Abstract
The mating reaction is triggered by specific pheromones in a wide variety of organisms. Small peptides are used as mating pheromones in yeasts and fungi. In the fission yeast Schizosaccharomyces pombe, M-factor is a C terminally farnesylated nonapeptide secreted from M-cells, and its counterpart, P-factor, is a simple peptide composed of 23 amino acids. The primary structure requirements for the biological activity of pheromone peptides remain to be elucidated. Here, we conducted comprehensive substitution of each of the amino acids in M-factor peptide and inspected the mating ability of these missense mutants. Thirty-five sterile mutants were found among an array of 152 mutants with single amino acid substitutions. Mapping of the mutation sites clearly indicated that the sterile mutants were associated exclusively with four amino acid residues (VPYM) in the carboxyl-terminal half. In contrast, the substitution of four amino-terminal residues (YTPK) with any amino acid had no or only a slightly deleterious effect on mating. Furthermore, deletion of the three N-terminal residues caused no sterility, although truncation of a fourth residue had a marked effect. We conclude that a farnesylated hexapeptide (KVPYMCFar–OCH3) is the minimal M-factor that retains pheromone activity. At least 15 nonfunctional peptides were found to be secreted, suggesting that these mutant M-factor peptides are no longer recognized by the cognate receptor.
Keywords: comprehensive mutagenesis, cognate G-protein-coupled receptor (GPCR), fission yeast, mating, pheromone peptide
SEXUAL reproduction in eukaryotes promotes genome diversity by genetic recombination and thus may accelerate evolution. As a result, mating behavior is important for reproduction. Like many fungi, the fission yeast Schizosaccharomyces pombe has two mating types, termed h+ (P) and h− (M) (Gutz et al. 1974; Egel 1989, 2004). Two haploid cells of opposite mating types mate and form a diploid zygote on starvation of nitrogen (Egel 1971). The diploid zygote undergoes meiosis and forms four haploid spores (Bresch et al. 1968; Gutz et al. 1974; Egel 1989; Nielsen 2004). Spores germinate in rich nutrient medium and proliferate as haploid vegetative cells.
The specificity of mating types in fission yeast is primarily determined by molecular recognition between a peptide mating pheromone and its cognate receptor. The mating reaction proceeds through several distinguishable steps (Egel 1989; Nielsen 2004). First, cells adhere to each other to form large cell aggregates in a reaction that is termed cell agglutination. Second, cells in aggregates protrude a conjugation tube toward a cell of opposite mating type. M- and P-cells make most contact at the cell tips, where the cell walls separating them are dissolved. Third, the juxtaposed plasma membranes are fused together. Last, after cell fusion, two nuclei derived from each mating type fuse together to form a diploid cell.
A wide variety of organisms use chemical signals to attract the opposite sex and facilitate mating with partners. In S. pombe, M-cells secrete the mating pheromone M-factor, which is specifically recognized by its cognate G-protein-coupled receptor (GPCR), Map3, on the surface of P-cells (Davey 1991, 1992; Tanaka et al. 1993). On the other hand, P-cells secrete P-factor, which activates the corresponding GPCR Mam2 on M-cells (Kitamura and Shimoda 1991; Imai and Yamamoto 1994). Mating pheromone signals stimulate cells of the opposite mating type to commence the mating reaction. Pheromone signaling evokes several responses in cells of the opposite mating type: G1 arrest of the cell cycle (Davey and Nielsen 1994; Imai and Yamamoto 1994), altered patterns of gene transcription (Mata and Bahler 2006; Xue-Franzen et al. 2006), sexual cell agglutination (Miyata et al. 1997), and polar cell elongation to form characteristic morphology (Fukui et al. 1986; Leupold 1987; Davey 1991). The mechanisms leading to cell agglutination followed by cell fusion are not well understood. So far, only Mam3 and Map4 have been proposed to be involved in cell–cell adhesion as putative M-specific and P-specific agglutinins, respectively (Sharifmoghadam et al. 2006). These proteins, which are necessary for mating, are thought to be transcriptionally activated downstream of the pheromone-signaling pathway.
M-factor is a nonapeptide whose C-terminal cysteine residue is S-farnesylated and carboxyl-methylated (Davey 1991, 1992; Davey and Nielsen 1994), whereas P-factor is a simple peptide composed of 23 amino acids (Imai and Yamamoto 1994). M-factor is encoded by three redundant genes, mfm1+, mfm2+, and mfm3+ (Davey 1992; Kjaerulff et al. 1994). These mfm+ genes produce precursor polypeptides composed of 41−44 amino acids, which can produce the same nonapeptide, mature M-factor. Any one of the three genes is sufficient for mating, but M-cells lacking all three genes fail to produce M-factor and are unable to mate, indicating that mfm1+, mfm2+, and mfm3+ are redundant in function but that M-factor is essential for mating (Kjaerulff et al. 1994). The biosynthetic pathway of M-factor has not been fully characterized, but is thought to be analogous to the pathway of Saccharomyces cerevisiae a-factor, which is also S-farnesylated and carboxyl-methylated at the C-terminal Cys residue (Anderegg et al. 1988; Davey 1992). The M-factor precursor protein is likely to undergo proteolytic processing and post-translational modifications to produce the mature, active pheromone (Michaelis 1993). Secretion of mature M-factor is mediated by an ATP-binding cassette (ABC) transporter, Mam1 (Christensen et al. 1997; Davey et al. 1997; Kjaerulff et al. 2005; Huyer et al. 2006). Importantly, the substrate specificity of the Mam1 transporter is thought to be low (Kjaerulff et al. 2005).
The pheromone signaling pathway downstream of the activated receptors is shared by both cell types. Therefore, mating-type specificity in the fission yeast is largely dependent on specific interaction between pheromone peptides and their cognate receptors. In this study, we attempted to generate a comprehensive array of missense M-factor mutants to identify amino acids that are essential for molecular recognition by the Map3 receptor. Except for the C-terminal essential Cys residue, which is chemically modified, we substituted each of the remaining 8 amino acids with the 19 other amino acids by using efficient in vitro site-directed mutagenesis with properly designed oligonucleotide primers. We then assayed the mating ability of the array of 152 missense M-factor mutants that were successfully created. As a result, 35 sterile mutants were identified among the 152 missense mutants. On the basis of our results, we discuss the primary structure vs. function relationship.
Materials and Methods
Yeast strains, media, and culture conditions
The parent strain of S. pombe used for mutagenesis was FS55 (h90 mfm1::LEU2 mfm2-D4 mfm3::ura4+ leu1-32 ura4-D18 ade6-M210), in which the mfm deletion alleles were derived from EG773 (Kjaerulff et al. 1994). The set of missense mfm1 mutants created in this study are listed in Supporting Information, Table S1. The S. pombe strains that originated differently from the standard laboratory strain (Leupold’s strain L972) and other closely related subspecies of the genus Schizosaccharomyces are listed in Table S2. Complete medium YE and synthetic media, SD and SSL+N were used for growth. Malt extract medium (ME) and synthetic media (SSL and SSL–N) were used for mating (Egel and Egel-Mitani 1974; Gutz et al. 1974; Moreno et al. 1991). S. pombe cells were grown at 30° and conjugated at 28° unless otherwise stated.
Construction of plasmids
The mfm1+ gene was amplified by PCR and cloned into pGEM-T vector (Promega Biotec, Madison, WI). The SacI/SalI fragment containing the mfm1+ gene from this vector was then recloned into the integration vector pBS(ade6) (K. Tamai, personal communication). The resultant plasmid, referred to as pBS(ade6–mfm1+), was used as a template for in vitro mutagenesis. After restriction with BamHI near the center of the ade6+ gene, pBS(ade6–mfm1+) was integrated at the ade6 locus on chromosome III by transformation of a recipient S. pombe ade6 auxotrophic strain.
Site-directed mutagenesis by in vitro DNA replication
Site-directed mutagenesis of the M-factor-coding gene, mfm1+, was conducted by using in vitro mutagenesis reagents (Quik-Change, Stratagene, San Diego, CA). DNA replication using Pfu DNA polymerase and pBS(ade6–mfm1+) as a template to generate mutated strands in vitro was performed as directed by the manufacturer’s manual. The degenerate oligonucleotides were designed to introduce various mutated triplet codons at specific sites of the mfm1+ ORF. The oligonucleotide primers are listed in Table S3. After replication, the reaction mixture was treated with restriction enzyme DpnI to digest the mfm1+ strands used as a template. Escherichia coli competent cells (DH5α) were then transformed with the DpnI-treated plasmids. Introduced mutations were confirmed by sequencing the recovered plasmids.
Quantitative assay of zygote formation
Cells were grown to 1×107 cells/ml in YE liquid medium and were then resuspended in sterilized water to a cell density of 1 × 108 cells/ml. A 50-μl aliquot of the suspension was spotted onto the ME agar medium, which was incubated for 2 days at 28°. Cells were counted under a differential interference contrast microscope or an ordinary light microscope. Cell types were classified into four groups: vegetative cells (V), zygotes (Z), asci (A), and free spores (S). The percentage of zygotes was calculated according to the following equation:
Usually, triplicate samples (at least 200 cells each) were counted, and the mean ± SD was calculated.
Quantitative assay of sexual cell agglutination
The intensity of cell agglutination in the mfm1+ strain (SS1001) and mfm1 mutants was estimated according to Shimoda and Yanagishima (1974). Overnight cultures grown in YE liquid medium were washed with sterilized water and resuspended in synthetic growth medium (SSL + N) at a cell density of 2 × 106 cells/ml. After cultivation at 30° for 20 hr with gentle shaking, cells were transferred to synthetic sporulation medium (SSL – N) at a cell density of 4 × 107 cells/ml and cultured at 28° for 4 hr with gentle shaking. An aliquot of the culture was diluted with water, and the optical density at 600 nm was measured (OD600 before sonication). Then cell suspension was subjected to sonication (20 kHz) for 10 sec to disperse cell aggregates completely. Immediately after sonication, the OD600 was determined (OD600 after sonication). The agglutination index was calculated according to the following equation:
This value is a function of an average size of cell aggregates (Yoshida and Yanagishima 1978).
Mass spectrometry
Mass spectrometry was applied to detect mutant M-factor peptides in the culture filtrates. Crude culture supernatants containing M-factor were prepared from homothallic strains as described previously (Davey 1991). Cells were grown in YE liquid medium at 30°, collected by centrifugation, washed three times with SSL, and then resuspended in SSL at a cell density of 1 × 106 cells/ml. Cells were grown for 24 hr at 29° with gentle shaking to the early stationary phase, Amberlite XAD-2 (Stratagene) resin was then added to 10% (v/v), and incubation was continued for another 72 hr. The Amberlite resin was collected by filtration and washed with excess ultrapure water. The absorbed material was eluted with methanol and then subjected to an ordinary desalting procedure suitable for MALDI-TOF samples using a ZipTip (Millipore, Bedford, MA) (Santos et al. 2007). A 0.5-μl aliquot was mixed with 0.5 μl of α-cyano-4-hydroxycinnamic acid, spotted on a stainless steel MALDI target plate, and then air-dried. Positive-ion MALDI-TOF spectra were acquired in reflectron mode using an AXIMA-CFR Plus instrument (Shimadzu, Kyoto, Japan).
Results
Comprehensive mutagenesis of mfm1+ encoding M-factor peptide
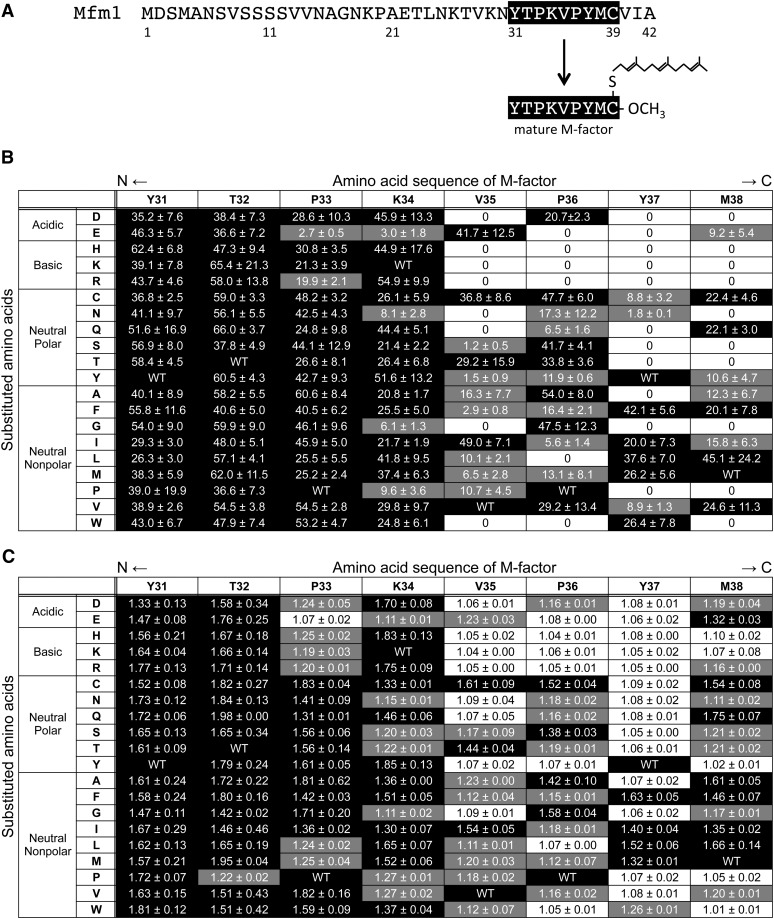
M-factor is a nonapeptide (YTPKVPYMCFar–OCH3) whose C-terminal Cys residue is S-farnesylated and carboxyl-methylated. The M-factor peptide is processed from precursor proteins encoded by mfm1+ (Figure 1A), mfm2+, and mfm3+. We attempted to create a set of single amino acid substitutions throughout the mature peptide. Chemical modifications of Cys are essential for both processing of the precursor protein and the biological activity of the mature peptide (Davey 1992), meaning that the Cys residue is indispensable. Therefore, each of the remaining eight amino acid residues was substituted in turn with the other 19 amino acids by in vitro site-directed mutagenesis. We chose the mfm1+ gene as a mutagenesis target, because its transcription is the most intense among the three mfm+ genes (Kjaerulff et al. 1994). We also took codon usage into consideration: for amino acids with multiple codons, the most preferable codon was chosen according to the S. pombe codon usage database (Forsburg 1994). The use of degenerate oligonucleotide primers was effective and the strategy was applied successfully; as a result, a full set of 152 missense mutant versions of M-factor were obtained (see Table S1). A homothallic strain lacking all three mfm+ genes (called a pheromone-less mutant) fails to produce M-factor and is unable to mate (Kjaerulff et al. 1994). Each of 152 mutated mfm1 genes was integrated at the ade6 locus of the h90 pheromone-less strain. Such integrant strains expressed only the mutated mfm1 gene and thus produced mutant M-factor.
Figure 1 .
Mating ability of 152 missense M-factor mutants. (A) Primary structure of the mfm1+ gene product. The mature M-factor peptide corresponds to residue Tyr31 to residue Cys39 in the precursor polypeptide. (B) Zygote formation. Frequency of zygotes of the missense mutants of mfm1 was determined after cultivation of cells on ME agar medium for 2 days. The amino acid sequence of mature M-factor (residues 31–38) is shown at the top and the substituted amino acids grouped according to their chemical nature are shown to the left. Three independent colonies were inspected for zygote formation. The mean zygote percentages with standard deviations are shown. Zygote formation is also indicated as a three-level grading: black, >20%; gray, 0−20%, and white, 0% (sterile). Zygote frequency of the SS1001 strain (mfm1+) was 73.1 ± 2.2%. (C) Cell agglutinability. The missense mutants were cultured in SSL−N for 4 hr with gentle shaking. Triplicate samples were taken and the intensity of sexual agglutination was determined. The mean agglutination index (AI) values with standard deviations are presented. The matrix table is constructed as in B. AI values are also indicated as a three-level grading: black, >1.30; gray, 1.10−1.30; and white, ≤1.10 (no aggregate). The AI of the SS1001 strain (mfm1+) was 1.73 ± 0.37.
Isolation of 35 sterile mutants carrying missense mfm1 mutations
Next, the mating efficiency of the 152 missense mfm1 mutants was inspected by determining the frequency of zygotes. The mutants were incubated on ME solid medium at 28° for 2 days. The SS1001 strain (mfm1Δ mfm2Δ mfm3Δ ade6-M210 << mfm1+) was included as positive control. This integrant strain showed a mating efficiency comparable to that of the wild-type (mfm1+ mfm2+ mfm3+) strain; i.e., the zygote frequency of SS1001 was 73.1 ± 2.2%. The results of 152 missense mutants are summarized in Figure 1B. Repeated microscopic observations revealed that 35 missense mutants failed to mate and were defined as sterile. Figure 1B showed that substitution of amino acid residues in the C-terminal half of M-factor led to a severe defect in mating ability, whereas the substitution of amino acids in the N-terminal half hardly disturbed conjugation. Furthermore, the substitution of amino acids in the C-terminal half of M-factor with basic amino acids led to marked inhibitory effects on mating.
As M-factor induces cell agglutinability in P-cells (Miyata et al. 1997), we next examined the sexual agglutinability of all mutants. Cells were first incubated in synthetic medium with a nitrogen source (SSL + N), and were then shifted to medium without nitrogen (SSL − N). The intensity of agglutination (AI) was assayed by photometry (see Materials and Methods). In the wild-type culture, strong agglutination was induced. The results of the mutants are presented in Figure 1C. The SS1001 strain (mfm1+) showed strong cell agglutination, with an AI of 1.73 ± 0.37. Notably, none of the 35 sterile mutants formed visible cell aggregates (AI < 1.10).
Correlation between zygote formation and agglutination intensity in the mutants
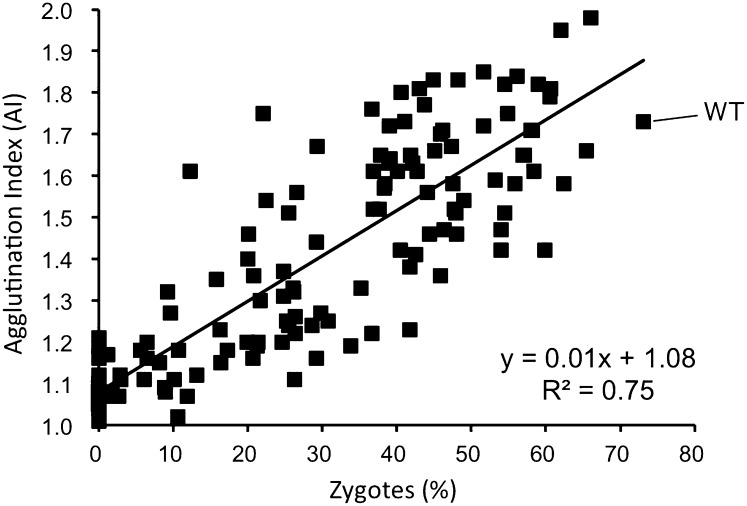
The sterile mfm1 mutants showed no sexual agglutination. In addition, some leaky mutants with reduced mating ability also showed poor agglutination. As a result, the correlation between efficiency of zygote formation and agglutination intensity was examined in more detail. Macroscopic cell agglutination reflects intense cell-to-cell adhesion between opposite mating-type cells. Each value of the two parameters in Figures 1, B and C was plotted with zygote frequency on the x-axis and AI on the y-axis. Figure 2 depicts a clear correlation between the two mating parameters, with a correlation coefficient R2 of 0.75 (n = 153). This linear positive correlation between zygote formation and agglutination intensity suggested that cell-to-cell adhesion is critical for the following cell fusion.
Figure 2 .
Correlation between zygote frequency and agglutination index of 152 missense M-factor mutants. Frequency of zygotes and AI values of the missense mutants presented in Figure 1 were used. The scatter diagram reveals a clear correlation between percentage of zygotes (x) and AI (y) of M-factor mutants. The values of the wild-type control were 73.1% (x) and 1.73 (y). The relationship between the two parameters is described by the linear expression: y = 0.01x + 1.08. The coefficient of determination, R2, is 0.75, indicating a strong correlation between them.
Stepwise deletion of the N-terminal residues of M-factor
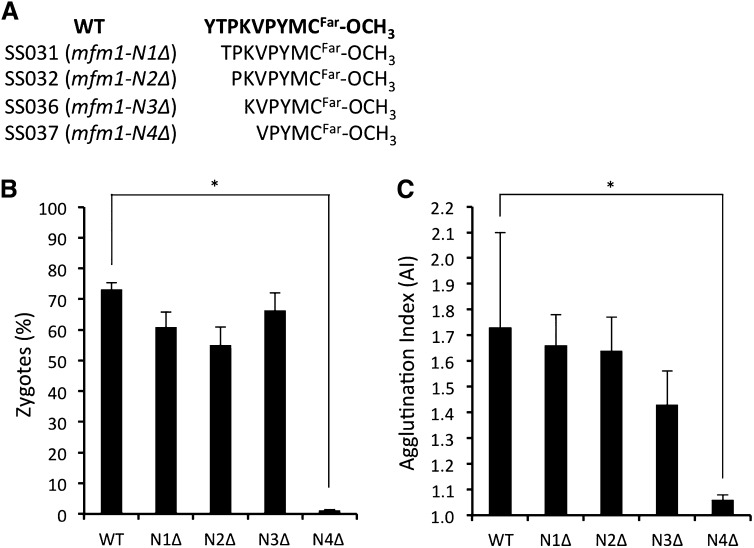
As the mating pheromone activity was largely dependent on the primary structure of the C-terminal half of M-factor, we next investigated the effect on mating of deleting residues from the N terminus of the M-factor peptide. We constructed a series of deletion mutants of M-factor, composed of only five to eight amino acids and lacking the N-terminal region of the wild-type peptide (Figure 3A). A mating assay of these deletion mutants revealed that deletion of three amino acid residues from the N terminus of M-factor did not cause a significant reduction in mating ability (Figure 3, B and C). However, removal of one more residue, Lys, dramatically reduced mating capability. Therefore, we conclude that the C-terminal half of M-factor, whose sequence is KVPYMCFar–OCH3 is sufficient for evoking the mating response. Secretion of this mini M-factor peptide was confirmed as mentioned below (Figure 4D). We also explored the consequence of adding alanine residues to the N terminus of M-factor peptide. An extension of up to two alanine residues affected neither zygote formation nor agglutination (data not shown).
Figure 3 .
Effect of the N-terminal truncation of M-factor on biological activity. (A) Amino acid sequence of the truncated M-factors. The wild-type strain and the deletion mutants were induced to mate as described in Figures 1, B and C. The percentage of zygotes (B) and agglutination index (C) were determined. Means with standard deviations of triplicate samples are presented. (*) P < 0.05, t-test.
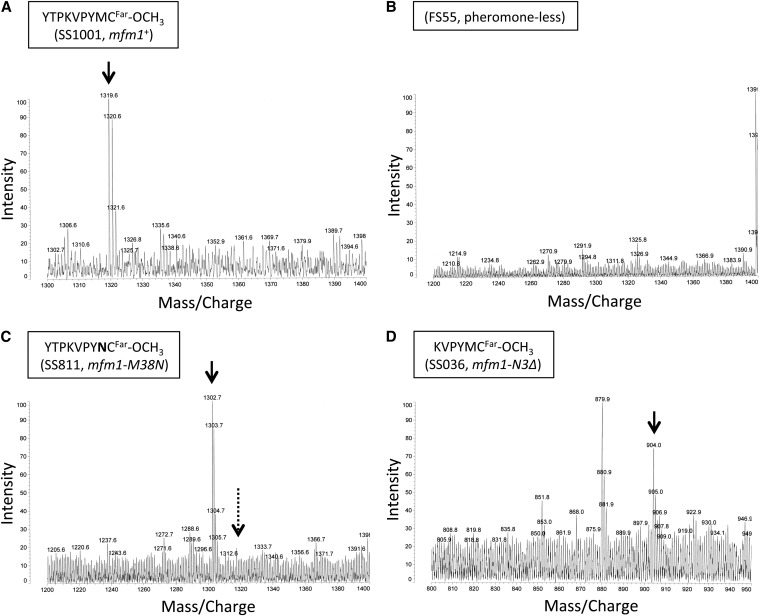
Figure 4 .
Secretion of mutant M-factors demonstrated by mass spectrometry. Mass spectrometry of M-factor from culture filtrates of a wild-type strain (A; SS1001, mfm1+), a pheromone-less mutant (B; FS55, mfm1Δ), a missense mutant (C; SS811, mfm1-M38N), and a truncated mutant (D; SS036, mfm1-N3Δ). The peak corresponding to the mass/charge value expected from the respective amino acid sequence is indicated by the solid downward arrow. The mass/charge position of wild-type M-factor is shown by a dotted arrow in B and C. The expected mass/charge values for M-factor from wild type, mfm1-M38N and mfm1-N3Δ, are m/z 1319.7, 1302.6, and 904.3, respectively. Some other examples of mass spectrometry data are shown in Figure S1.
Secretion of sterile-type mutant M-factor peptides
To determine whether the mutant M-factor peptides were secreted by Mam1, we attempted to detect M-factor peptides with altered molecular mass in the culture filtrate by mass spectrometry. As the MALDI–TOF system was not sensitive enough to detect any peaks of M-factor in the culture filtrate, partial purification and concentration of M-factor seemed to be necessary prior to mass spectrometry. Polystyrene resin Amberlite XAD-2 was used to partially purify M-factor according to Davey (1992). As a result, M-factor was detected in the wild-type culture filtrates at a peak in accordance with the expected molecular mass of 1319.6 (Figure 4A). This M-factor peak was absent in the culture filtrate from the mfm1 mfm2 mfm3 triple deletant, FS55 (Figure 4B).
Next, secretion of the 35 different mutant M-factor peptides in the culture filtrates was examined. Secretion was demonstrated by detecting a peak of expected molecular mass; for example, the M-factor mutant M38N (Met38 substituted with Asn) had a molecular mass of 1302.7 instead of 1319.6 of wild-type M-factor. The appearance of this expected peak coincided with an absence of the wild-type peak (Figure 4C). The above-described mini M-factor peptide composed of six amino acids was also detected (Figure 4D). Likewise, several mutant M-factor peptides were discerned as shown in Figure S1. In summary, among the 35 missense mutants, the following 15 substituted peptides were successfully detected: V35R, V35W, P36E, Y37D, Y37H, Y37Q, Y37R, M38D, M38G, M38H, M38K, M38N, M38R, M38S, and M38W. The other 20 mutant M-factor peptides were not detected, probably owing to an actual reduction in the amount in the culture filtrates or to loss during the purification/concentration procedures. We concluded that at least 15 nonfunctional M-factor peptides are likely to be secreted but not recognized by Map3 receptor proteins.
The N-terminal Tyr residue is not essential for processing of M-factor
Processing of the S. cerevisiae a-factor mating pheromone from a precursor polypeptide has been studied extensively (Marcus et al. 1990; Hrycyna et al. 1991; Boyartchuk and Rine 1998; Schmidt et al. 1998; Tam et al. 1998, 2001). By contrast, only limited information is available for S. pombe M-factor. The last two sequential steps of a-factor processing involve proteolytic cleavage of the N-terminal extension. These steps are catalyzed by different proteases, Ste24p (Fujimura-Kamada et al. 1997; Tam et al. 2001) and Axl1p (Adames et al. 1995) (Figure 5A). In most fungal and yeast pheromones, an N-terminal Tyr residue is highly conserved in the mature peptides (cf. Figure 5A). This Tyr residue is thought to be a recognition key for proteolysis. In S. pombe, however, our substitution analysis clearly indicated that the N-terminal Tyr31 residue was not required for the biological activity of M-factor (see Figures 1, B and C).
Figure 5 .
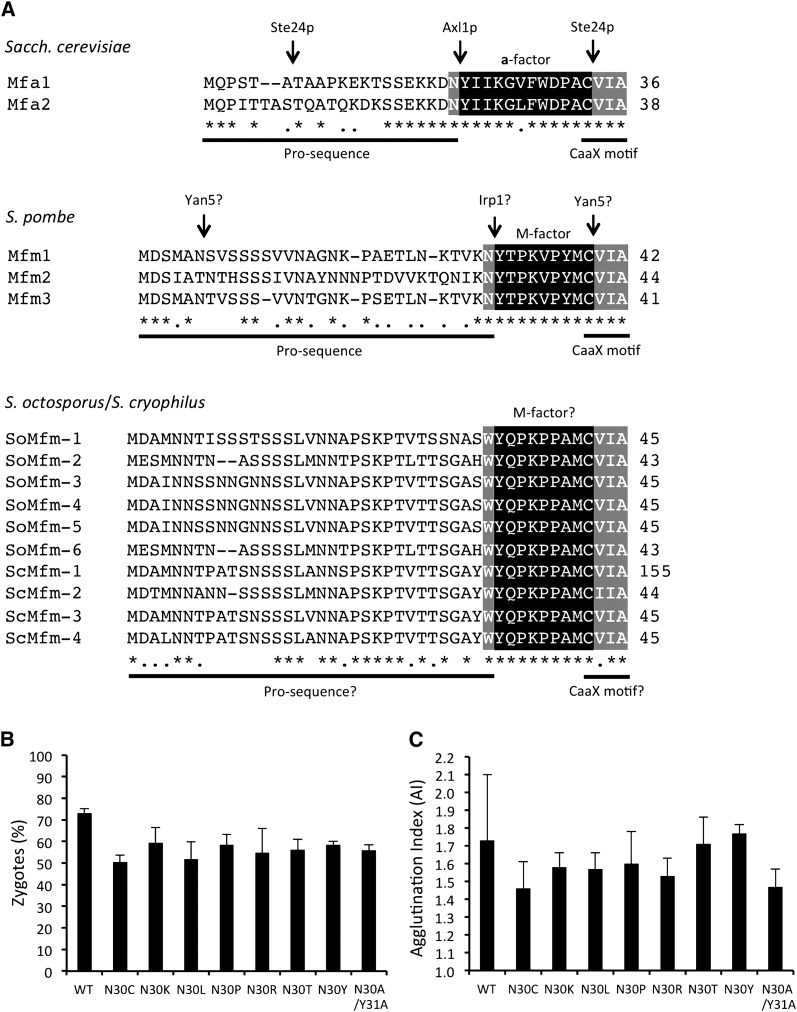
Substitution of amino acid residues at a putative cleavage site of M-factor processing. (A) Sequence alignment of the precursors derived from the different genes of a-factor in S. cerevisiae (top), of M-factor in S. pombe (middle), and of putative M-factor in S. octosporus (6 genes) and in S. cryophilus (4 genes) (bottom). M-factor-coding genes of S. octosporus and S. cryophilus were annotated by the Schizosaccharomyces group Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/) (Rhind et al. 2011). Putative M-factor precursor proteins of S. octosporus are tentatively termed SoMfm-1 to SoMfm-6; similarly, those of S. cryophilus are termed ScMfm-1 to ScMfm-4. Numerals to the right of the sequence represent the length of the precursor proteins. Homology of amino acids is shown by asterisks (identical) or dots (similar). Multiple sequence alignment was done by a standard algorithm (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Mature peptide sequences are shown by white letters on black. Flanking conserved residues are indicated by white letters on gray. Experimentally identified proteases involved in the specific cleavage of precursors are shown above the a-factor sequence, and the hypothesized proteases for M-factor are also shown. (B and C) Mating ability of the missense mutants was determined as described in Figures 1, B and C. Percentage of zygotes (B) and agglutination index (C) are shown. Means with standard deviations of triplicate samples are presented.
We noted that an Asn residue at the C terminus of the pro-sequence (i.e., Asn30 adjacent to Tyr31) is also conserved among most fungal pheromone peptides (cf. Figure 5A) (Martin et al. 2011). Interestingly, this residue is Trp instead of Asn in 10 putative M-factor precursor proteins of Schizosaccharomyces octosporus and Schizosaccharomyces cryophilus (Figure 5A). To examine whether this Asn residue is needed for proteolytic cleavage, we introduced missense mutations at this codon (Table S1; SS002−SS019). No M-factor with substitution at the Asn30 residue showed reduced mating efficiency (Figure 5, B and C). In addition, the substitution of Asn30–Tyr31 with Ala30–Ala31 was not inhibitory (SS033). These observations imply that the Asn–Tyr sequence at the putative protease target site of M-factor is not important for its biological activity, and thus processing of M-factor can proceed normally.
Conservation of the primary structure of mature M-factor in S. pombe strains derived from different origins
The primary structure of M-factor is likely to be important for its biological activity (Figures 1, B and C). In fact, substitutions of amino acid residues in the C-terminal half (VPYM) impaired completely or partially the function of M-factor. Surprisingly, amino acid substitutions in the N-terminal half (YTPK) had only a limited effect on mating. Our finding that M-factor is robust to alterations of the primary structure suggests that amino acid substitutions may have occurred quite rapidly during evolution. To test this possibility, we examined 33 strains of S. pombe var. pombe and close subspecies whose origins differ from that of the standard laboratory strain (L972) first established by U. Leupold (see Table S2). These strains were derived from different sources or geographic locations across the world. All of the mfm1, mfm2, and mfm3 genes of these strains were sequenced and compared with the nucleotide sequences of L972 registered at the public database (Schizosaccharomyces pombe GeneDB; http://old.genedb.org/genedb/pombe/).
Notably, all of the mfm genes of the 34 strains including L972 (i.e., 102 genes in total) were predicted to produce mature M-factor peptides whose primary structure is identical to the known sequence, YTPKVPYMC. Meanwhile, many probable neutral mutations were found in the pro-sequences and intronic sequences (Table 1). One exceptional mutation in the mature M-factor coding region caused no amino acid substitution and thus was a synonymous change. We conclude that the primary structure of mature M-factor is highly conserved in these S. pombe strains, although a few neutral mutations have been accumulated in the mfm genes.
Table 1 . Polymorphic alleles in the three M-factor-encoding genes of S. pombe strains with different origins from that of the standard laboratory strain L972.
| Genes | Base substitution and insertion | Amino acid substitution | Regions | No. of strains | Strains |
|---|---|---|---|---|---|
| mfm1 | T11A | M4K | Propeptide | 1 | YHL274 |
| G12A | M4I | Propeptide | 3 | EF6, NBRC0354, YHL280 | |
| T24C | V8V | Propeptide | 1 | NBRC0354 | |
| A88G | N30D | Propeptide | 3 | MS9-1, SP-6, JCM8262 | |
| G147T | Intron | 1 | YHL271 | ||
| G187A | Intron (3′ consensus) | 5 | JCM8274, YHL264, YHL272, YHL273, YHL274 | ||
| mfm2 | T12C | I4I | Propeptide | 5 | EF6, NBRC0345, NBRC354, YHL274, YHL280 |
| A26C | H9P | Propeptide | 8 | MS9-1, SP-6, JCM8262, JCM1846, YHL267, YHL275, YHL277, YHL282 | |
| T28C | S10P | Propeptide | 2 | EF6, YHL280 | |
| T39C | I13I | Propeptide | 1 | EF6 | |
| 133 (ins- GCT) | Intron | 8 | MS9-1, SP-6, JCM8262, JCM1846, YHL267, YHL275, YHL277, YHL282 | ||
| T148A | Intron | 3 | MS9-1, SP-6, JCM8262 | ||
| C149T | Intron | 3 | MS9-1, SP-6, JCM8262 | ||
| T160C | Intron | 3 | MS9-1, SP-6, JCM8262 | ||
| A161G | Intron | 3 | MS9-1, SP-6, JCM8262 | ||
| mfm3 | A9T | S3S | Propeptide | 3 | JCM8274, JCM1846, YHL282 |
| G99A | K33K | Mature peptide | 3 | JCM8274, JCM1846, YHL282 | |
| A121G | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| T127C | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| T138C | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| C140T | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| G148A | Intron | 1 | JCM8274 | ||
| 151 (ins A) | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| T153C | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| 159 (ins CAAA) | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| A161G | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| T162C | Intron | 3 | JCM8274, JCM1846, YHL282 | ||
| T165A | Intron | 1 | JCM8274 |
The coding regions of mfm1, mfm2, and mfm3 were amplified by PCR. The products were subjected to nucleotide sequencing and compared with the corresponding sequence of L972. Differences from the L972 sequence are listed, and the deduced amino acid substitutions are indicated. The mutation sites are classified into the following three categories: propeptide, intron, and mature peptide. Strains harboring the same alterations are grouped together.
Discussion
A novel mutational approach to determine the structural requirement of a small peptide pheromone for receptor recognition
Mating-type specificity in yeasts is controlled at several different levels, but the most critical one is the molecular recognition between the peptide-mating pheromone and its cognate receptor protein. To obtain clues to how these molecules specifically interact, mutational analyses have been conducted with budding yeast (Huyer et al. 2006). This study took a similar approach, but was more comprehensive in that each of the amino acid residues of this small peptide was substituted by all of the other amino acids. As a result, we succeeded in the creation of 152 such missense mutants. This unprecedented approach enabled us to investigate more thoroughly the structure vs. function relationship of M-factor.
Our comprehensive substitution approach clearly indicated that a simple alanine-scanning approach must be applied with care. The results presented here show that the effect of amino acid substitutions at specific residues depends on the amino acid that is substituted. For example, P36A substitution showed no deleterious effect, but P36L caused complete sterility. Conversely, Y37A exhibited a sterile phenotype, but Y37F substitution led to normal mating. Similar examples of the different consequences of the substituted amino acids are seen in Figures 1, B and C. In this study, we developed an efficient method applying in vitro mutagenesis with degenerate oligonucleotides. This technique will be useful and applicable to biologically active peptides and particular domains of polypeptides to elucidate the function of specific residues or domains reliably.
The N-terminal half of M-factor is dispensable for molecular recognition by its cognate receptor Map3
We isolated 35 sterile mutants among the set of 152 single-substituted missense mutants (Figures 1, B and C). M-factor is secreted by the ABC transporter Mam1, which is highly homologous to S. cerevisiae Ste6p, the transporter responsible for the export of a-factor mating pheromone (Christensen et al. 1997). Mature M-factor peptides, which are formed from the precursor polypeptide by proteolysis, must be recognized by Mam1 transporter on the plasma membrane for secretion. It is possible that some of the nonfunctional mutant M-factor peptides are poorly recognized by Mam1. This seems unlikely, however, because the substrate specificity of the Mam1 transporter is known to be low, as indicated by the following observations: green fluorescent protein is exported by Mam1 when sandwiched between the propeptide sequence and a CAAX motif of M-factor (Kjaerulff et al. 2005), and ectopically expressed S. cerevisiae Ste6p facilitates the export of M-factor in S. pombe cells (Christensen et al. 1997). These observations imply that the M-factor-specific transporter Mam1 is probably dependent on the farnesylated Cys residue, but not on other amino acid residues of M-factor. As a result, most of the mutant M-factor peptides should be recognized by the Mam1 transporter and secreted into the culture medium.
In fact, 15 nonfunctional M-factor peptides were successfully detected by mass spectrometry (Figure 4 and Figure S1), indicating that these peptides are indeed secreted by the Mam1 transporter. For the other 20 mutant M-factors, insufficient recognition by the Map3 receptor will need to be confirmed directly by using chemically synthesized peptides. It is probable that most of the 35 sterile M-factors are nonfunctional due to poor recognition by the wild-type receptor Map3, although other possibilities cannot be completely ruled out. We propose the possibility that certain missense mutations of the map3 gene might enable the receptor to recognize the mutant M-factors. Hopefully, searching for such suppressor map3 mutants will be feasible, because an efficient screening strategy for isolating mating-sufficient revertants in S. pombe is available (Kitamura and Shimoda 1991). Mapping of such suppressor mutations in the map3 ORF may enable us to identify the ligand-binding sites of the receptor. This kind of genetic analysis of mutant ligand/receptor combinations will hopefully contribute to GPCR biology.
As shown in Figures 1, B and C, the primary structure of M-factor is likely to be associated with its biological activity. Amino acid substitution of the Y31 and T32 residues with other amino acids did not cause a marked effect on mating efficiency. Amino acid substitution of the P33 and K34 residues led to only a partial mating defect, depending on substituted amino acids (Figure 1B). Interestingly, however, some mutants with substitutions of residues in the C-terminal half (V35, P36, Y37, and M38) showed significantly reduced mating ability. Notably, substitution with basic amino acids caused severe mating defects (Figures 1, B and C), suggesting that the electric charge of amino acids may play a role in the interaction of pheromone peptides with their corresponding receptor Map3. M-factor contains a long hydrophobic farnesyl group at the carboxyl terminus. The importance of the C-terminal half for activity may relate to the hydrophobicity of the farnesyl group. One possible mechanism is that the hydrophobic tail is involved in association of M-factor with the plasma membrane; this association may then facilitate interaction of the C-terminal half of the peptide moiety of M-factor with ligand-binding residues of the Map3 receptor. Mutational analysis of S. cerevisiae a-factor suggested that the N-terminal region is important for function (Huyer et al. 2006). This inconsistency may come from the difference in amino acids recognized by the receptor, although it is also possible that the mutagenesis of a-factor was not extensive enough to draw a firm conclusion. Further comprehensive mutagenesis seems necessary to adequately compare the functional sites of these two pheromone peptides.
Our mating assay indicated that amino acid substitutions in the N-terminal half of M-factor can be made without affecting the biological activity. In addition, a mini M-factor composed of 6 amino acid residues without the three N-terminal residues retained activity. Despite these observations, the putative M-factor peptides are fully conserved in 34 strains of S. pombe strains collected from different areas. We speculate that the wild-type sequence confers a selective advantage under certain conditions, especially in nature. Mating assays with different experimental conditions may verify such a selective advantage of wild-type M-factor.
Necessity of the conserved dipeptide sequence at a cleavage site of the precursor protein
Prenylation of the C-terminal Cys residue is required for both processing and biological function in S. pombe (Davey 1992). Comparison of the primary structure of the pheromone peptides suggests that the two consecutive amino acids, Asn and Tyr, at the N-terminal cleavage site are well conserved. In fact, the cleavage site of M-factor precursors encoded by the three different genes of S. pombe is Asn-Tyr, and this is also the case for two a-factor precursor proteins of S. cerevisiae (Figure 5A). Despite this conservation, substitutions of these two amino acids with others did not affect mating activity in S. pombe, indicating that proteolytic cleavage may occur normally. The cutting site appears to be determined by a broader sequence. In support of this idea, even distant deletions within the N-terminal extension block Axl1p processing in S. cerevisiae (Huyer et al. 2006). The structure and/or length of the pro-sequence might be important for this cleavage step.
Why are there three redundant genes for M-factor?
The composition of genes encoding pheromone peptides differs between the two mating types in yeasts. In S. pombe, P-factor is encoded by a single gene (map2+), which contains four tandem copies of mature P-factor-coding sequences (Imai and Yamamoto 1994); by contrast, M-factor is encoded by three unlinked genes, mfm1+, mfm2+, and mfm3+ (Figure 5A). All three genes produce a polypeptide precursor that is then processed to yield a small mature peptide with the same amino acid sequence. Deletion of any one or two of the mfm genes causes no remarkable mating defects (Kjaerulff et al. 1994). Such multiple structural genes for mating pheromones are often found in other yeasts and fungi (Martin et al. 2011): for instance, S. cerevisiae contains two redundant pheromone genes, S. octosporus, the species most closely related to S. pombe, has six genes, and S. cryophilus also has four genes (Figure 5A). The redundant genes of S. octosporus and S. cryophilus also encode M-factor peptides of the same amino acid sequence. Furthermore, the primary structures of the putative M-factors of S. octosporus and S. cryophilus are the same. Three of the nine amino acid residues (T32, V35, and Y37) of S. pombe M-factor are different from those of putative S. octosporus and S. cryophilus M-factors (Q32, P35, and A37) (Figure 5A). Synthetic M-factor peptides of the latter were found to be ineffective on S. pombe P-cells (data not shown). Our assay also showed that a Y37A substitution caused complete sterility (Figure 1B). Therefore, S. pombe is reproductively isolated from S. octosporus and S. cryophilus, at least in terms of the compatibility of M-factor and its cognate receptor.
Why are three redundant genes for M-factor present in the S. pombe genome? The identity of amino acid sequences between any pair of the mfm gene products is high, ranging from 74 to 94%. All three mfm genes contain one intron, and the location of this single intron is also conserved (Kjaerulff et al. 1994). Thus, the question is, Why are three redundant genes necessary for the synthesis of M-factor peptides? We can propose the three following possibilities: first, pheromone peptides must be produced quickly in large amounts under environmental conditions favorable for sexual reproduction; second, expression of the respective genes may be differentially controlled, enabling fine-tuning; third, if the receptors are mutated, such redundancy enables the cells to alter one copy to become well adapted to receptor changes, while keeping the others unchanged. In this way, haploid S. pombe cells are able to adapt flexibly to various mutational changes in receptor proteins.
Supplementary Material
Acknowledgments
We thank Olaf Nielsen, Juerg Kohli, Matthias Sipiczki, Gerry Smith, Henry Levin, Japan Collection of Microorganisms (JCM), and National BioResource Project Japan (YGRC/NBRP) for strains and plasmids. This study was supported in part by Grant-in-Aid for Publication of Scientific Research Results to T.N. from the Ministry of Education, Culture, Sports, Science and Technology of Japan. T.S. was supported by the exploratory research grant from the Japan Society for the Promotion of Science (JSPS).
Footnotes
Communicating editor: J. Heitman
Literature Cited
- Adames N., Blundell K., Ashby M. N., Boone C., 1995. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science 270: 464–467 [DOI] [PubMed] [Google Scholar]
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W., 1988. Structure of Saccharomyces cerevisiae mating hormone a-factor: identification of S-farnesyl cysteine as a structural component. J. Biol. Chem. 263: 18236–18240 [PubMed] [Google Scholar]
- Boyartchuk V. L., Rine J., 1998. Roles of prenyl protein proteases in maturation of Saccharomyces cerevisiae a-factor. Genetics 150: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresch C., Muller G., Egel R., 1968. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 102: 301–306 [DOI] [PubMed] [Google Scholar]
- Christensen P. U., Davey J., Nielsen O., 1997. The Schizosaccharomyces pombe mam1 gene encodes an ABC transporter mediating secretion of M-factor. Mol. Gen. Genet. 255: 226–236 [DOI] [PubMed] [Google Scholar]
- Davey J., 1991. Isolation and quantitation of M-factor, a diffusible mating factor from the fission yeast Schizosaccharomyces pombe. Yeast 7: 357–366 [Google Scholar]
- Davey J., 1992. Mating pheromones of the fission yeast Schizosaccharomyces pombe: purification and structural characterization of M-factor and isolation and analysis of two genes encoding the pheromone. EMBO J. 11: 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Nielsen O., 1994. Mutations in cyr1 and pat1 reveal pheromone-induced G1 arrest in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 26: 105–112 [DOI] [PubMed] [Google Scholar]
- Davey J., Christensen P. U., Nielsen O., 1997. Identification of the transporter for the M-factor mating pheromone in fission yeast. Biochem. Soc. Trans. 25: 224S. [DOI] [PubMed] [Google Scholar]
- Egel R., 1971. Physiological aspects of conjugation in fission yeast. Planta 98: 89–96 [DOI] [PubMed] [Google Scholar]
- Egel R., 1989. Mating-type genes, meiosis and sporulation, pp. 31–73 Molecular Biology of the Fission Yeast, edited by Nasim A., Young P., Johnson B. F. Academic Press, San Diego, CA [Google Scholar]
- Egel R., 2004. Fission yeast in general genetics, pp. 1–12 The Molecular Biology of Schizosaccharomyces pombe, edited by Egel R. Springer, Berlin [Google Scholar]
- Egel R., Egel-Mitani M., 1974. Premeiotic DNA synthesis in fission yeast. Exp. Cell Res. 88: 127–134 [DOI] [PubMed] [Google Scholar]
- Forsburg S. L., 1994. Codon usage table for Shizosaccharomyces pombe. Yeast 10: 1045–1047 [DOI] [PubMed] [Google Scholar]
- Fujimura-Kamada K., Nouvet F. J., Michaelis S., 1997. A novel membrane-associated metalloprotease, Ste24p, is required for the first step of NH2-terminal processing of the yeast-a-factor precursor. J. Cell Biol. 136: 271–285. 29 [DOI] [PMC free article] [PubMed]
- Fukui Y., Kaziro Y., Yamamoto M., 1986. Mating pheromone-like diffusible factor released by Schizosaccharomyces pombe. EMBO J. 5: 1991–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H., Heslot H., Leupold U., Loprieno N., 1974. Schizosaccharomyces pombe, pp. 395–446 in Handbook of Genetics, Vol. 1, edited by R. C. King. Plenum Press, London/New York
- Hrycyna C. A., Sapperstein S. K., Clarke S., Michaelis S., 1991. The Saccharomyces cerevisiae STE14 gene encodes a methyltransferase that mediates C-terminal methylation of a-factor and RAS proteins. EMBO J. 10: 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyer G., Kistler A., Nouvet F. J., George C. M., Boyle M. L., et al. , 2006. Saccharomyces cerevisiae a-factor mutants reveal residues critical for processing, activity, and export. Eukaryot. Cell 5: 1560–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Yamamoto M., 1994. The fission yeast mating pheromone P-factor: its molecular structure, gene structure, and ability to induce gene expression and G1 arrest in the mating partner. Genes Dev. 8: 328–338 [DOI] [PubMed] [Google Scholar]
- Kitamura K., Shimoda C., 1991. The Schizosaccharomyces pombe mam2 gene encodes a putative pheromone receptor which has a significant homology with the Saccharomyces cerevisiae Ste2 protein. EMBO J. 10: 3743–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff S., Davey J., Nielsen O., 1994. Analysis of the structural genes encoding M-factor in the fission yeast Schizosaccharomyces pombe: identification of a third gene, mfm3. Mol. Cell. Biol. 14: 3895–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff S., Muller S., Jensen M. R., 2005. Alternative protein secretion: the Mam1 ABC transporter supports secretion of M-factor linked GFP in fission yeast. Biochem. Biophys. Res. Commun. 338: 1853–1859 [DOI] [PubMed] [Google Scholar]
- Leupold U., 1987. Sex appeal in fission yeast. Curr. Genet. 12: 543–545 [Google Scholar]
- Marcus S., Caldwell G. A., Xue C. B., Naider F., Becker J. M., 1990. Total in vitro maturation of the Saccharomyces cerevisiae a-factor lipopeptide mating pheromone. Biochem. Biophys. Res. Commun. 172: 1310–1316 [DOI] [PubMed] [Google Scholar]
- Martin S. H., Wingfield B. D., Wingfield M. J., Steenkamp E. T., 2011. Causes and consequence of variability in peptide mating pheromones of ascomycete fungi. Mol. Biol. Evol. 28: 1987–2003 [DOI] [PubMed] [Google Scholar]
- Mata J., Bahler J., 2006. Global roles of Ste11p, cell type, and pheromone in the control of gene expression during early sexual differentiation in fission yeast. Proc. Natl. Acad. Sci. USA 103: 15517–15522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., 1993. STE6, the yeast a-factor transporter. Semin. Cell Biol. 4: 17–27 [DOI] [PubMed] [Google Scholar]
- Miyata M., Metsuoka M., Inada T., 1997. Induction of sexual co-flocculation of heterothallic fission yeast (Schizosaccharomyces pombe) cells by mating pheromones. J. Gen. Appl. Microbiol. 43: 169–174 [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P., 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Nielsen O., 2004. Mating type control and differentiation, pp. 281–296 The Molecular Biology of Schizosaccharomyces pombe, edited by Egel R. Springer, Berlin [Google Scholar]
- Nielsen O., Davey J., Egel R., 1992. The ras1 function of Schizosaccharomyces pombe mediates pheromone-induced transcription. EMBO J. 11: 1391–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N., Chen Z., Yassour M., Thompson D. A., Haas B. J., et al. , 2011. Comparative functional genomics of the fission yeasts. Science 332: 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos H. M., Rial-Otero R., Femandes L., Vale G., Rivas M. G., et al. , 2007. Improving sample treatment for in-solution protein identification by peptide mass fingerprint using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Proteome Res. 6: 3393–3399 [DOI] [PubMed] [Google Scholar]
- Schmidt W. K., Tam A., Fujimura-Kamada K., Michaelis S., 1998. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc. Natl. Acad. Sci. USA 95: 11175–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifmoghadam M. R., Bustos-Sanmamed P., Valdivieso M. H., 2006. The fission yeast Map4 protein is a novel adhesin required for mating. FEBS Lett. 580: 4457–4462 [DOI] [PubMed] [Google Scholar]
- Shimoda C., Yanagishima N., 1974. Mating reaction in Saccharoromyces cerevisiae. VI. Effect of 2-deoxyglucose on conjugation. Plant Cell Physiol. 15: 767–778 [Google Scholar]
- Tam A., Nouvet F. J., Fujimura-Kamada K., Slunt H., Sisodia S. S., et al. , 1998. Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing. J. Cell Biol. 142: 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A., Schmidt W. K., Michaelis S., 2001. The multispanning membrane protein Ste24p catalyzes CAAX proteolysis and NH2-terminal processing of the yeast a-factor precursor. J. Biol. Chem. 276: 46798–46806 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Davey J., Imai Y., Yamamoto M., 1993. Schizosaccharomyces pombe map3+ encodes the putative M-factor receptor. Mol. Cell. Biol. 13: 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue-Franzen Y., Kjaerulff S., Holmberg C., Wright A., Neilsen O., 2006. Genomewide identification of pheromone-targeted transcription in fission yeast. BMC Genet. 7: 303 [DOI] [PMC free article] [PubMed]
- Yoshida K., Yanagishima N., 1978. Intra- and intergeneric mating behavior of ascosporogenous yeasts. I. Quantitative analysis of sexual agglutination. Plant Cell Physiol. 19: 1519–1533 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.