Doppler US examination was performed during hospitalization and after discharge, in outpatient clinic. The level of catecholamine metabolites in 24-h urine collection was assessed.
Results
In the analyzed group, 4 patients were born in a good condition. They received 9/10 points in Apgar scale. Three newborns received 5–6 points at first minute, and 7 points at fifth minute. Two were born with asphyxia and received 1 point at first minute, and 5/6 at fifth minute. Comorbidities were presented in Table 1. Hemorrhage was more frequent in boys (7 out of 9 cases). Bilateral hematomas were found in 3 patients. Unilateral bleeding on the right side appeared in 6 full-term newborns. Ultrasound examination evaluated the hemorrhagic lesions as inhomogeneous, with decreased echogenicity, and with hyperechoic outlines (Figure 1). Some lesions were hyperechoic and non-echoic or solitary-cystic. Color Doppler US did not reveal any foci of vascular flow (Figure 2). The level of catecholamine acid in 24-h urine collection was normal (on average: 1.4 mg/24 h, reference value: below 13.6 mg). The level was assessed at day 7. The diagnosis of adrenal gland hemorrhage was made between the 1st and the 7th day of life.
Table 1.
Comorbidities in the analyzed group of newborns.
| No. | Comorbidity | Quantity |
|---|---|---|
| 1. | Sepsis | 4 |
| 2. | Pulmonitis | 5 |
| 3. | Intrauterine infection | 3 |
| 4. | Circulatory failure | 2 |
| 5. | Respiratory failure | 2 |
| 6. | Necrotizing enterocolitis | 1 |
| 7. | Complex heart disease | 1 |
| 8. | Jaundice | 2 |
| 9. | Ischemia | 2 |
Figure 1.
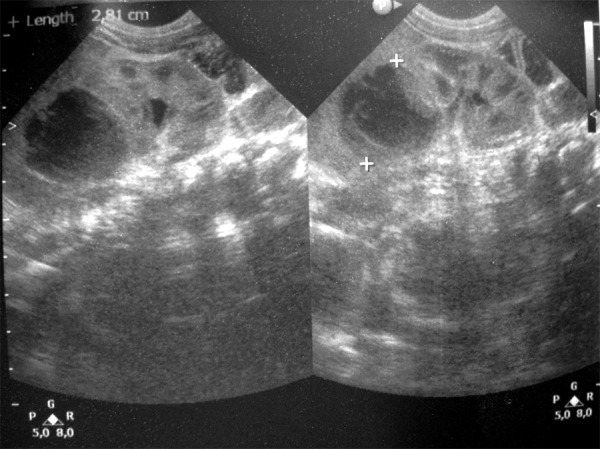
Adrenal gland hemorrhage; kidney (US image).
Figure 2.
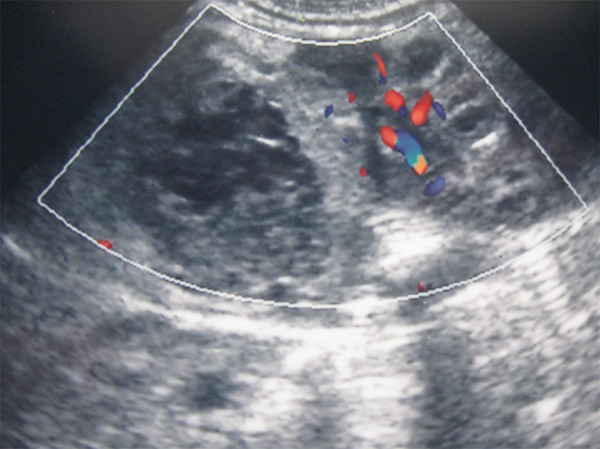
Vascular flow in the kidney: no foci of blood flow within the area of hemorrhage in the adrenal gland (color Doppler image).
A complete regression of lesions was found in 5 cases, between the 20th and 90th day of life, and in 2 cases between the 113th and 165th day of life. One child was admitted for examination at the age of 16 months. No focal lesions were found in adrenal glands. One patient died on day 7 due to a difficult, inoperable malformation of the heart. The level of vanillyl-mandelic acid in 24-h urine collection was not evaluated in that case.
Discussion
The analyzed group of 9 newborns with adrenal gland hemorrhage included mostly male patients, and in the majority of cases the hemorrhage appeared on the right side. This is consistent with other reports in the literature [1,4,9,10]. Most of the hemorrhages were within the right adrenal gland due to a higher probability of adrenal gland compression by the liver (located on the right side) and a direct connection between the vein of the right adrenal gland and the inferior vena cava, which is connected with transmission of pressure fluctuations to vessels of the right adrenal gland [1,4,5,11].
The most important issue in suspected bleeding is to differentiate these lesions from neuroblastoma, especially in unilateral hemorrhages [1,3–5,9–11]. Neuroblastoma is a malignancy originating from cells of the neural tube, i.e. neuroblasts. The tumor produces catecholamines. The level of catecholamine metabolites (vanillyl-mandelic acid) in 24-h urine collection was normal in the analyzed group. In some cases, the level of catecholamine metabolites remains within the normal range, in spite of the neoplasm. This may be i.a. due to a low mass of the tumor in the fetus. Therefore, it is suggested to repeat tests at a later time [5]. Unenhanced level of catecholamine metabolites does not exclude the possibility of neuroblastoma [4,5]. The following factors predisposing to adrenal gland hemorrhage are essential: birth asphyxia, sepsis, intrauterine infections, serious condition of the neonate [1,4,5,11]. Among our patients, a severe and moderate asphyxia was present in the majority of cases. There were also other risk factors, including: sepsis, intrauterine infections, respiratory and circulatory failure, and necrotizing enterocolitis. Jaundice (including the one which required exchange transfusion) and ischemia found in our material could be related to adrenal gland hemorrhages but could also follow from comorbidities.
Color Doppler US examination seems to be of the highest significance for a correct diagnosis of adrenal gland hemorrhage. Ultrasound examination is the basic diagnostic modality; when repeated over time, it may allow for monitoring of the evolution of changes and for differentiation with other causes of changes within adrenal glands, such as: teratomas, neuroblastoma, malignant and benign tumors [1,6,9–11]. When differentiated from neuroblastoma, hemorrhage showed no vascular flow in color Doppler US and a gradual regression of lesions over time, during sequential examinations [9–11]. We observed a complete regression of lesions within the period from the 20th to the 165th day of life. Most of the cases of adrenal gland lesions are diagnosed in fetal life, during standard examinations in mothers [4,5,11].
Minor calcifications of the lesions appear at a later time in case of adrenal gland hemorrhages [4–6,11]. In our material, no such calcifications were found on US performed during hospitalization of the neonates. The evolution of lesions in subsequent color Doppler US examinations, up to a complete regression, observed in our patients, confirmed the diagnosis of adrenal gland hemorrhage.
The level of catecholamine metabolites determined two times did not fulfill the role of a screening test which would identify abnormalities or decrease mortality in neuroblastoma [8]. In case of this tumor, lesions intensify in advanced stages of the disease, together with appearance of metastases. The prognosis is bad [8,11].
Conclusions
In case of adrenal gland lesions found in newborns, it is of the highest importance to differentiate bleeding from a malignancy common in newborns, i.e. neuroblastoma.
Color Doppler ultrasound findings, observation of the evolution of changes and no vascular flow in adrenal glands, are suggestive of bleeding. Color Doppler ultrasound seems to be the most important diagnostic method.
The level of urinary catecholamine metabolites (vanillyl-mandelic acid) does not fulfill the role of a screening test for neuroblastoma which would reduce the mortality. However, increased levels of these metabolites may suggest a diagnosis other than the adrenal gland hemorrhage.
References:
- 1.Brzewski M, Biejat A, Rumińska M, et al. Miejsce ultrasonografii w algorytmie diagnostyczno-leczniczym noworodków z wylewem do nadnerczy. Ultrasonografia. 2008;(35):32–34. [in Polish] [Google Scholar]
- 2.Rajtar-Leontiew Z, Kuszyk R. Hemorrhage into the adrenal glands in the neonates. New Medicine. 2001;(1):22–26. [Google Scholar]
- 3.Rozsai B, Szasz M, Ottoff G, et al. A neonate with abdominal mass. Acta Pediatr. 2006;95(10):1326–28. doi: 10.1080/08035250600644754. [DOI] [PubMed] [Google Scholar]
- 4.Eo H, Kim JH, Jang KM, et al. Comparison of clinico-radiological features between congenital cystic neuroblastoma and neonatal adrenal hemorrhagic pseudocyst. Korean J Radiol. 2011;12(1):52–58. doi: 10.3348/kjr.2011.12.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauvat F, Sarnacki S, Brisse H, et al. Outcome of suprarenal localized masses diagnosed during the perinatal period. A retrospecitive multicenter study. Cancer. 2002;94(9):2474–80. doi: 10.1002/cncr.10502. [DOI] [PubMed] [Google Scholar]
- 6.Marciński A. Choroby układu moczowego i nadnerczy u noworodków. A może ultrasonografia? Ultrason Pol. 1991;1(2–3):123–39. [in Polish] [Google Scholar]
- 7.Lange M, Maciejewska J, Rumińska M, et al. Wylew do nadnerczy u noworodków – obraz kliniczny i ultrasonograficzny. Endokrynol Pol. 2006;5(Supl.5):74–78. [in Polish] [Google Scholar]
- 8.Woods WG. Wykonywanie badań przesiewowych w kierunku zwojaka zarodkowego w wieku 3 tygodni, a następnie 6 miesięcy, nie zmniejszyło śmiertelności z powodu tego nowotworu u dzieci do 8 roku życia. N Engl J Med. 2002;346:1041–46. [in Polish] [Google Scholar]
- 9.Jakubowski W. Diagnostyka ultrasonograficzna w chorobach nerek. Praktyczna Ultrasonografia, Warszawa–Zamość. 2004:112–26. [in Polish] [Google Scholar]
- 10.Gharagozlos AM, Katz DS. Badania obrazowe nadnerczy. Sekrety radiologii. Medycyna Praktyczna (Diagnostyka Obrazowa) 2002;2(6):92–98. [in Polish] [Google Scholar]
- 11.Chong-Hsin Ch, Tzu-Ang Ch. The value of ultrasound in the diagnosis and management of neonatal adrenal hemorrhage. Chin J Radiol. 1999;24(3):107–11. [Google Scholar]


