Abstract
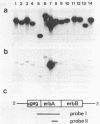
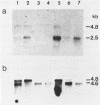
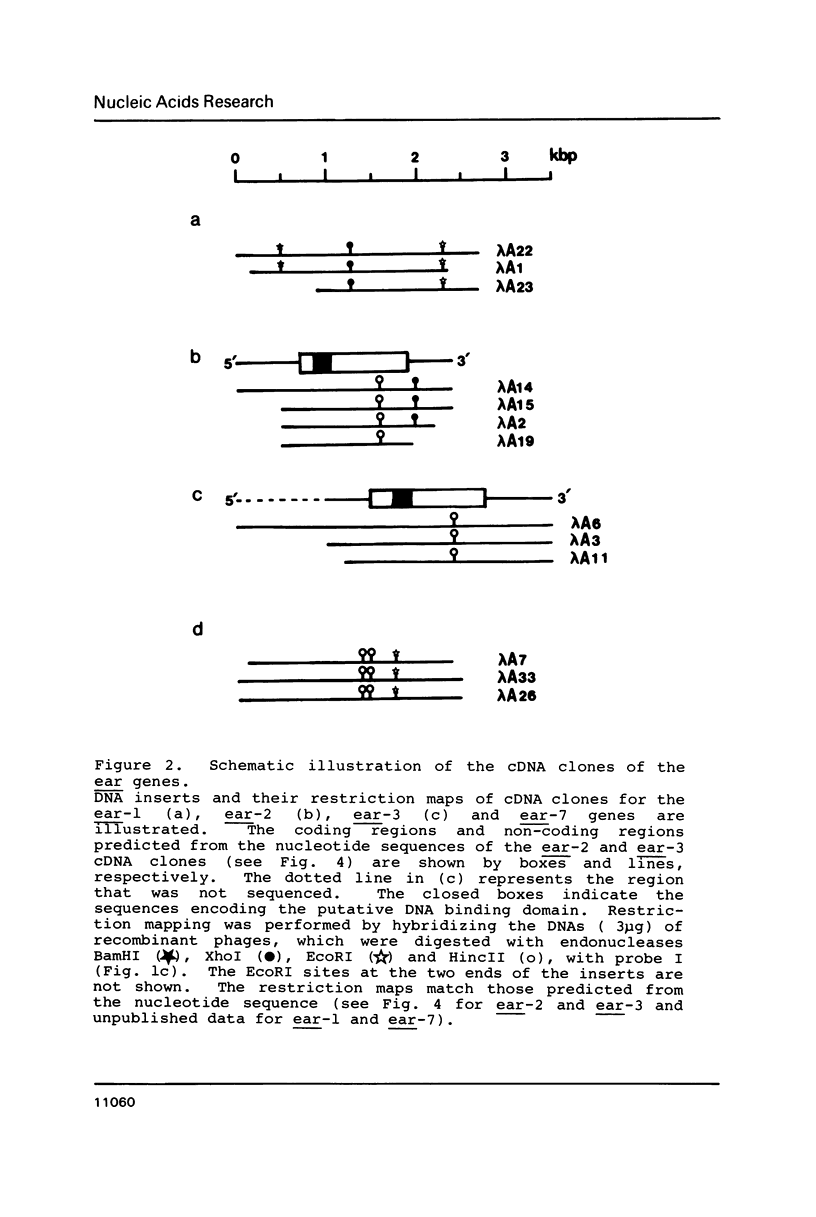
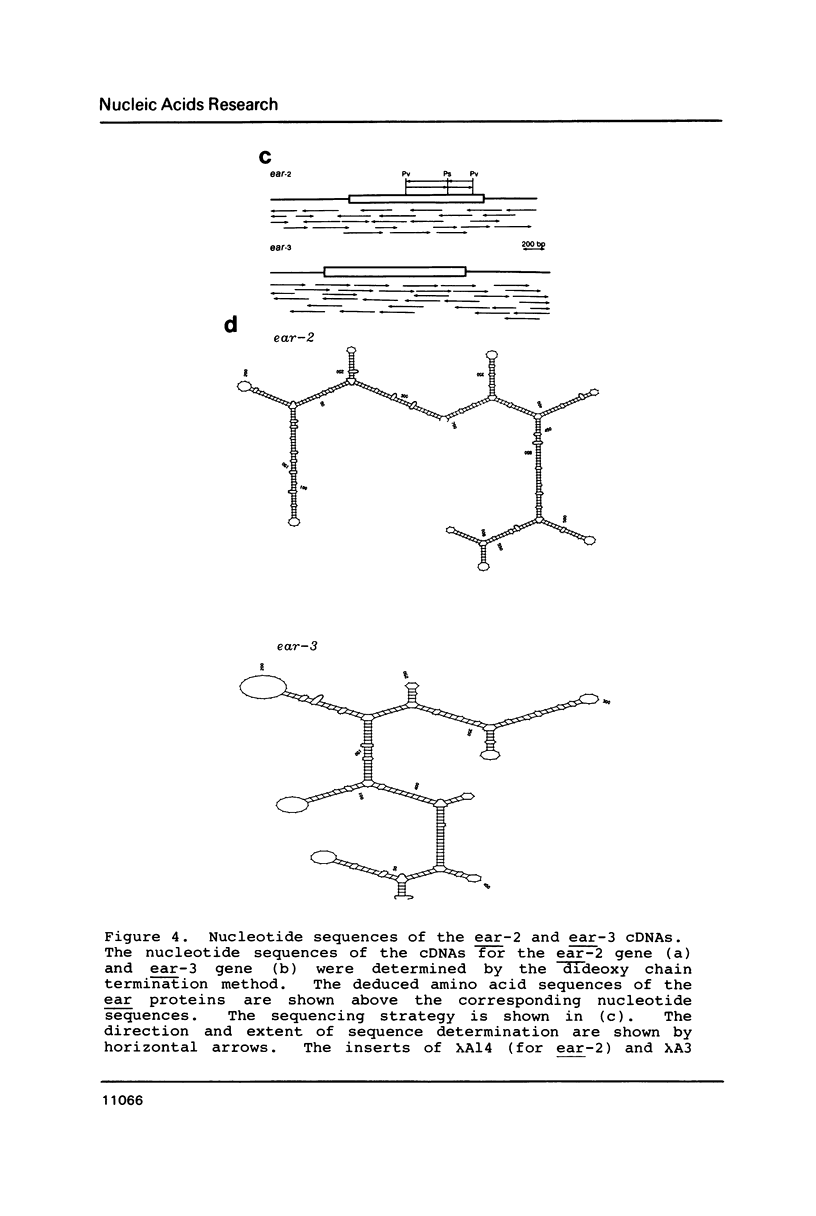
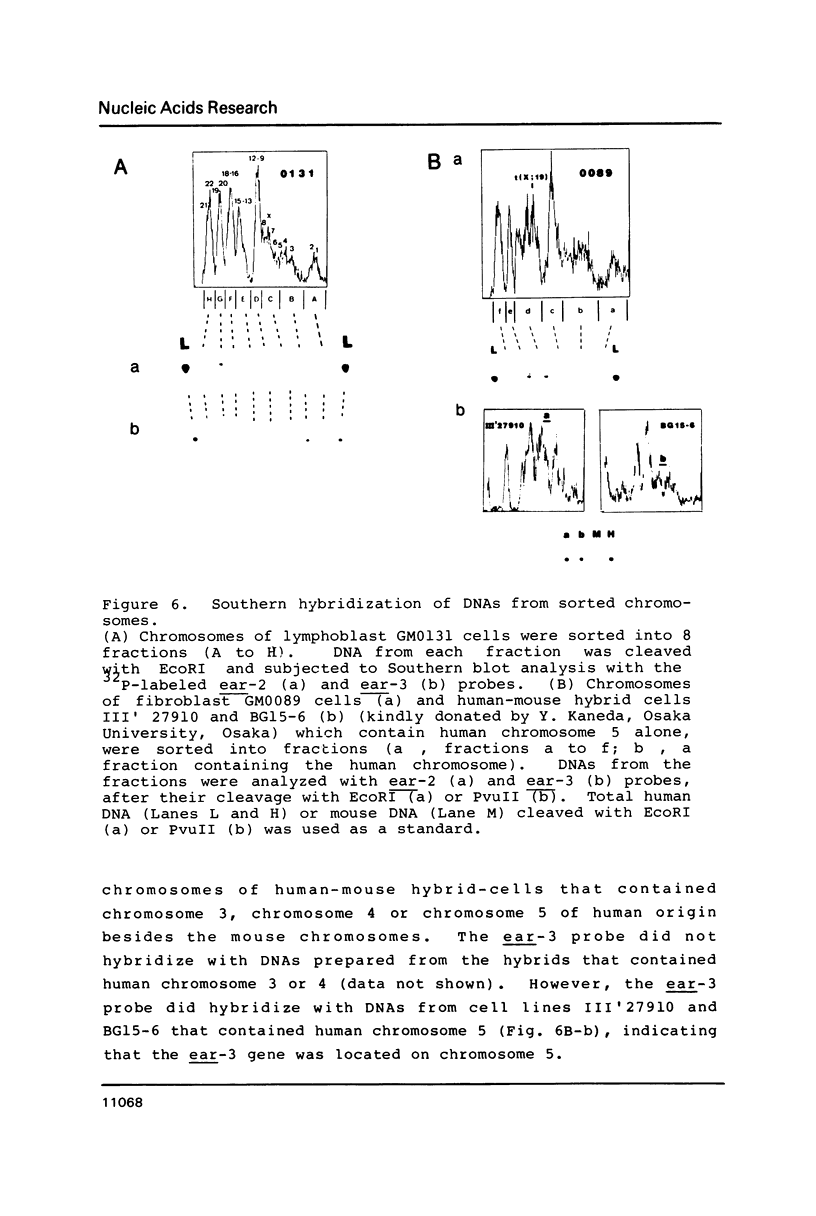
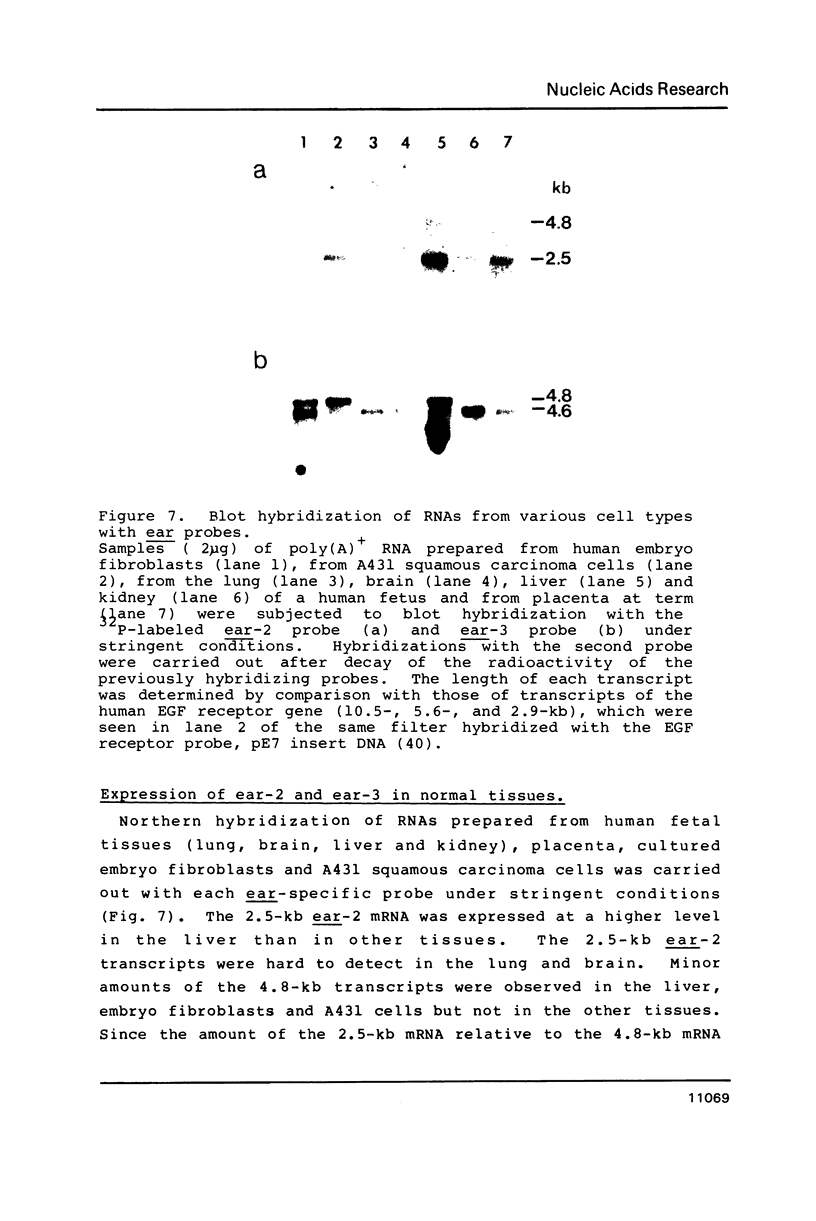
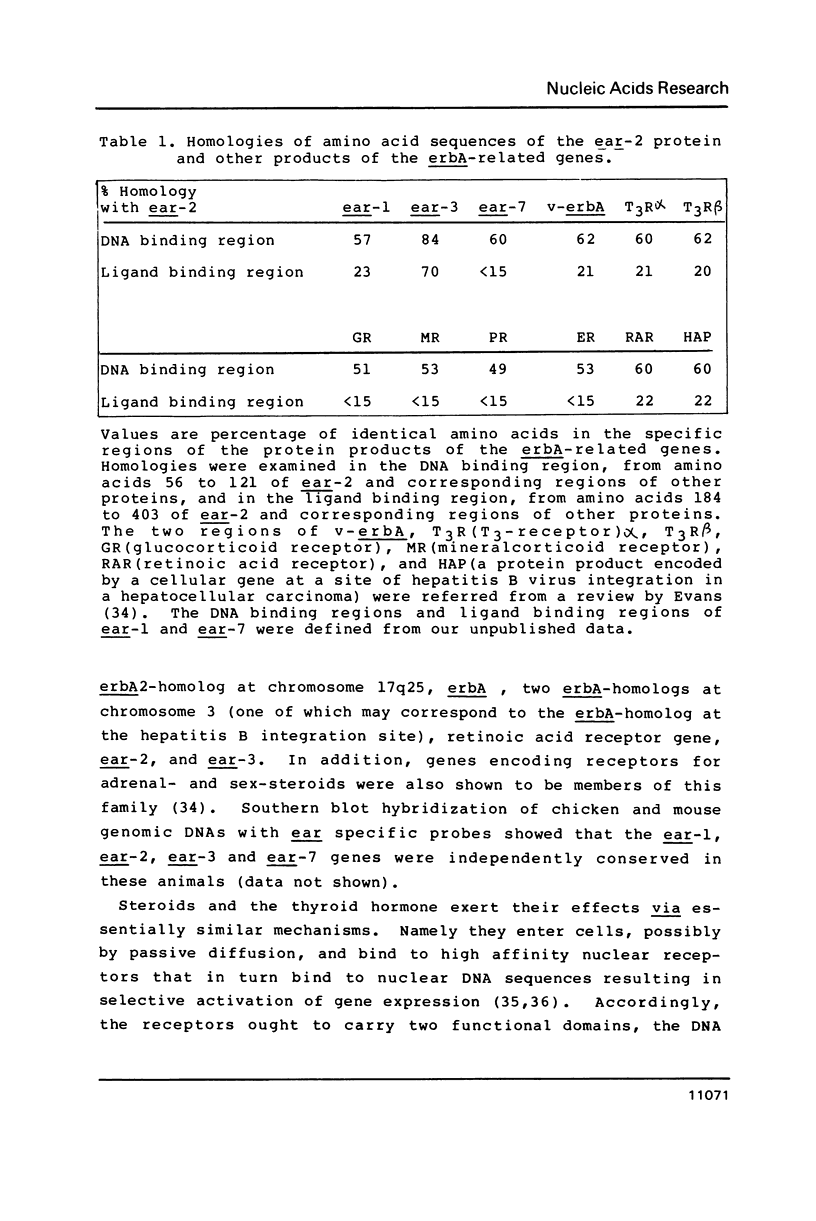
Two v-erbA-related genes, named ear-2 and ear-3, have been identified in the human genome and characterized by cDNA cloning. These genes are predicted to encode proteins that are very similar in primary structure to receptors for steroid hormones or thyroid hormone (T3). In addition, amino acid sequences of the ear-2 and ear-3 gene products are very similar each other especially at the DNA binding domain (86% homology) and at the putative ligand binding domain (76% homology). Northern hybridization with ear DNA probes of RNAs from various tissues of a human fetus reveals that the expression of ear-2 is high in the liver whereas the expression of ear-3 is relatively ubiquitous. Hybridization analysis of DNAs from sorted chromosomes shows that the ear-2 gene is located on chromosome 19 and ear-3 on chromosome 5, indicating that the two genes are clearly different from each other.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986 Jan 16;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coussens L., Yang-Feng T. L., Liao Y. C., Chen E., Gray A., McGrath J., Seeburg P. H., Libermann T. A., Schlessinger J., Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985 Dec 6;230(4730):1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Selden J. R., Laws G., Dorney D. J., Finan J., Tripputi P., Emanuel B. S., Rovera G., Nowell P. C., Croce C. M. A human c-erbA oncogene homologue is closely proximal to the chromosome 17 breakpoint in acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4495–4499. doi: 10.1073/pnas.81.14.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Fukushige S., Murotsu T., Matsubara K. Chromosomal assignment of human genes for gastrin, thyrotropin (TSH)-beta subunit and C-erbB-2 by chromosome sorting combined with velocity sedimentation and Southern hybridization. Biochem Biophys Res Commun. 1986 Jan 29;134(2):477–483. doi: 10.1016/s0006-291x(86)80445-4. [DOI] [PubMed] [Google Scholar]
- Gosden J. R., Middleton P. G., Rout D., De Angelis C. Chromosomal localization of the human oncogene ERBA2. Cytogenet Cell Genet. 1986;43(3-4):150–153. doi: 10.1159/000132313. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Jansson M., Philipson L., Vennström B. Isolation and characterization of multiple human genes homologous to the oncogenes of avian erythroblastosis virus. EMBO J. 1983;2(4):561–565. doi: 10.1002/j.1460-2075.1983.tb01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- MacLeod K. M., Baxter J. D. Chromatin receptors for thyroid hormones. Interactions of the solubilized proteins with DNA. J Biol Chem. 1976 Dec 10;251(23):7380–7387. [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Sakamoto S., Yamamoto T., Hayman M., Kawai S., Toyoshima K. Comparison of genome structures among three different strains of avian erythroblastosis virus. Gan. 1984 Apr;75(4):325–333. [PubMed] [Google Scholar]
- Ohashi M., Aizawa S., Ooka H., Ohsawa T., Kaji K., Kondo H., Kobayashi T., Noumura T., Matsuo M., Mitsui Y. A new human diploid cell strain, TIG-1, for the research on cellular aging. Exp Gerontol. 1980;15(2):121–133. doi: 10.1016/0531-5565(80)90083-2. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Semba K., Kamata N., Toyoshima K., Yamamoto T. A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6497–6501. doi: 10.1073/pnas.82.19.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheer D., Sheppard D. M., le Beau M., Rowley J. D., San Roman C., Solomon E. Localization of the oncogene c-erbA1 immediately proximal to the acute promyelocytic leukaemia breakpoint on chromosome 17. Ann Hum Genet. 1985 Jul;49(Pt 3):167–171. doi: 10.1111/j.1469-1809.1985.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Spurr N. K., Solomon E., Jansson M., Sheer D., Goodfellow P. N., Bodmer W. F., Vennstrom B. Chromosomal localisation of the human homologues to the oncogenes erbA and B. EMBO J. 1984 Jan;3(1):159–163. doi: 10.1002/j.1460-2075.1984.tb01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hihara H., Nishida T., Kawai S., Toyoshima K. A new avian erythroblastosis virus, AEV-H, carries erbB gene responsible for the induction of both erythroblastosis and sarcomas. Cell. 1983 Aug;34(1):225–232. doi: 10.1016/0092-8674(83)90153-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ikawa S., Akiyama T., Semba K., Nomura N., Miyajima N., Saito T., Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986 Jan 16;319(6050):230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]