Abstract
Activator/Dissociation (Ac/Ds) transposable elements from maize are widely used as insertional mutagenesis and gene isolation tools in plants and more recently also in medaka and zebrafish. They are particularly valuable for plant species that are transformation-recalcitrant and have long generation cycles or large genomes with low gene densities. Ac/Ds transposition frequencies vary widely, however, and in some species they are too low for large-scale mutagenesis. We discovered a hyperactive Ac transposase derivative, AcTPase4x, that catalyzes in the yeast Saccharomyces cerevisiae 100-fold more frequent Ds excisions than the wild-type transposase, whereas the reintegration frequency of excised Ds elements is unchanged (57%). Comparable to the wild-type transposase in plants, AcTPase4x catalyzes Ds insertion preferentially into coding regions and to genetically linked sites, but the mutant protein apparently has lost the weak bias of the wild-type protein for insertion sites with elevated guanine–cytosine content and nonrandom protein-DNA twist. AcTPase4x exhibits hyperactivity also in Arabidopsis thaliana where it effects a more than sixfold increase in Ds excision relative to wild-type AcTPase and thus may be useful to facilitate Ac/Ds-based insertion mutagenesis approaches.
Keywords: Activator (Ac), Dissociation (Ds), DDE, hyperactive transposase, hAT
DNA transposons are widely used in plants and animals as functional genomics tools and gene transfer vehicles. In plants, transposable elements are particularly valuable when large-scale T-DNA insertion mutagenesis is not feasible, e.g., for transformation-recalcitrant species or plants with long generation cycles. The success of transposon insertion mutagenesis strategies depends on high forward mutagenesis rates and a favorable distribution of novel insertions. As forward mutagenesis rates are frequently limited by transposase activity, attempts were made to find hyperactive transposase mutants. For some transposons, such mutants were fortuitously found; in other instances, systematic screening approaches and molecular evolution were successful (Goryshin and Reznikoff 1998; Beall et al. 2002; Baus et al. 2005; Keravala et al. 2006; Mates et al. 2009).
The maize Activator/Dissociation (Ac/Ds) transposable elements have been widely used in plants for gene tagging and functional genomics approaches because they are active in numerous plant species, integrate preferentially into or near to coding regions, and frequently transpose to genetically linked sites, enabling local saturation mutagenesis approaches (reviewed in Kunze and Weil 2002). The successful introduction of Ac/Ds elements into yeast revealed that application of these elements is not restricted to plants (Weil and Kunze 2000). Their recent adoption as tools for transgenesis and the generation of gene trap lines in the teleost fishes zebrafish and medaka further emphasized the wide range functionality of Ac/Ds elements (Emelyanov et al. 2006; Boon Ng and Gong 2011; Froschauer et al. 2012).
An impediment for a universal application is the variability of Ac/Ds transposition frequency in different plant species. In tobacco, Ac transposition frequency is similar as that in maize, with the majority of plants showing between 1 and 5% germinal excisions (Jones et al. 1990). In tomato and the monocot crops rice and barley, transposition frequencies range from 2 to 40% with a strong variance in individual lines (Belzile et al. 1989; Enoki et al. 1999; Koprek et al. 2000; Lazarow and Lütticke 2009). In contrast, germinal transposition frequencies in Arabidopsis typically are low, ranging from 0.07 to 5.7% (Schmidt and Willmitzer 1989; Dean et al. 1992). The varying transposition frequencies result from the complex regulation of Ac activity, including epigenetic inactivation by the host plant. The DNA methylation state affects the Ac promoter activity (Kunze et al. 1988) and the binding of the AcTPase to the subterminal binding sites (Wang et al. 1996; Wang and Kunze 1998; Ros and Kunze 2001). Moreover, there is no linear relationship between AcTPase levels and transposition frequency. McClintock (1951) has noted already that an increase of Ac copy number can result in developmentally delayed transposition and an overall decrease in transposition frequency (“inverse dose effect”). The AcTPase is active only in a limited concentration range because with increasing expression the protein aggregates into large, filament-like structures that are transpositionally inactive (Heinlein et al. 1994). The tight regulation of Ac transposition may be an evolutionary beneficial mechanism to protect the host against harmful transposition frequencies; however, for transposon tagging, it is a drawback.
Ac belongs to the eukaryotic hAT transposon superfamily (Kempken and Windhofer 2001; Kunze and Weil 2002) that had long been thought to be unique (Capy et al. 1997). Recently, it was suggested that the transposase catalytic centers of all eukaryotic transposon superfamilies, including the hAT elements, share a conserved aspartate-aspartate-glutamate (DDE) catalytic triad in an RNaseH-like fold with prokaryotic transposons like Tn5 and retroviral integrases (Kulkosky et al. 1992; Yuan and Wessler 2011). To this end, however, among the hAT elements, the basic necessity of the DDE motif was proven only for Hermes (Zhou et al. 2004).
In this work, we studied several mutant derivatives of the maize Activator transposase. We showed that the putative AcTPase DDE motif residues are essential for transposase activity. We found four amino acid substitutions that result in enhanced AcTPase activity. Their combination in the quadruple mutant AcTPase4x leads in yeast cells to a 100-fold increase in Ds element excision compared to the unaltered AcTPase. In stably transformed Arabidopsis plants, AcTPase4x also triggers an elevated Ds excision activity, suggesting that this protein might be suitable to improve Ac/Ds transposon-based insertion mutagenesis approaches.
Materials and Methods
Generation of AcTPase mutants
Amino acid substitutions were introduced into the AcTPase ORF by site-directed mutagenesis of plasmid pWL80 (Weil and Kunze 2000). Mutagenesis primer sequences are listed in the Supporting Information, Table S3. Double mutants were generated from the single mutants by inserting a SpeI/PflMI fragment from pWL80-E249A into pWL80-E336A and an EcoRI fragment from pWL80-D545A into pWL80-D459A, respectively. For construction of the AcTPase4X expression vector, a NarI/NheI AcTPase fragment from pWL80-E249A/E336A was inserted into pWL80-D459A/D545A.
Transposition assay in yeast
CWY1 yeast cells (ade2:Ds1; Weil and Kunze 2000) were transformed with the wild-type and mutant AcTPase expression vectors. Growth of yeast cells, selection of Ade+ revertants, and amplification of Ds excision sites were performed according to Weil and Kunze (2000).
DNA gel-blot analyses of Ds transposition in yeast
Genomic DNA from yeast was prepared with the E.Z.N.A. yeast DNA kit (Omega Bio-tek) according to the manufacturer’s instructions. For DNA gel-blot analysis, 1 µg yeast genomic DNA was digested with PvuI, fractionated by gel electrophoresis, and transferred to Amersham Hybond-NX membrane (GE Healthcare). Ds elements were detected by hybridization with a 404-bp DNA probe labeled with DIG-11-dUTP (Roche Diagnostics GmbH).
Analysis of Ds insertion sites in yeast
Splinkerette-PCR was performed according to Uren et al. (2009) with minor modifications. The first base (cytosine) of the Splink 1 primer and the long-strand adaptor were omitted. Gene-specific primer sequences are listed in Table S3. For amplification of Ds insertion sites, 300 ng yeast genomic DNA was digested with Sau3AI. Following adaptor ligation, the DNA was purified and used in the primary PCR.
Plant material and transformation
Arabidopsis thaliana Col-0 plants were transformed with the Ds donor construct by the floral-dip method (Clough and Bent 1998). Transgenic plants were selected on solid half-strength MS medium supplemented with 50 µg/ml kanamycin. Two independent Ds-transgenic lines, Ds5 and Ds24, were transformed with constructs carrying AcTPase and AcTPase4X expression cassettes. AcTPase and Ds transgenic plants were selected on half-strength MS medium supplemented with 50 µg/ml kanamycin and 25 µg/ml hygromycin B.
Construction of binary vectors
The 3-kb Ds element from pD16 was excised with PstI and PauI, blunted with T4-DNA-Polymerase (Fermentas), and inserted into the SmaI restriction site of pPGTKan3 (Kasaras and Kunze 2010). pD16 was derived from pKU4 (Baker et al. 1987) by insertion of the Ds element into the BamHI restriction site of pUC19. AcTPase expression constructs were generated by inserting the transposase expression cassette containing the AcTPase103–807 cDNA from pcATG10 (Kunze et al. 1993) into pCAMBIA1200, resulting in pCAM10ATG. Mutant AcTPase4X expression constructs were generated by exchanging an NsiI fragment from pcATG10 with the respective fragment region from pWL80-E249A/E336A/D459A/D545A and subsequent transfer as a BamHI fragment into pCAM10ATG.
Analysis of Ds excision sites in Arabidopsis
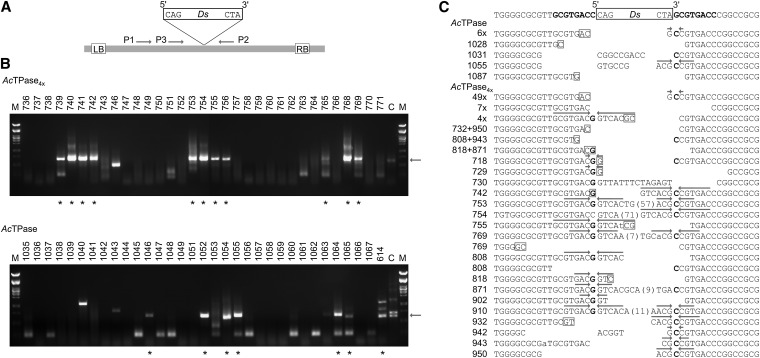
For analysis of Ds excision sites, 10 ng genomic DNA were amplified with primers P1 and P2, followed by a nested PCR with primers P3 and P2 (Figure 6A; Table S3). PCR products were fractionated by gel electrophoresis, and amplificates approximating the size of empty Ds donor sites (687 bp) were purified and sequenced either directly or after subcloning.
Figure 6 .
Analysis of Ds excision sites in Arabidopsis. (A) Schematic of the T-DNA in pCAM10ATG. LB, left T-DNA borders. RB, right T-DNA borders. Ds, 3-kb Ds element. P1–P3, primers used for amplification of empty Ds donor sites. (B) Screen for somatic Ds excision events. For each transposase, a representative agarose gel for screening and recovery of Ds excision sites from individual Arabidopsis plants is shown. Arrows at the right indicate the expected size for empty Ds donor-site amplificates of ∼687 bp. Asterisks indicate PCR products from individual plants that approximate this size and were subjected to sequence analysis. (C) Ds excision footprints formed by AcTPase and AcTPase4x in Arabidopsis. The top line shows the sequence at the Ds donor site. Letters in boldface flanking the Ds indicate the 8-bp target-site duplication. The lines below show transposon footprints in individual plants after Ds excision by AcTPase or AcTPase4x. The predominant footprint gc was detected in 6 AcTPase (“6×”) and in 49 AcTPase4x individuals (“49×”). A “restoring” excision product was recovered from seven AcTPase4x plants (“7×”). Four individual AcTPase4x plants share the same footprint (“4×”). From plant nos. 769, 818, 871, 943, and 950 two and from plant no. 808 three distinct footprints were isolated, respectively. Bases with weak signal strength in the sequence reads are indicated in lowercase. Putative microhomologies at flanking DNA fusion sites are indicated as boxed nucleotides. Arrows above sequences highlight inverted repeats centered around the complementary bases G and C of the nucleotides bordering the Ds element that result from resolution of intermediate hairpin structures formed at the Ds-flanking host DNA during excision.
Results
Substitution of the putative DDE motif residues abolishes AcTPase activity
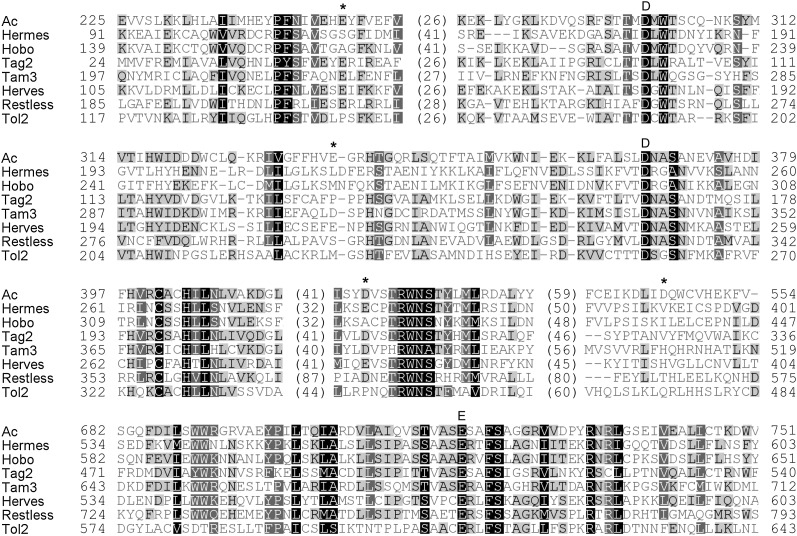
The amino acid sequence alignment of the AcTPase with the Hermes transposase and six other hAT element transposases from plants, insects, fungi, and fish suggests that the highly conserved AcTPase residues D301, D367, and E719, located in the conserved hAT1 and hAT3 regions, form the DDE motif in the catalytic center of the protein (Figure 1 and Figure 2A). We substituted each of these residues by alanine in the N-terminally truncated AcTPase103–807 (subsequently referred to as AcTPase) (Kunze et al. 1993) and expressed the mutant proteins in the yeast (Saccharomyces cerevisiae) strain CWY1, which carries a nonautonomous Ds element in the yeast ADE2 gene (Weil and Kunze 2000). The activity of AcTPase derivatives was measured by the number of Ade+ revertants. No Ds excision events were detected among 1011 cells for any of the three transposase mutants AcTPaseD301A, AcTPaseD367A, and AcTPaseE719A (Figure 2B). Proper expression and integrity of the AcTPase derivatives was verified by protein blot analysis (Figure 2C). The complete loss of AcTPase activity demonstrates that the residues D301, D367, and E719 are essential for AcTPase catalytic function and supports the notion that these residues form the DDE motif of the AcTPase. Mutation of any of the catalytic DDE residues should completely abolish transposase activity as has been shown for retroviral integrases, bacterial and Tc1/mariner superfamily transposases, and the hAT element Hermes transposase (reviewed in Haren et al. 1999; Zhou et al. 2004).
Figure 1 .
Alignment of eight hAT transposases. The DDE triad is marked with letters above the alignment. Asterisks indicate the four amino acids E249, E336, D459, and D545 mutated in the hyperactive AcTPase4X.
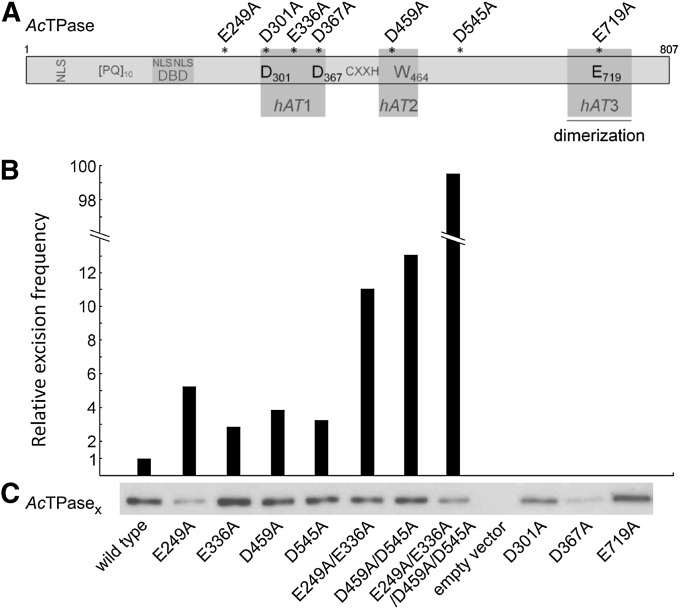
Figure 2 .
Ds excision activities of AcTPase mutants. (A) Schematic of the Ac transposase. The three amino acid sequence regions hAT1 (aa 293–376), hAT2 (aa 442–490) and hAT3 (aa 678–759) are conserved in hAT transposases. The hAT3 region is a dimerization domain. Residues D301, D367, and E719 presumably form the catalytic triad, and tryptophan W464 is supposedly involved in hairpin formation. NLS, nuclear localization signals. DBD, DNA-binding domain. (PQ)10, dipeptide repeat essential for AcTPase function. (B) Relative Ds excision frequencies triggered by AcTPase derivatives in yeast. The values are the median of nine independent experiments for the wild-type AcTPase and the quadruple AcTPase mutant and of five independent experiments for the other AcTPase derivatives. For the relative values, Ds excision frequency induced by wild-type AcTPase was set to 1. The absolute median excision frequency promoted by wild-type AcTPase was 2.9 × 10−5 Ade+ cells/total living cells and for the quadruple mutant 288 × 10−5 Ade+ cells/total cells. (C) Protein gel-blot analysis of wild-type AcTPase and mutant derivative expression in yeast.
Quadruple mutant AcTPase4x is hyperactive in yeast
In the course of functional analyses of the AcTPase, we have generated various mutant AcTPase proteins with single amino acid substitutions and screened their excision activities in the yeast transposition assay (Figure 2). Of these, the four AcTPase derivatives E249A, E336A, D459A, and D545A induced three- to fivefold increases in Ds excision frequency when compared to the native AcTPase (Figure 2B). To investigate if the combination of these mutations leads to a further enhancement, we first constructed the two double mutants AcTPaseE249A/E336A and AcTPaseD459A/D545A. With 11- and 13-fold increases in Ds excision frequency, both double mutants exhibit a more-than-additive rise in activity (Figure 2B). Subsequently, we generated the quadruple mutant AcTPaseE249A/E336A/D459A/D545A (AcTPase4x). The expression of this protein resulted in a 10-fold increase in Ds excisions relative to the two double mutants and an almost 100-fold increase compared to wild-type AcTPase (Figure 2B), indicating a strong synergistic effect of the mutations. The transposase amounts in the yeast cells expressing AcTPase4x and AcTPaseE249A are slightly reduced (Figure 2C). Although AcTPase is subject to overexpression inhibition (Heinlein et al. 1994; Weil and Kunze 2000), this slightly reduced transposase level is unlikely to account for the significant increase in Ds mobilization activity of AcTPase4x.
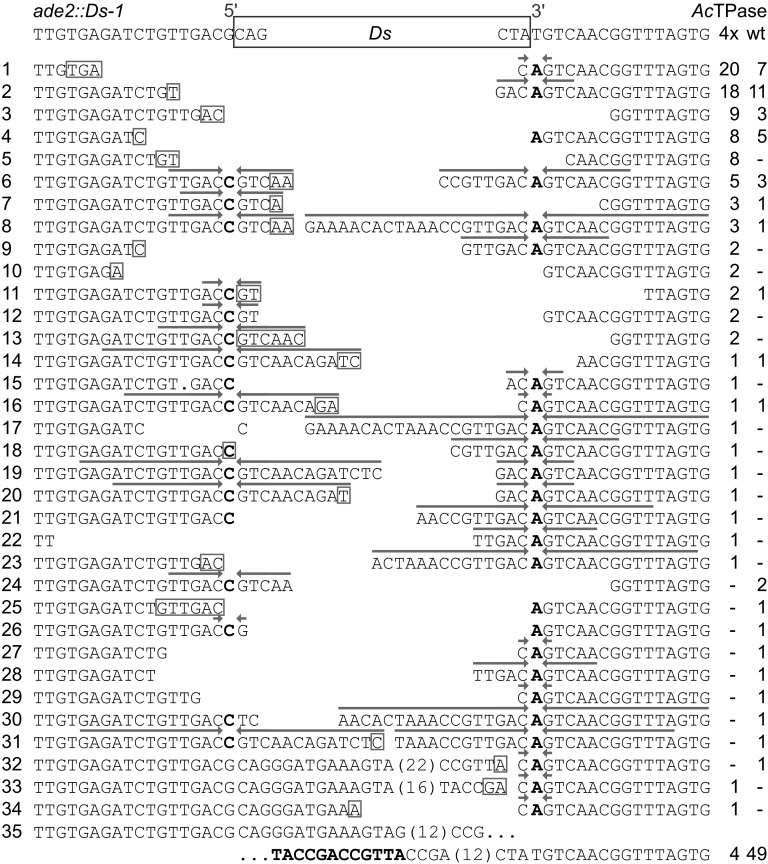
To investigate if the enhanced AcTPase4x activity is accompanied by altered processing of the Ds excision site, we analyzed the empty donor sequences in 93 and 100 Ade+ revertants generated by AcTPase and AcTPase4x, respectively (Figure 3). In 43 AcTPase- and 92 AcTPase4x-derived Ade+ revertants, the Ds excision site sequences are consistent with the DNA hairpin model for Ds footprint formation (Kunze and Weil 2002). Two footprints formed by AcTPase4x (Figure 3, rows 15 and 17) and one formed by AcTPase (Figure 3, row 30) conflict with this model. We cannot distinguish if these sequences result from aberrant DNA repair after Ds excision or from PCR errors. In three other Ade+ revertants, two generated by AcTPase4x and one by the unmutated AcTPase, 11–42 bases from the transposon 5′ end are left behind at the excision site (Figure 3, rows 32–34), whereas the 3′ end has been processed according to the hairpin model. In these revertants, no reintegration of Ds was detected, suggesting that they result from abortive excision events. In the remaining 4 AcTPase4x- and 49 AcTPase-generated Ade+ revertants, Ds was only partially lost (Figure 3, row 35). In all these “type 35” revertants, the same 42 bases from the Ds 5′ end and 19 bases from the Ds 3′end, including both terminal inverted repeats, are left behind. These revertants could result from homologous recombination at the 12-bp direct repeat TACCGACCGTTA present in the Ds 5′ and 3′ subterminal regions. Consistent with this assumption, no reintegration of Ds was observed in any of these revertants. The wild-type AcTPase apparently gave rise to 12-fold more type 35 revertants than the AcTPase4x. Although the overall number of the 93 AcTPase-generated Ade+ revertants were isolated from seven independent experiments with, on average, 16 revertants per culture, we cannot exclude that the high number of type 35 revertants partially results from clonal propagation of early events after induction of transposase expression in the yeast culture.
Figure 3 .
Ds excision footprints formed by AcTPase and AcTPase4x in yeast. The top row shows the sequence at the Ds insertion site in the yeast ADE2 gene. Rows 1–35 show the recovered Ds excision footprints and at the right their incidence in independent Ade+ revertants from wild-type AcTPase (wt) or hyperactive AcTPase4X (4x) expressing cells. Putative microhomologies at flanking DNA fusion sites are indicated as boxed nucleotides. Arrows above sequences highlight inverted repeats centered around the complementary bases C and A of the nucleotides bordering the Ds element that result from resolution of intermediate hairpin structures formed at the Ds-flanking host DNA during excision. Boldface letters in row 35 indicate the remaining copy of the 12-bp direct repeat in the Ds ends.
Not counting the type 35 revertants, in AcTPase4x- and AcTPase-expressing cells, 25 and 19 different Ds excision footprint sequences were isolated. Both transposases generate the same two predominant footprint types accounting for 40% (n = 96) of the footprints formed by AcTPase4x and 41% (n = 44) of the footprints formed by AcTPase (Figure 3, rows 1 and 2), suggesting that AcTPase4x does not differ from AcTPase in the biochemistry of Ds excision. Moreover, at most Ds excision sites, generated by either version of the transposase, the chromosome breaks seem to have been resolved by microhomology-dependent end-joining (Yu et al. 2004) (Figure 3).
Ds reintegration rate affected by AcTPase4x is unchanged in yeast
Although the two DNA single-strand cleavage steps at the transposon donor site and the strand-transfer step to the target DNA in nonreplicative transposition are catalyzed by the same active center of the transposase, they can be uncoupled in transposase mutants (Bolland and Kleckner 1996). We therefore compared the reintegration frequency of Ds elements in Ade+ revertants obtained with AcTPase or AcTPase4x. DNA gel-blot analysis revealed that AcTPase4x catalyzes Ds reintegration as efficiently as the wild-type protein: ignoring the type 35 revertants, 57% (n = 44) of the excision events conducted by the native AcTPase and 57% (n = 88) of the events induced by AcTPase4x were followed by reintegration of Ds into a new genomic position. In all cases, a single reintegrated Ds copy was observed (Figure 4A). Consequently, not only Ds excisions but also the total number of Ds reintegrations per yeast cell effected by the AcTPase4x protein is ∼100-fold higher than with the AcTPase.
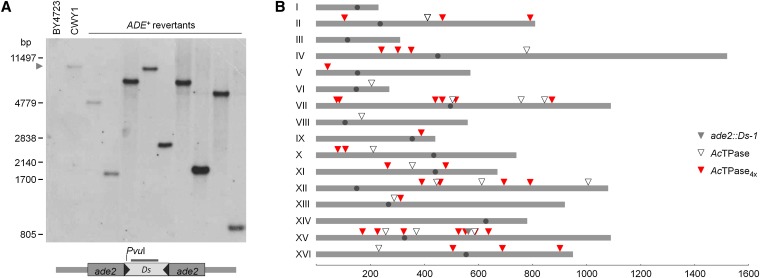
Figure 4 .
Reintegration of Ds elements in yeast. (A) Examples for reintegrated Ds elements from AcTPase4x-promoted transposition. DNA gel blot with PvuI-digested genomic DNA from yeast strains BY4723, CWY1, and nine individual Ade+ revertants, hybridized with a Ds-specific probe. The gray arrowhead marks the Ds donor site in the ade2 locus of yeast strain CWY1. (B) Distribution of Ds insertion sites in the yeast genome. The 16 yeast chromosomes are drawn to scale as gray bars with the centromere for each chromosome indicated by a dot. The gray triangle marks the initial position of the Ds element in the ade2 locus on yeast chromosome XV. Red triangles indicate reintegration of the Ds element promoted by the hyperactive AcTPase4X, and open triangles by the AcTPase. Scale bar is in kilobase pairs.
AcTPase4x exhibits an altered Ds insertion sequence preference
Upon integration at new genomic positions, hAT transposable elements generate 8-bp target site duplications (TSD). By sequencing the flanking genomic DNA on both sides of 14 Ds reinsertions formed by AcTPase4x and 7 reinsertions formed by wild-type AcTPase, we confirmed that in all cases the expected TSD was created (Table S1), indicating that the architecture of the transpososome is not or is only marginally altered with the AcTPase4x protein.
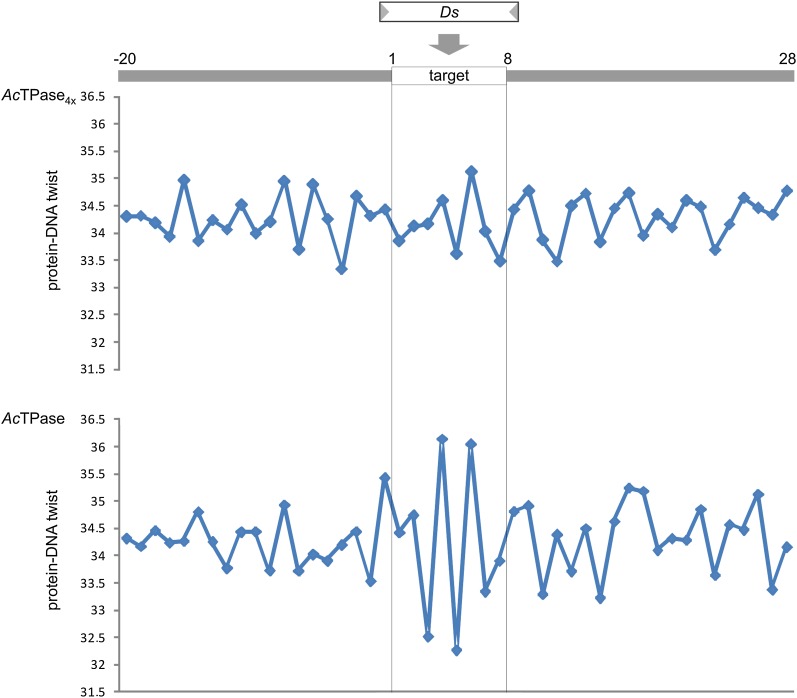
Recently, in maize a preference for Ds insertions into DNA target sites with an elevated guanine–cytosine (GC) content and highly nonrandom protein–DNA twist values was detected (Vollbrecht et al. 2010). The average GC content of the 18 and 35, 8-bp target sites chosen by AcTPase and AcTPase4x is 42.4 and 38.9%, respectively (Table S1). The GC content in a 400-bp window centered on the 18 Ds insertion sites chosen by AcTPase is 40.2% and that for the 35 Ds reinsertions promoted by AcTPase4x is 38.9%. Thus, the GC content of 8-bp Ds insertion sites and the chromosomal vicinity targeted by wild-type AcTPase is slightly higher than that of the AcTPase4x landing regions, which closely match the average yeast genomic GC content of 39%. We then analyzed the 8-bp Ds insertion site sequences ±20 bp on either side for protein DNA-twist prediction (Olson et al. 1998). The insertion sites chosen by the AcTPase display a pattern of alternating protein–DNA twist values with a twofold symmetry about the center of the target site that is highly similar to the pattern observed in maize. Interestingly, the target sites chosen by the AcTPase4x do not exhibit such a pattern (Figure 5).
Figure 5 .
Predicted twist-angle torsion at Ds insertion sites in yeast. Average values for protein-DNA twist (y axis) are plotted for each nucleotide position against the sequence of the 8-bp Ds insertion sites ±20 bp adjoining sequence (x-axis) from 35 AcTPase4x-generated Ds insertions (top) and 18 AcTPase-generated Ds insertions (bottom). The borders of the 8-bp Ds target sites are indicated by the vertical lines.
Preference for linked transposition and insertion into genes is conserved for AcTPase4x
Mapping of the Ds reinsertion sites on the yeast chromosomes indicates for both transposases a weak preference for linked transposition (Figure 4B). Seven of the 34 mapped AcTPase4X-driven Ds transpositions (21%) reinserted on chromosome XV, 4 of them <72 kb distant from the Ds launch pad in the ade2 locus (Figure 4B; Table S1 and Table S2). When Ds was mobilized by the wild-type AcTPase, 3 of 17 reintegrations (18%) occurred on chromosome XV, one of them at a distance of only 20 kb (Figure 4B; Table S1 and Table S2). Accordingly, both transposases deployed twice as many Ds insertions into the chromosomal vicinity than expected for random distribution in the genome (Table S2). Even though the yeast genome with 70% open reading frames has a much higher fraction of coding sequences than plants, it is conspicuous that 33 (94%) of the 35 analyzed AcTPase4X-promoted and 17 (94%) of the 18 AcTPase-promoted Ds insertions occurred in annotated genes or predicted open reading frames. Each transposase produced one insertion into moderately repeated sequences, yeast retrotransposon TYA, and the long terminal repeat of TY3, respectively.
Hyperactive AcTPase4X induces more frequent Ds excision in planta
To investigate whether AcTPase4X is also hyperactive in plants, we generated two independent A. thaliana starter lines that carry a 3-kb Ds element flanked by short, GC-rich sequences from the original wx-m7 maize locus including the TSD. The Ds starter lines were transformed with expression cassettes for either AcTPase or AcTPase4x under control of the Agrobacterium tumefaciens TR-octopine DNA 2′ promoter. One to two rosette leaves from 382 individual Arabidopsis plants containing both the Ds element and the AcTPase or AcTPase4x expression construct were analyzed for somatic excision of Ds (Table 1 and Figure 6).
Table 1 . Ds excision in Arabidopsis.
| Ds excision sitea | |||||||
|---|---|---|---|---|---|---|---|
| Transposase | Lineb | Plantsc | Excisionsd | A | B | C | D |
| AcTPase | 30 | 54 | — | — | — | — | — |
| 31 | 50 | — | — | — | — | — | |
| 20 | 66 | 10 | 6 | 4 | — | — | |
| Σe | 170 | 10 | 6 | 4 | — | — | |
| AcTPase4x | 29 | 68 | 21 | 7 | 5 | 6 | 3 |
| 42 | 72 | 21 | 13 | — | 5 | 3 | |
| 41 | 72 | 28 | 17 | 3 | 4 | 4 | |
| Σ | 212 | 70 | 37 | 8 | 15 | 10 | |
Classification of individual plants according to the Ds excision sites isolated. A: number of plants with “gc” footprint. B: number of plants showing unique footprints including “restoring” excision products. C: number of plants with multiple footprints. D: sequence was unreadable distal to the Ds excision site.
The three independent plant lines analyzed for each transposase are, with the exception of 29 (Ds5), all derived from Ds starter line Ds24.
Number of plants analyzed for excision of Ds.
Number of individual plants with empty Ds donor sites.
Σ = total number of AcTPase and AcTPase4x plants and excisions, respectively.
From 10 (6%) of the 170 AcTPase-expressing plants, Ds footprints could be amplified and sequenced (Table 1). These samples were all derived from progeny of parental plant no. 20. In progeny from parental lines no. 30 and 31, no empty donor sites could be amplified although they expressed AcTPase. In contrast, from 70 (33%) of the 212 descendants of the three independent AcTPase4x-expressing parental plants, empty donor sites were amplified (Table 1). From 15 of these plants, two to five different Ds excision products were recovered, indicating that multiple independent somatic Ds excisions had occurred in the leaves. Ten empty donor-site amplificates from AcTPase4x-expressing plants could not be sequenced across the Ds excision site because several sequences were superimposed distal to it. However, all 10 sequence reads, starting from primer P2 in the 3′-flanking DNA (Figure 6A), terminate at the former Ds position with a cytosine that is the complement of the guanine immediately flanking the Ds 3′ end. This cytosine is generated during hairpin resolution and thus proves that transposase-promoted Ds excision had occurred at these sites. Taken together, we detected a more than sixfold higher somatic Ds excision frequency in Arabidopsis plants expressing AcTPase4x compared to plants expressing the unmutated protein, indicating that the AcTPase4x is also hyperactive in plants.
Both transposases give rise to the same predominant “gc” footprint (Table 1 and Figure 6C). It was detected in 60% of the AcTPase-generated Ds excision sites and in 52% of the AcTPase4x-generated Ds excision sites (49 of 95 sequence reads including those with superimposed sequences behind the Ds). These frequencies closely match the results from a recent deep-sequencing survey of Ac/Ds excision from a donor locus flanked by the identical 8-bp TSD in Arabidopsis, where the same predominant gc footprint was found to account for ∼55% of all reads (Huefner et al. 2011).
Unique Ds excision footprints were detected in four AcTPase- and in 21 AcTPase4x-expressing plants, including 13 plants with multiple excision events (Figure 6C). The generation of the gc footprint and the majority of unique footprints can be explained by the hairpin model in conjunction with the microhomology-dependent nonhomologous end-joining (NHEJ) model for DNA break repair (Figure 6C) (Yu et al. 2004). The residual nine footprints (plant nos. 1031, 1055, 730, 753, 754, 769, 871, 910, and 942) consist of segments derived by processing of the flanking DNA hairpin and additional nonpalindromic nucleotides that originate neither from the Ds nor from the flanking DNA. Ds excision sites from plant nos. 753 and 754 (Figure 6C) contain insertions of 57 and 71 bases, respectively, which partially match Arabidopsis genomic sequences. In 7 AcTPase4x plants (7.4% of the 95 sequence reads), we detected excision sites where the Ds element and one copy of the duplicated target site were precisely eliminated, resulting in restoration of the original target-site sequence (“restoring excision”; Figure 6C).
Discussion
The efficiency of transposon insertional mutagenesis approaches is frequently hampered by low transposase activity, which may have evolved to protect the host genome against unfavorably high mutation frequencies. Consistent with this idea, various hyperactive transposases were identified by mutagenesis in prokaryotic and animal transposons (Weinreich et al. 1994; Lampe et al. 1999; Beall et al. 2002; Baus et al. 2005; Mates et al. 2009; Yusa et al. 2011). The maize Activator transposase mutants reported here are the first examples of a hyperactive plant transposase. The alignment of eight hAT element transposases, most of which were shown to be active, illustrates that none of the amino acids substituted in the hyperactive AcTPase4x are in highly conserved positions (Figure 1). The hyperactive mutations act synergistically as their combination into a quadruple mutant AcTPase4x results in a 100-fold higher Ds excision activity in yeast. Synergistic enhancement of hyperactivity following the combination of two to nine single amino acid substitutions has also been observed for Tn5, piggyBac, Himar1, and Sleeping Beauty transposases (Weinreich et al. 1994; Lampe et al. 1999; Baus et al. 2005; Yusa et al. 2011).
Except for Tn5, little is known about the biochemical mechanisms of transposase hyperactivity. In the case of SB100X, it is hypothesized that the mutations alter the folding properties of the transposase (Mates et al. 2009). As the four amino acid substitutions in AcTPase4x are located in a region that is assumed to contain a multimerization interface (Essers et al. 2000) and with increasing concentration the AcTPase activity is limited by progressive aggregation into nonfunctional complexes (Heinlein et al. 1994), we tested whether AcTPase4x has altered aggregation properties. However, upon expression in Escherichia coli, yeast, and petunia protoplasts, we did not detect any difference in the wild-type AcTPase (our unpublished results). The hyperactive mutation D459A is only two amino acids apart from a tryptophan residue that is highly conserved among hAT transposases (Figure 1; Zhou et al. 2004). In Hermes and piggyBac transposases, this tryptophan supposedly acts in DNA hairpin formation and base flipping (Zhou et al. 2004; Mitra et al. 2008).
AcTPase4x is also hyperactive in Arabidopsis, although apparently not as pronounced as in yeast. However, the comparative quantification of AcTPase activity in sporophytic plant tissue by amplification of Ds excision sites is inherently imprecise and underestimates the excision frequency, as excisions in single cells and very small tissue sectors may remain undetected and multiple independent excisions generating the same (predominant) footprint are counted as one event. On the other hand, it is also possible that hyperactivity is unequally expressed in different host systems. Of 18 piggyBac transposase mutants showing hyperactivity in yeast, only five exhibited hyperactivity in mammalian cells (Yusa et al. 2011). Similarly, hyperactivity of Sleeping Beauty transposase SB10 in HeLa cells was stronger than in mice (Baus et al. 2005).
DNA hairpin formation, resolution, repair, and end joining at the empty donor site, first suggested by Coen et al. (1986) for the snapdragon Tam3 element, is the universal excision site processing pathway for hAT transposons (Coen et al. 1986; Kunze and Weil 2002; Yu et al. 2004). This mechanism results in footprints with short deletions and palindromes at the repaired DNA joint centered around the complement of the base adjacent to the transposon ends. The vast majority (90%) of footprints, including the predominant ones, generated by AcTPase4x and AcTPase in yeast and in Arabidopsis, can be explained by this model. Fifteen of the 22 distinct footprint types that had been reported by Yu et al. (2004) using the wild-type AcTPase were also recovered from wild-type AcTPase- and AcTPase4x-expressing yeast cells in this study, suggesting that the basic excision mechanism remains unaffected by the four amino acid substitutions in AcTPase4x. This conclusion is corroborated by the Ds excision footprint spectrum in planta. The frequency of the predominant gc footprint generated by the quadruple mutant AcTPase4x, the low frequency of 4% restoring Ds excisions, and the occurrence of excision products with short sequence insertions, suggesting that transposon excision-induced double-strand break repair is not strictly dependent on the canonical NHEJ pathway factors Ku70 and DNA ligase IV (Lig4), are concordant with the results from a recent deep sequencing survey of somatic Ds excision catalyzed by the wild-type Ac transposase in Arabidopsis (Huefner et al. 2011).
Among the Ade+ revertants from AcTPase4x and, more frequently, from AcTPase-expressing cells, we detected the same type of apparently incomplete Ds excision products (Figure 3, row 35) that had also been found by Yu et al. (2004). This product could emerge either from a homologous recombination event between two perfect 12-bp direct repeats in both transposon ends or from gene conversion and microhomology-dependent NHEJ after excision of Ds during replication from one daughter chromatid. As this product was never obtained in the absence of transposase, we speculate that its formation is promoted by the transposase in a similar way as in plants, where the presence of an active Ac element can greatly enhance intramolecular homologous recombination between direct repeat sequences in maize and Arabidopsis (Athma and Peterson 1991; Xiao et al. 2000). In the transpososome, the AcTPase synapses the transposon ends, which might stimulate recombination between directly repeated sequences in the flanking host DNA and possibly also within the transposon ends.
Consistent with other Ac/Ds transposition studies in plants and yeast, we do not recognize a target sequence preference for the Ds insertion site selection with the AcTPase or AcTPase4x. The analysis of 1741 Ds insertion sites in maize revealed, however, that the elements have a preference for insertion into sequences with a high GC content and that the insertion sites exhibit a pattern of alternating nucleotide pairs with more and less than average DNA deformability, respectively (Vollbrecht et al. 2010). This suggests that Ac/Ds insertion is guided by structural features of the DNA rather than by the primary DNA sequence. Interestingly, for the wild-type AcTPase, we observe in yeast, similar to that in maize, a slightly elevated GC content at the insertion sites and a periodicity in the protein-DNA twist, whereas the hyperactive AcTPase4x apparently has largely lost the preference for target sites with these features.
In plants, 50–80% of excised Ac/Ds elements reinsert into the genome (reviewed in Kunze et al. 1997; Kunze and Weil 2002). The hyperactive AcTPase4x induces the same reintegration frequency as the wild-type protein, suggesting that excision and reintegration activities are effected by different amino acids. Similarly, in a hyperactive piggyBac transposase, the excision reaction was enhanced, whereas the integration reaction remained unchanged (Yusa et al. 2011).
In plants, the propensity of Ac and Ds elements to transpose to genetically linked sites and to insert into coding regions and the lack of insertion-site specificity have proven to be advantageous. In this study, we find that these properties are largely conserved in yeast. In comparison, the transposase of the insect transposon Hermes directs only 40 to 45% of insertions into ORFs of the Saccharomyces genome (Gangadharan et al. 2010). Although it has not been shown yet whether the AcTPase4x excision and reintegration properties are quantitatively similar in plants, the protein is a promising candidate to improve transposon mutagenesis and gene-tagging efficiencies.
Supplementary Material
Acknowledgments
We thank Anne Herrmann for excellent technical support. This work was supported by Deutsche Forschungsgemeinschaft grant KU-715/9.
Footnotes
Communicating editor: S. E. Bickel
Literature Cited
- Athma P., Peterson T., 1991. Ac induces homologous recombination at the maize P locus. Genetics 128: 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B., Coupland G., Fedoroff N. V., Starlinger P., Schell J., 1987. Phenotypic assay for excision of the maize controlling element Ac in tobacco. EMBO J. 6: 1547–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baus J., Liu L., Heggestad A. D., Sanz S., Fletcher B. S., 2005. Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol. Ther. 12: 1148–1156 [DOI] [PubMed] [Google Scholar]
- Beall E. L., Mahoney M. B., Rio D. C., 2002. Identification and analysis of a hyperactive mutant form of Drosophila P-element transposase. Genetics 162: 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzile F., Lassner M. W., Tong Y., Khush R., Yoder J. I., 1989. Sexual transmission of transposed Activator elements in transgenic tomatoes. Genetics 123: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland S., Kleckner N., 1996. The three chemical steps of Tn10/IS10 transposition involve repeated utilization of a single active site. Cell 84: 223–233 [DOI] [PubMed] [Google Scholar]
- Boon Ng G. H., Gong Z., 2011. Maize Ac/Ds transposon system leads to highly efficient germline transmission of transgenes in medaka (Oryzias latipes). Biochimie 93: 1858–1864 [DOI] [PubMed] [Google Scholar]
- Capy P., Langin T., Higuet D., Maurer P., Bazin C., 1997. Do the integrases of LTR-retrotransposons and class II element transposases have a common ancestor? Genetica 100: 63–72 [PubMed] [Google Scholar]
- Clough S. J., Bent A. F., 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R., Martin C., 1986. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell 47: 285–296 [DOI] [PubMed] [Google Scholar]
- Dean C., Sjodin C., Page T., Jones J. D. G., Lister C., 1992. Behavior of the maize transposable element Ac in Arabidopsis thaliana. Plant J. 2: 69–81 [Google Scholar]
- Emelyanov A., Gao Y., Naqvi N. I., Parinov S., 2006. Trans-kingdom transposition of the maize Dissociation element. Genetics 174: 1095–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki H., Izawa T., Kawahara M., Komatsu M., Koh S., et al. , 1999. Ac as a tool for the functional genomics of rice. Plant J. 19: 605–613 [DOI] [PubMed] [Google Scholar]
- Essers L., Adolphs R. H., Kunze R., 2000. A highly conserved domain of the maize Activator transposase is involved in dimerization. Plant Cell 12: 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froschauer A., Sprott D., Gerwien F., Henker Y., Rudolph F., et al. , 2012. Effective generation of transgenic reporter and gene trap lines of the medaka (Oryzias latipes) using the Ac/Ds transposon system. Transgenic Res. 21: 149–162 [DOI] [PubMed] [Google Scholar]
- Gangadharan S., Mularoni L., Fain-Thornton J., Wheelan S. J., Craig N. L., 2010. DNA transposon Hermes inserts into DNA in nucleosome-free regions in vivo. Proc. Natl. Acad. Sci. USA 107: 21966–21972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryshin I. Y., Reznikoff W. S., 1998. Tn5 in vitro transposition. J. Biol. Chem. 273: 7367–7374 [DOI] [PubMed] [Google Scholar]
- Haren L., Ton-Hoang B., Chandler M., 1999. Integrating DNA: transposases and retroviral integrases. Annu. Rev. Microbiol. 53: 245–281 [DOI] [PubMed] [Google Scholar]
- Heinlein M., Brattig T., Kunze R., 1994. In vivo aggregation of maize Activator (Ac) transposase in nuclei of maize endosperm and Petunia protoplasts. Plant J. 5: 705–714 [DOI] [PubMed] [Google Scholar]
- Huefner N. D., Mizuno Y., Weil C. F., Korf I., Britt A. B., 2011. Breadth by depth: expanding our understanding of the repair of transposon-induced DNA double strand breaks via deep-sequencing. DNA Repair (Amst.) 10: 1023–1033 [DOI] [PubMed] [Google Scholar]
- Jones J. D. G., Carland F., Harper L., Lim E., Dooner H., 1990. Genetic Properties of the Maize Transposon Activator (Ac) in Tobacco, pp. 59–64 in Plant Gene Transfer - UCLA Symposia on Molecular and Cellular Biology, New Series, Vol. 129, edited by C. J. Lamb and R. N. Beachy. Wiley-Liss Inc., New York [Google Scholar]
- Kasaras A., Kunze R., 2010. Expression, localisation and phylogeny of a novel family of plant-specific membrane proteins. Plant Biol (Stuttg) 12(Suppl. 1): 140–152 [DOI] [PubMed] [Google Scholar]
- Kempken F., Windhofer F., 2001. The hAT family: a versatile transposon group common to plants, fungi, animals, and man. Chromosoma 110: 1–9 [DOI] [PubMed] [Google Scholar]
- Keravala A., Liu D., Lechman E. R., Wolfe D., Nash J. A., et al. , 2006. Hyperactive Himar1 transposase mediates transposition in cell culture and enhances gene expression in vivo. Hum. Gene Ther. 17: 1006–1018 [DOI] [PubMed] [Google Scholar]
- Koprek T., McElroy D., Louwerse J., Williams-Carrier R., Lemaux P. G., 2000. An efficient method for dispersing Ds elements in the barley genome as a tool for determining gene function. Plant J. 24: 253–263 [DOI] [PubMed] [Google Scholar]
- Kulkosky J., Jones K. S., Katz R. A., Mack J. P. G., Skalka A. M., 1992. Residues critical for retrovirus integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol. Cell. Biol. 12: 2331–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R., Weil C. F., 2002. The hAT and CACTA superfamilies of plant transposons, pp. 565–610 in Mobile DNA II, edited by Craig N. L., Craigie R., Gellert M., Lambowitz A. M. ASM Press, Washington, D.C [Google Scholar]
- Kunze R., Starlinger P., Schwartz D., 1988. DNA methylation of the maize transposable element Ac interferes with its transcription. Mol. Gen. Genet. 214: 325–327 [Google Scholar]
- Kunze R., Behrens U., Courage-Franzkowiak U., Feldmar S., Kühn S., et al. , 1993. Dominant transposition-deficient mutants of maize Activator (Ac) transposase. Proc. Natl. Acad. Sci. USA 90: 7094–7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R., Saedler H., Lönnig W.-E., 1997. Plant transposable elements, pp. 331–470 in Advances in Botanical Research, Vol. 27, edited by J. A. Callow. Academic Press, San Diego [Google Scholar]
- Lampe D. J., Akerley B. J., Rubin E. J., Mekalanos J. J., Robertson H. M., 1999. Hyperactive transposase mutants of the Himar1 mariner transposon. Proc. Natl. Acad. Sci. USA 96: 11428–11433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow K., Lütticke S., 2009. An Ac/Ds-mediated gene trap system for functional genomics in barley. BMC Genomics 10: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates L., Chuah M. K., Belay E., Jerchow B., Manoj N., et al. , 2009. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41: 753–761 [DOI] [PubMed] [Google Scholar]
- McClintock B., 1951. Chromosome organization and genic expression. Cold Spring Harb. Symp. Quant. Biol. 16: 13–47 [DOI] [PubMed] [Google Scholar]
- Mitra R., Fain-Thornton J., Craig N. L., 2008. piggyBac can bypass DNA synthesis during cut and paste transposition. EMBO J. 27: 1097–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W. K., Gorin A. A., Lu X. J., Hock L. M., Zhurkin V. B., 1998. DNA sequence-dependent deformability deduced from protein-DNA crystal complexes. Proc. Natl. Acad. Sci. USA 95: 11163–11168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros F., Kunze R., 2001. Regulation of Activator/Dissociation transposition by replication and DNA methylation. Genetics 157: 1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Willmitzer L., 1989. The maize autonomous element Activator (Ac) shows a minimal germinal excision frequency of 0.2%–0.5% in transgenic Arabidopsis thaliana plants. Mol. Gen. Genet. 220: 17–24 [Google Scholar]
- Uren A. G., Mikkers H., Kool J., van der Weyden L., Lund A. H., et al. , 2009. A high-throughput splinkerette-PCR method for the isolation and sequencing of retroviral insertion sites. Nat. Protoc. 4: 789–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht E., Duvick J., Schares J. P., Ahern K. R., Deewatthanawong P., et al. , 2010. Genome-wide distribution of transposed Dissociation elements in maize. Plant Cell 22: 1667–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Kunze R., 1998. Transposase binding site methylation in the epigenetically inactivated Ac derivative Ds-cy. Plant J. 13: 577–582 [DOI] [PubMed] [Google Scholar]
- Wang L., Heinlein M., Kunze R., 1996. Methylation pattern of Activator (Ac) transposase binding sites in maize endosperm. Plant Cell 8: 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil C. F., Kunze R., 2000. Transposition of maize Ac/Ds transposable elements in the yeast Saccharomyces cerevisiae. Nat. Genet. 26: 187–190 [DOI] [PubMed] [Google Scholar]
- Weinreich M. D., Gasch A., Reznikoff W. S., 1994. Evidence that the cis preference of the Tn5 transposase is caused by nonproductive multimerization. Genes Dev. 8: 2363–2374 [DOI] [PubMed] [Google Scholar]
- Xiao Y. L., Li X., Peterson T., 2000. Ac insertion site affects the frequency of transposon-induced homologous recombination at the maize p1 locus. Genetics 156: 2007–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Marshall K., Yamaguchi M., Haber J. E., Weil C. F., 2004. Microhomology-dependent end joining and repair of transposon-induced DNA hairpins by host factors in Saccharomyces cerevisiae. Mol. Cell. Biol. 24: 1351–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y. W., Wessler S. R., 2011. The catalytic domain of all eukaryotic cut-and-paste transposase superfamilies. Proc. Natl. Acad. Sci. USA 108: 7884–7889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa K., Zhou L., Li M. A., Bradley A., Craig N. L., 2011. A hyperactive piggyBac transposase for mammalian applications. Proc. Natl. Acad. Sci. USA 108: 1531–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Mitra R., Atkinson P. W., Hickman A. B., Dyda F., et al. , 2004. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature 432: 995–1001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.