Summary
Background:
Intraosseous lipoma is a very rare lesion, which constitutes not more than 0.1% of bone tumors. The introduction of cross-sectional imaging, especially MRI, seems to have increased the detection rate of these lesions.
Case Report:
The authors presented 6 cases of intraosseous lipomas in bones of the lower extremities. All lesions were detected incidentally and presented radiographically as radiolucent lesions with sclerotic borders and internal trabeculations. One lesion caused bone expansion. CT and MRI identified fatty tissue in all lesions. Cystic degeneration was present in one lesion and dystrophic calcifications in two.
Discussion:
The radiographic appearance of intraosseous lipomas is not characteristic and requires differential diagnostics conducted for a long time. However, CT and MRI allow for a tissue-specific diagnosis. The detection of a predominant fatty component in a lesion confirms its benign character and no further diagnostic work-up is required.
Keywords: bone tumor, intraosseous lipoma, imaging
Background
Bone lipomas are rare tumours, that constitute up to 0.1% of bone tumours [1,2]. With an ever increasing use of cross-sectional imaging in the diagnostics of the musculoskeletal system, and especially of MRI, the detection rate of those lesions has increased [3]. The aim of this work was to present diagnostic imaging findings in 6 cases of intraosseous lipomas consulted at our Facility in the years 2005–2010, and to review the literature concerning this rare pathology.
Case Report
Case 1 – proximal femur
A 68-year-old male qualified for an arthroplasty of the right hip joint due to osteoarthritis. An AP image of the hip joints showed a centrally located cystic lesion in the right proximal femoral shaft, surrounded by a thick sclerotic rim, without a periosteal reaction. CT and MRI showed the presence of fatty tissue within the lesion (Figure 1). Arthroplasty of the right hip joint was performed with an intraoperative collection of sample material for histopathological examination, which revealed mature adipose tissue without features of necrosis. Intraosseous lipoma was diagnosed.
Figure 1.
A 68-year-old male. (A) Plain film showing a radiolucent lesion in the proximal femoral diaphysis surrounded by a thick, irregular rim of osteosclerosis. (B) MRI. The lesion exhibiting high signal intensity on T1-weighted images, identical with the signal of subcutaneous fat.
Case 2 and 3 – distal femur
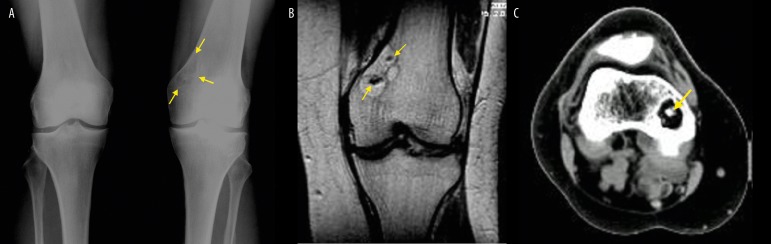
A 58-year-old male with knee pain, who had a stress AP radiograph of both knee joints, and a lateral radiogram of the right knee joint. The images showed osteoarthritis in the medial compartments of both knee joints in the form of joint space reduction, especially on the left side, and a cystic lesion in the lateral epicondyle of the right femur. Eccentrically located lesion was well circumscribed with a sclerotic rim, caused bone expansion and was abutting the posterior part of the articular surface of the lateral femoral condyle. It contained internal trabeculations. An MRI revealed the presence of an adipose tissue signal within the lesion, identical to that of subcutaneous fat and medullary cavity. In the central part of the lesion, there was a fluid-filled space (Figure 2).
Figure 2AB.
A 58-year-old male. (A,B) Plain films demonstrating a cystic lesion in the lateral femoral condyle and epicondyle. The eccentrically located lesion is surrounded by a sclerotic rim. It causes a moderate bone expansion, and contains internal trabeculations.
Figure 2C–E.
A 58-year-old male. MRI, T1-weighted (C), T2-weighted (D), and T2-weighted images with fat suppression (E): the signal of the peripheral part of the lesion showing the same pattern as the signal of the subcutaneous fat, whereas the central part contains fluid-filled space (low on T1, high on T2) corresponding to cystic degeneration (arrows in E).
A 58-year-old female with knee pain, who had a stress AP radiograph of both knee joints. Apart from osteoarthritis, there was also a cystic lesion surrounded by a sclerotic rim in the medial epicondyle of the left femur, not communicating with the articular surface. CT and MRI of the left knee revealed adipose tissue and centrally located calcifications within the lesion (Figure 3).
Figure 3.
A 58-year-old female. (A) Plain AP film of both knee joints showing a cystic lesion surrounded with a thin sclerotic rim, located in the medial epicondyle of the left femur (arrows). In MRI (B) and CT (C) examinations, the lesion is composed exclusively of adipose tissue. Small foci of low signal intensity in MRI correspond to calcifications as shown by CT imaging (arrows in B and C).
Case 4, 5 and 6 – bones of the feet
A 49-year old male with forefoot pain. An AP and lateral x-ray images of both feet showed hallux valgus with secondary osteoarthritis in MTP I joints and a polycyclic cystic lesion in the head of the talus surrounded by a sclerotic rim. An MRI showed adipose tissue within the lesion. The lesion communicated with the articular surface of the talonavicualar joint and the anterior part of the subtalar joint (Figure 4).
Figure 4.
A 49-year-old male. AP radiogram (A) of the feet showing bilateral hallux valgus and mild osteoarthritis of tarsal joints. Lateral radiogram of the right foot (B) showing a cystic lesion in the head of the talus, surrounded by a sclerotic rim (arrows). MRI: T1- and T2-weighted images (C,D) and T2-weighted images with fat saturation (E) displaying a fatty nature of the lesion.
A 28-year-old female with a cystic lesion in the central part of the calcaneus (Ward’s triangle), found on x-ray of the feet performed due to faulty posture. CT images provided for evaluation revealed adipose tissue within the lesion, with a central area of soft tissue including dystrophic calcifications (Figure 5)
Figure 5.
A 28-year-old female. (A) Lateral radiogram of the foot showing a cystic lesion with a central calcification in the middle of the calcaneus (Ward’s triangle). (B) CT demonstrated a predominantly fatty content of the lesion with some soft tissue containing dystrophic calcifications in the center of the lesion.
A 55-year-old female diagnosed with first-stage thoracic sarcoidosis. The disease started with erythema nodosum in the region of the ankles. Imaging examinations were carried out due to pain in the metatarsal area present for a few months. X-ray and MRI examinations showed inflammatory lesions in the tarsal joints, particularly intense at the level of the Lisfranc joint, and a cystic lesion composed of adipose tissue in the right cuboid bone (Figure 6).
Figure 6A,B.
A 55-year-old female with sarcoidosis. (A,B) plain AP film of the feet and lateral film of the right foot showing bilateral hallux valgus, hazy delineation of Lisfranc joints and a cystic lesion in the right cuboid bone.
Figure 6C–F.
A 55-year-old female with sarcoidosis. The lesion has a high signal intensity on T1- and T2-weighted images (C,D), which becomes attenuated on PD- and T1-weighted fat-saturated images (E,F). No contrast enhancement is observed in the lesion (F). Effusions and enhancement in the tarsal joints is due to inflammation in the course of sarcoidosis (arrows in E,F).
Discussion
Intraosseous lipomas constitute very rare bone tumours, with 200 cases reported thus far [1–3]. The largest number of cases was presented by Milgram (61 cases) and Campbell et al., who described 35 cases collected by the members of the British Society of Skeletal Radiology and who carried out a meta-analysis of cases presented in the English literature since 1966 [4,3].
Histologically, these tumours are composed of mature adipose cells, which are slightly larger than the non-tumorous ones, and may include single spindle cells, regressive lesions (foci of fat necrosis, cystic spaces, and dystrophic calcifications) and bone trabeculae undergoing resorption [4–7]. Milgram divided these lesions into three stages, depending on the histopathological appearance [4]. Stage I includes lesions composed of viable, mature lipocytes, similar to the cells of subcutaneous adipose tissue. In stage II, necrotic foci are present in the lesion; foamy macrophages and foci of reactive osteogenesis may also appear. Stage III is a completely necrotic lesion with focal calcifications and cystic degeneration that resembles a bone infarct, the only features differentiating it from lipoma being lack of trabecular resorption or possible bone expansion [4,8].
Intraosseous lipomas are found only slightly more often in men. They may develop at all ages, although they are usually diagnosed around the age of 40 [3]. Most of the cases presented in the literature (about 70%) were associated with clinical symptoms, usually pain [3]. This is contrary to the data from our material, in which all lesions were diagnosed incidentally on radiographs performed due to problems that undoubtedly were clinically connected with another pathology: osteoarthritis of hip (n=1) or knee joints (n=2), hallux valgus and osteoarthritis within the tarsus (n=1), sarcoid arthritis (n=1) and faulty posture (plano-valgus foot). The direct connection between lipomas and patients’ symptoms was also questioned by Campbell et al., who found that the manifestations recurred after tumour removal or subsided spontaneously without treatment, which pointed to their relation to some other pathology [3]. In one case visualised with an MRI, they found an increased signal intensity within the lesion, which could be related to overload (microfracture of bone trabeculae). Unfortunately, correlation of the image with patient’s symptoms was not possible [3].
The lesions tend to locate within the lower limb (71%), usually within the calcaneus (about 30% of cases). Other frequent locations include: subtrochanteric femur, as well as femur and tibia in the region of the knee joint [3]. Our cases also seem to confirm the predilection of lesions for bones of the lower limb. A less frequent location is the upper limb (7%) and the axial skeleton (cranium – 4%, mandible – 3%, spine – 4%, pelvis – 5%) [3,9–12].
Radiographic picture of intraosseous lipomas is usually uncharacteristic and requires differentiation with other lesions, including non-ossifying fibroma, fibrous dysplasia, solitary cyst, giant-cell bone tumor, bone infarct and cartilaginous tumours [3,5]. Intraosseous lipomas usually present as cystic lesions with an increased radiolucency, surrounded by a sclerotic rim (74%) [3,4,6]. Lipomas located in the proximal femur may be surrounded by a relatively extensive area of sclerotisation [13]. Lesions in the intertrochanteric area of the femur need to be differentiated from liposclerosing myxofibrous tumor, a very rare, benign fibro-osseous lesion with mixed histology, which may include components of lipoma [14]. Inside the cysts, internal trabeculations may be present, while in lipomas with regressive lesions (stage II and III according to Milgram) – foci of calcifications, usually located centrally [3,4]. An image of a cystic lesion in the calcaneus with a central calcification is pathognomonic for intraosseous lipoma (Figure 5). In the collected material, we could identify a sclerotic rim surrounding lesions in every case, with the greatest intensity in case of focus located in the proximal femur (Figure 1A). Most of the tumours in our material had slightly polycyclic borders and internal septa. Two lesions contained calcifications in the tumour matrix. In one case there was a slight bone expansion (Figure 2A, B). This symptom may be found in some cases of intraosseous lipomas (about 14%), and is usually of minor degree. In cases of greater expansion, it is necessary to differentiate it from an aneurysmal cyst [3,7,9,16].
Computed tomography (CT) and magnetic resonance imaging (MRI) allow for a more accurate evaluation of lesion morphology [17–22]. Finding that the lesion is composed mostly of adipose tissue is actually diagnostic. In CT, adipose tissue has a low attenuation coefficient, ranging from −110 to −40 HU. In fast spin-echo sequences of an MRI, adipose tissue signal is high both in T1- and T2-weighted images, and becomes extinguished in fat-suppression sequences. Calcifications are visible in CT as hyperdense areas, while in MRI as areas of low signal intensity (Figure 3). Fluid-filled spaces found in about 67% of lesions show a low-to-medium signal intensity in T1-weighted images, and a very high signal intensity in T2-weighted images (Figure 2) [3]. No contrast enhancement is detected within the lesions (Figure 6) [21].
It should also be mentioned, that some authors do not consider intraosseous lipomas true bone tumors [5,23]. One of the arguments is that these lesions are usually found within areas with physiologically lower number of trabeculae and a higher yellow bone marrow content, such as the trochanteric region of the femur or the anterior part of calcaneus. In the heel bone, intraosseous lipomas are almost exclusively found within a so-called Ward’s triangle, between the load-bearing trabeculae [24,25]. Campbell et al. suggested that in some cases, resorption of the trabeculae within an unloaded area may occur together with a peripheral trabecular hypertrophy, producing an image of a seemingly delineated focal lesion [3] (Figure 5). Other authors point to the fact that Ward’s triangle is characterised by a specific vascularization, which may predispose to bone marrow infarction and development of regressive lesions [5,23]. Solitary cysts and intraosseous lipomas found in the same location in the calcaneus led some authors to a conclusion, that these lesions may present a continuum of one disease process [26,27]. It is also believed that lipomas may constitute the last phase of involution of other focal lesions of bones, a bone infarct in particular. Stage III intraosseous lipoma described by Milgram may be difficult to distinguish from a bone infarct in histopathological examination [4]. Lipomas causing bone expansion may be a continuation of a regressing aneurysmal bone cyst [28]. There was a report of a focal lesion causing bone expansion with a mixed histopathological picture of an aneurysmal bone cyst and intraosseous lipoma [29]. Recently, there has appeared a report on a solitary cyst of the humerus in an 18-year-old man becoming filled with an adipose tissue after alcohol ablation [30]. So far, only one case of periosteal lipoma has been subjected to a cytogenetic analysis that revealed a translocation identical with that of soft tissue lipomas, which are considered true neoplasms [31]. Soft tissue lipomas are much more common in women (8:1). However, epidemiology of intraosseous lipomas, with a slightly higher incidence in men, does not support the relationship between these two types of tumors [3]. It seems that the nature of intraosseous lipomas is yet to be unequivocally defined.
Irrespectively of the above mentioned controversies, establishing prognosis as well as further diagnostic and therapeutic management is of most importance to a radiologist and a clinician Milgram described four cases of malignant transformation of intraosseous lipomas [32]. One of them concerned a 19-year-old boy with a stage I lipoma. Three other cases included older individuals with stage III lipomas. In all patients, malignant histiocytic fibroma was diagnosed. As already mentioned, stage III lipomas appear very similar to bone infarcts. A malignant transformation of bone infarct is a common complication. Cases of various histological types of sarcomas, mainly of mesenchymal origin (malignant fibrous histiocytoma, fibrosarcoma, liposarcoma) have been described in single bone infarcts as well as in multiple infarcts complicating systemic diseases (alcoholism, anaemia) [33–38]. As the literature lacks other reports of malignant transformations of intraosseous lipomas, we believe that the report by Milgram should be considered only as a proof of difficulties associated with differentiation between bone infarct and an intraosseous lipoma, rather than of the malignant potential of this tumour. It is also worth mentioning that the world literature includes only over a dozen of case reports of bone liposarcoma, and if we consider only the lesions with an epicentre located within the medullary cavity, this number decreases below ten [39–41]. In radiographic examinations, these tumours present as poorly delineated osteolytic lesions with a nonreactive bone degeneration or an accompanying malignant periosteal reaction and a large tumour in soft tissues. In one case, a CT examination revealed an extensive soft-tissue mass without macroscopically visible adipose tissue [41]. Therefore, liposarcoma should not be included in the differential diagnosis of intraosseous lipoma.
To sum up, similarly to other authors, we believe that imaging of a tumour composed of adipose tissue proves its benign nature and concludes the diagnostic process [3]. Additional proof for the validity of this approach is presented in papers analysing the presence of adipose tissue in benign and malignant bone tumours of various histological structures, which indicate that the existence of adipose tissue in malignant tumours is extremely rare [42,43]. We believe that, due to the previously mentioned complications of bone infarct, diagnostics should be continued only in cases where pain seems to be directly related to the presence of the lesion, the radiographic presentation is difficult to differentiate from a bone infarct, and contrast enhancement or aggressive bone destruction are found in CT or MRI.
References:
- 1.Schajowicz F. Tumors and tumorlike lesions of bone: pathology, radiology and treatment. 2nd ed. Springer-Verlag; 1994. [Google Scholar]
- 2.Dahlin DC, Unni KK. Bone tumors: general aspects and data on 8,542 cases. 4th ed. Charles C Thomas; Springfield IL: p. 1986. [Google Scholar]
- 3.Campbell RSD, Grainger AJ, Mangham DC, et al. Intraosseus lipoma: report of 35 new cases and a review of the literature. Skeletal Radiol. 2003;32:209–22. doi: 10.1007/s00256-002-0616-7. [DOI] [PubMed] [Google Scholar]
- 4.Milgram JW. Intraosseous lipomas. A clinicopathologic study of 66 cases. Clin Orthop. 1988;231:277–302. [PubMed] [Google Scholar]
- 5.Greenspan A, Jundt G, Remagen W. Diagnostyka różnicowa w onkologii ortopedycznej. Medipage. 2008:438–42. [in Polish] [Google Scholar]
- 6.Barcelo M, Pathria MN, Abdul-Karim FW. Intraosseous lipoma. A clinicopathologic study of four cases. Arch Pathol Lab Med. 1992;116:947–50. [PubMed] [Google Scholar]
- 7.Gero MJ, Kahn LB. Case report 498: Intraosseous lipoma of the di end of the fibula with focal infarction. Skeletal Radiol. 1988;17:443–46. doi: 10.1007/BF00361667. [DOI] [PubMed] [Google Scholar]
- 8.Miller WB, Ausich JE, McDaniel RK, et al. Mandibular intraosseous lipoma. J Oral Maxillofac Surg. 1982;40:594–96. doi: 10.1016/0278-2391(82)90291-9. [DOI] [PubMed] [Google Scholar]
- 9.Chow LT, Lee KC. Intraosseous lipoma. A clinicopathologic study of nine cases. Am J Surg Pathol. 1992;16:401–10. [PubMed] [Google Scholar]
- 10.Heir GM, Geron PR. Mandibular intraosseous lipoma related to trigeminal neuropathy. Clin Prev Dent. 1983;5:13–15. [PubMed] [Google Scholar]
- 11.Miller WB, Ausich JE, McDaniel RK, et al. Mandibular intraosseous lipoma. J Oral Maxillofac Surg. 1982;40:594–96. doi: 10.1016/0278-2391(82)90291-9. [DOI] [PubMed] [Google Scholar]
- 12.Pande KC, Ceccherini AF, Webb JK, et al. Intraosseous lipomata of adjacent vertebral bodies. Eur Spine J. 1998;7:344–47. doi: 10.1007/s005860050086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milgram JW. Intraosseous lipomas with reactive ossification in the proximal femur. Report of eight cases. Skeletal Radiol. 1981;7:1–13. doi: 10.1007/BF00347165. [DOI] [PubMed] [Google Scholar]
- 14.Ragsdale BD. Polymorphic fibroosseous lesions of bone: an almost sitespecific diagnostic problem of the proximal femur. Hum Pathol. 1993;24:505–12. doi: 10.1016/0046-8177(93)90162-a. [DOI] [PubMed] [Google Scholar]
- 15.Kransdorf MJ, Murphey MD, Sweet DE. Liposclerosing Myxofibrous Tumor: A Radiologic-Pathologic-Distinct Fibro-osseous Lesion of Bone with a Marked Predilection for the Intertrochanteric Region of the Femur. Radiology. 1999;212:693–98. doi: 10.1148/radiology.212.3.r99se40693. [DOI] [PubMed] [Google Scholar]
- 16.Ehara S, Kattapuram SV, Rosenberg AE. Case report 619: Intraosseous lipoma of the sacrum. Skeletal Radiol. 1990;19:375–76. doi: 10.1007/BF00193096. [DOI] [PubMed] [Google Scholar]
- 17.Reig-Boix V, Guinot-Tormo J, Risent-Martinez F, et al. Computed tomography of intraosseous lipoma of os calcis. Clin Orthop. 1987;221:286–91. [PubMed] [Google Scholar]
- 18.Ketyer S, Brownstein S, Cholankeril J. CT diagnosis of intraosseous lipoma of the calcaneus. J Comput Assist Tomogr. 1983;7:546–47. doi: 10.1097/00004728-198306000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Ramos A, Castello J, Sartoris DJ, et al. Osseus lipoma: CT appearance. Radiology. 1985;157:615–19. doi: 10.1148/radiology.157.3.4059548. [DOI] [PubMed] [Google Scholar]
- 20.Richardson AA, Erdmann BB, Beier-Hanratty S, et al. Magnetic resonance imagery of a calcaneal lipoma. J Am Podiatr Med Assoc. 1995;85:493–96. doi: 10.7547/87507315-85-9-493. [DOI] [PubMed] [Google Scholar]
- 21.Blacksin MF, Ende N, Benevenia J. Magnetic resonance imaging of intraosseous lipomas: a radiologicpathologic correlation. Skeletal Radiol. 1995;24:37–41. doi: 10.1007/BF02425945. [DOI] [PubMed] [Google Scholar]
- 22.Dooms GC, Hricak H, Sollitto RA, et al. Lipomatous tumors and tumors with fatty component: MR imaging potential and comparison of MR with CT results. Radiology. 1985;157:479–83. doi: 10.1148/radiology.157.2.4048459. [DOI] [PubMed] [Google Scholar]
- 23.Remagen W, Lampérth BE, Jundt G, et al. Das sogennante “osteolytische” Dreick des Calcaneus. Radiologische und pathoanatomische Befunde. Osteologie. 1994;3:275–83. [in German] [Google Scholar]
- 24.Appenzeller J, Weitzner S. Intraosseous lipoma of os calcis. Case report and review of literature of intraosseous lipoma of extremities. Clin Orthop. 1974;101:171–75. [PubMed] [Google Scholar]
- 25.Jensen NC, Madsen LP, Linde F. Topographical distribution of trabecular bone strength in the human os calcanei. J Biomech. 1991;24:49–55. doi: 10.1016/0021-9290(91)90325-h. [DOI] [PubMed] [Google Scholar]
- 26.Abdelwahab IF, Lewis MM, Klein MJ, et al. Case report 515. Simple (solitary) bone cysts of the calcaneus. Skeletal Radiol. 1989;17:607–10. doi: 10.1007/BF02569412. [DOI] [PubMed] [Google Scholar]
- 27.Lagier R. Case report 128: lipoma of the calcaneus with bone infarct. Skeletal Radiol. 1980;5:267–69. doi: 10.1007/BF00580603. [DOI] [PubMed] [Google Scholar]
- 28.Coquerelle P, Cotten A, Flipo RM, et al. Intraosseous lipoma: role and limitations of modern imaging techniques. Rev Rhum Engl Ed. 1995;62:147–50. [PubMed] [Google Scholar]
- 29.Michota RS, Perdiue RL, McGee TP. Aneurysmal bone cyst/lipoma of toe: case study and presentation. J Am Podiatr Med Assoc. 1978;68:725–31. doi: 10.7547/87507315-68-10-725. [DOI] [PubMed] [Google Scholar]
- 30.Wada R, Lambert RGW. Deposition of intraosseus fat in a degenerating simple bone cyst. Skeletal Radiol. 2005;34:415–18. doi: 10.1007/s00256-004-0856-9. [DOI] [PubMed] [Google Scholar]
- 31.Bridge JA, DeBoer J, Walker CW, et al. Translocation t(3;12)(q28;q14) in parosteal lipoma. Genes Chromosomes Cancer. 1995;12:70–72. doi: 10.1002/gcc.2870120113. [DOI] [PubMed] [Google Scholar]
- 32.Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiology. 1990;19:347–52. doi: 10.1007/BF00193088. [DOI] [PubMed] [Google Scholar]
- 33.Furey JG, Ferrer-Torells M, Reagan JW. Fibrosarcoma arising at the site of bone infarcts. A report of two cases. J Bone Joint Surg. 1960;42:802–10. [PubMed] [Google Scholar]
- 34.Galli SJ, Weintraub HP, Proppe KH. Malignant fibrous histiocytoma and pleomorphic sarcoma in association with medullary bone infarcts. Cancer. 1978;41:607–19. doi: 10.1002/1097-0142(197802)41:2<607::aid-cncr2820410227>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 35.Abrahams TG, Hull M. Case report 394: malignant fibrous histiocytoma (MFH) arising in an infarct of bone. Skeletal Radiol. 1986;15:578–83. doi: 10.1007/BF00361060. [DOI] [PubMed] [Google Scholar]
- 36.Frierson HF, Jr, Fechner RE, Stallings RG, et al. Malignant fibrous histiocytoma in bone infarct. Association with sickle cell trait and alcohol abuse. Cancer. 1987;59:496–500. doi: 10.1002/1097-0142(19870201)59:3<496::aid-cncr2820590324>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Arnold WH. Hereditary bone dysplasia with sarcomatous degeneration. Study of a family. Ann Int Med. 1973;78:902–6. doi: 10.7326/0003-4819-78-6-902. [DOI] [PubMed] [Google Scholar]
- 38.Dorfman HD, Norman A, Wolff H. Fibrosarcoma complicating bone infarction in a caisson worker. J Bone Joint Surg. 1966;48:528–32. [PubMed] [Google Scholar]
- 39.Stewart FW. Primary liposarcoma of bone. Am J Pathol. 1931;7:87–93. [PMC free article] [PubMed] [Google Scholar]
- 40.Addison AK, Payne SR. Primary liposarcoma of bone. Case report J Bone Joint Surg Am. 1982;64:301–4. [PubMed] [Google Scholar]
- 41.Retz LD., Jr Primary liposarcoma of bone: Report of a Case and a Review of Literature. J Bone Joint Surg Am. 1961;43:123–29. [Google Scholar]
- 42.Simpfendorfer CS, Ilaslan H, Davies AM, et al. Does the presence of focal normal marrow fat signal within a tumor on MRI exclude malignancy? An analysis of 184 histologically proven tumors of the pelvic and appendicular skeleton. Skeletal Radiol. 2008;37:797–804. doi: 10.1007/s00256-008-0523-7. [DOI] [PubMed] [Google Scholar]
- 43.Stacy GS. An analysis of malignant bone tumors for the presence of internal fat-signal-intensity on magnetic resonance examinations. Skeletal Radiol. 2007;36:355–75. [Google Scholar]