Abstract
Comparative genetic mapping provides insights into the evolution of the reproductive barriers that separate closely related species. This approach has been used to document the accumulation of reproductive incompatibilities over time, but has only been applied to a few taxa. House mice offer a powerful system to reconstruct the evolution of reproductive isolation between multiple subspecies pairs. However, studies of the primary reproductive barrier in house mice—hybrid male sterility—have been restricted to a single subspecies pair: Mus musculus musculus and Mus musculus domesticus. To provide a more complete characterization of reproductive isolation in house mice, we conducted an F2 intercross between wild-derived inbred strains from Mus musculus castaneus and M. m. domesticus. We identified autosomal and X-linked QTL associated with a range of hybrid male sterility phenotypes, including testis weight, sperm density, and sperm morphology. The pseudoautosomal region (PAR) was strongly associated with hybrid sterility phenotypes when heterozygous. We compared QTL found in this cross with QTL identified in a previous F2 intercross between M. m. musculus and M. m. domesticus and found three shared autosomal QTL. Most QTL were not shared, demonstrating that the genetic basis of hybrid male sterility largely differs between these closely related subspecies pairs. These results lay the groundwork for identifying genes responsible for the early stages of speciation in house mice.
Keywords: house mouse, speciation, hybrid male sterility, pseudoautosomal region, reproductive isolation
THE genetic dissection of reproductive barriers between species is a powerful approach to understanding speciation. In some cases, genetic mapping has revealed the identities and functions of the gene networks responsible for reproductive isolation (Sawamura and Yamamoto 1997; Ting et al. 1998; Barbash et al. 2003; Presgraves et al. 2003; Brideau et al. 2006; Bayes and Malik 2009; Ferree and Barbash 2009; Mihola et al. 2009; Phadnis and Orr 2009; Tang and Presgraves 2009). By providing a list of genomic locations that contribute to reproductive barriers, mapping also allows investigation of the role of genomic context, including local recombination rate (Noor et al. 2001; Rieseberg 2001; Butlin 2005; Nachman and Payseur 2012), in speciation. Phenotypes associated with reproductive isolation have been mapped in a variety of species, with an emphasis on hybrid sterility and hybrid inviability (Hollocher and Wu 1996; True et al. 1996; Tao et al. 2003; Sweigart et al. 2006; Bomblies et al. 2007; Masly and Presgraves 2007; Moyle 2007; Chen et al. 2008; Lee et al. 2008; Long et al. 2008; Kao et al. 2010; Martin and Willis 2010).
The comparison of reproductive isolation among species pairs has revealed general patterns that characterize speciation. For example, hybrid sterility tends to evolve before hybrid inviability (Coyne and Orr 1989). A worthwhile extension of this comparative framework focuses on the loci responsible for reproductive barriers (Moyle and Payseur 2009). By comparing loci mapped in different species pairs, genetic changes that increase reproductive isolation can be assigned to specific phylogenetic lineages, revealing the evolutionary history of reproductive barriers (Moyle and Nakazato 2008). This information enables the evaluation of models that describe the accumulation of reproductive isolating mutations (Orr 1995), the distinction between classes of incompatibilities (e.g., “derived-derived” and “ancestral-derived”; Orr 1995; Cattani and Presgraves 2009), and the temporal ordering of genetic changes that contribute to different reproductive barriers.
Recent applications of this comparative genetics approach have produced new insights into the evolution of reproductive isolation. Overlapping quantitative trait loci (QTL) control hybrid pollen/seed sterility in two species pairs of Solanum, suggesting common evolutionary origins for the underlying mutations (Moyle and Graham 2005; Moyle and Nakazato 2008). The number of incompatibilities involved in hybrid dysfunction may increase faster than linearly with divergence time in Drosophila (Matute et al. 2010; but see Barbash 2011) and in Solanum (Moyle and Nakazato 2010), as predicted by theory assuming the Dobzhansky–Muller model (Orr 1995; Turelli and Orr 2000; Orr and Turelli 2001). Additional comparative genetic studies are needed, especially for recently diverged species where genetic factors closely tied to speciation can be identified.
House mice provide a powerful system for understanding the evolution of reproductive isolation during the early stages of speciation. The clade is composed of three closely related subspecies (Mus musculus musculus, Mus musculus domesticus, and Mus musculus castaneus), which rapidly diverged from a common ancestor only 500,000 generations ago (She et al. 1990; Boursot et al. 1996; Suzuki et al. 2004; Salcedo et al. 2007; Geraldes et al. 2008). The geographic ranges of these subspecies extend throughout the Mediterranean and Western Europe (M. m. domesticus), Eastern Europe and Northern Asia (M. m. musculus), and Southern Asia (M. m. castaneus) (Boursot et al. 1993). The best-studied region of geographical overlap is in central Europe, where M. m. musculus and M. m. domesticus hybridize in a zone of secondary contact (Boursot et al. 1993; Sage et al. 1993). Patterns of gene flow in this hybrid zone and reproductive characteristics in hybrid mice indicate that M. m. musculus and M. m. domesticus are partially reproductively isolated. Diagnostic loci show steep allele frequency clines across the hybrid zone (Vanlerberghe et al. 1986; Dod et al. 1993, 2005; Munclinger et al. 2002; Payseur et al. 2004; Payseur and Nachman 2005; Raufaste et al. 2005; Macholán et al. 2007, 2008; Teeter et al. 2008, 2010), suggesting that multiple genomic regions confer reproductive barriers (Payseur 2010). Hybrid males from this zone (Turner et al. 2012) and from the laboratory (Iványi et al. 1969; Forejt and Iványi 1974; Storchová et al. 2004; Britton-Davidian et al. 2005; Vyskočilová et al. 2005, 2009; Good et al. 2008a,b) exhibit reproductive phenotypes that indicate subfertility or sterility. Hybrid male sterility has been linked to loci on the X chromosome (Oka et al. 2004; Storchová et al. 2004; Britton-Davidian et al. 2005; Good et al. 2008a,b and 2010 Vyskočilová et al. 2009) and the autosomes (Forejt and Iványi 1974; Forejt 1996; Oka et al. 2007; Gregorová et al. 2008; Mihola et al. 2009). The only known vertebrate hybrid sterility gene, Prdm9, was found in crosses between these two subspecies (Forejt and Iványi 1974; Forejt 1996; Mihola et al. 2009). Female hybrid sterility (Britton-Davidian et al. 2005), reduced immunological function in hybrids (Sage et al. 1986; Moulia et al. 1991), faster fertilization rates of conspecific sperm (Dean and Nachman 2009), and female mating preferences for conspecific males (Laukaitis et al. 1997) provide additional reproductive barriers between these subspecies.
Reproductive barriers between M. m. domesticus and M. m. castaneus have not been directly evaluated, but some evidence suggests that isolation may be less severe than that between M. m. musculus and M. m. domesticus. In natural populations, hybridization has occurred between the two subspecies in human-mediated zones of secondary contact (Orth et al. 1998). Hybridization may have also occurred in Iran during subspecies range expansion (Duvaux et al. 2011). Patterns of genetic differentiation indicate higher levels of introgression from M. m. domesticus into M. m. castaneus than between M. m. musculus and M. m. domesticus (Geraldes et al. 2008, 2011). Inbred strains of M. m. castaneus have been repeatedly crossed to classical inbred strains of house mice (mostly of M. m. domesticus origin) to map phenotypes (e.g., Janaswami et al. 1997; Anunciado et al. 2000; Ishikawa et al. 2000; Lyons et al. 2003, 2004; Yi et al. 2006). Mapping populations usually have been established using backcross and intercross designs, suggesting that F1 hybrids between these two subspecies are effectively fertile. However, the unusual history of the classical strains, which includes hybridization between different subspecies (Beck et al. 2000; Frazer et al. 2007; Yang et al. 2007; Keane et al. 2011), might have shaped these patterns. Additionally, even if F1 hybrid sterility does not block reproduction between M. m. castaneus and M. m. domesticus, barriers could appear in subsequent generations. Recessive-recessive incompatibilities that isolate these subspecies would not be visible in F1’s. Such incompatibilities contribute to hybrid dysfunction in other taxa (Presgraves 2003; Oka et al. 2004; White et al. 2011) and are predicted to be more common than other types of disrupted interactions (Muller 1942). Support for the existence of recessive-recessive incompatibilities in crosses involving M. m. castaneus and M. m. domesticus comes from the identification of multiple genomic regions in M. m. castaneus that reduce fertility only when homozygous. Several independent segments of the M. m. castaneus (CAST/EiJ strain) genome caused reduced fecundity when introgressed on to the genomic background of a strain primarily descended from M. m. domesticus (Davis et al. 2007).
Here, we provide the first detailed characterization of the genetic architecture of hybrid male sterility between M. m. castaneus and M. m. domesticus. We report QTL for a broad range of male fertility traits in the F2 generation. We compare these QTL to those discovered in an equivalent study of hybrid male sterility between M. m. domesticus and M. m. musculus (White et al. 2011) to understand the evolution of reproductive isolation in house mice.
Materials and Methods
Animal husbandry and crossing design
Two wild-derived inbred strains purchased from Jackson Laboratories (www.jax.org) were used to conduct the intercrosses: M. m. castaneus (CAST/EiJ) and M. m. domesticus (WSB/EiJ). Parents were crossed in reciprocal directions to generate the F1 hybrids (M. m. castaneusCAST × M. m. domesticusWSB and M. m. domesticusWSB × M. m. castaneusCAST). The F2 intercross was generated by crossing F1 siblings from both parental directions: (M. m. castaneusCAST × M. m. domesticusWSB) F1 × (M. m. castaneusCAST × M. m. domesticusWSB) F1 and (M. m. domesticusWSB × M. m. castaneusCAST) F1 × (M. m. domesticusWSB × M. m. castaneusCAST) F1. All crosses occurred within the University of Wisconsin School of Medicine and Public Health mouse facility according to animal care protocols approved by the University of Wisconsin Animal Care and Use Committee. Mice were provided with food and water ad libitum. Pups were weaned into same-sex sibling groups at 21 days and males were separated into individual cages at ∼56 days. Males were killed for phenotyping at 70 days of age (±5 days) using carbon dioxide.
Quantification of male fertility phenotypes
Five morphological characters were quantified to diagnose subfertility and sterility in males: testis weight (Iványi et al. 1969; Forejt and Iványi 1974), sperm density (Searle and Beechey 1974; Storchová et al. 2004; Vyskočilová et al. 2005), proportion of abnormal sperm (Kawai et al. 2006), sperm head morphology (Oka et al. 2004; Storchová et al. 2004; Kawai et al. 2006), and stage VII seminiferous tubule area. Variation in these phenotypes will stem from two sources: strain-specific differences in fertility characteristics and hybrid incompatibilities that are not present in the parent strains. Testes were weighed fresh immediately upon dissection, fixed overnight in Bouin’s, and washed in an ethanol series. The right testis was embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin following standard procedures. Testis weight was positively correlated with body weight in F2 males (Pearson’s r = 0.255, P < 0.001). To account for this correlation, testis weight was divided by body weight prior to QTL analyses. We also mapped QTL for absolute right testis weight and for the residual trait scores of testis weight regressed on body weight. Sperm was extracted from the left and right cauda epididymides to measure sperm density, sperm head morphology, and abnormal sperm type as previously outlined (White et al. 2011). Cross-sectional seminiferous tubule area was only quantified in stage VII tubules to control for variance in area among the different stages of spermatogenesis (Russell et al. 1990) as previously described (White et al. 2011). The area of seminiferous tubules was positively correlated with testis weight (Pearson’s r = 0.407, P < 0.001). To account for this correlation, all QTL analyses were conducted using the residual trait scores from a least squares regression of seminiferous tubule area on testis weight.
To quantify X and Y chromosome synapsis, Dumont and Payseur (2011) collected early meiotic cells for CAST/EiJ, WSB/EiJ, and F1 hybrids. X and Y chromosome synapsis was recorded as a binary indicator for each cell in late pachytene. Synapsis was defined by the formation of a synaptonemal complex between the pseudoautosomal regions of the two chromosomes, as revealed by the merger of SYCP3 signals across the pseudoautosomal region (PAR). Within each parental strain and F1, cells were pooled across multiple males [CAST/EiJ: three males, 91 total cells; WSB/EiJ: five males, 99 total cells; (CAST/EiJ × WSB/EiJ) F1: four males, 96 total cells; and (WSB/EiJ × CAST/EiJ) F1: one male, 63 total cells]. The proportion of X and Y chromosomes that were not synapsed was calculated across the total pool of cells.
Genotyping and quality control procedures
Genomic DNA extraction, SNP design, genotyping, and quality control procedures, were conducted as previously described (White et al. 2011). After quality control, there were 188 SNPs across the autosomes and X chromosome, one Y-linked SNP, one mitochondrial SNP, and two SNPs within the M. m. castaneus PAR. Pairwise genotypic similarity of males fell between 13.8 and 85.8% with the exception of two males who were identical at >98.9% of their markers. These males likely reflected duplicate DNA samples and were removed from the analysis. After quality control, the final data set retained 313 males. Only 4.9% of markers were missing from the entire data matrix. All genotypes within the PAR were verified by amplifying ∼1 kb across the SNPs with polymerase chain reaction (PCR) and Sanger sequencing the PCR fragment.
QTL analyses
The genetic map was estimated from a total of 579 males and females with the est.map function of R/qtl (Broman et al. 2003; Broman and Sen 2009), using a Carter–Falconer mapping function (Carter and Falconer 1951; Broman et al. 2002). Because the stringent filtering scheme for markers ensured few genotyping errors (Dumont et al. 2011), the map was estimated assuming a genotyping error rate of zero. SNPs were spaced at an average distance of 8.06 cM. Marker order matched that of the reference mouse genome (Mouse Genome Sequencing Consortium 2002), suggesting no large chromosomal rearrangements. Physical positions were interpolated using the physical and genetic map positions of flanking markers. Crossing design, the number of males, and the number of SNPs were nearly identical to a previous study between M. m. musculus and M. m. domesticus (White et al. 2011). This enabled a direct comparison of the genetic architecture of hybrid male sterility.
Interval mapping was conducted using the scanone function in R/qtl (Lander and Botstein 1989; Broman and Sen 2009) as previously described (White et al. 2011). Genotype probabilities were calculated between markers every 2 cM using a genotyping error rate of 0.001. Phenotypes were mapped using standard interval mapping except for the abnormal sperm types, which were mapped using the extended Haley–Knott method (Feenstra et al. 2006).
Joint analyses of multiple QTL were conducted with two-dimensional, two-QTL scans and multiple QTL modeling as previously described (White et al. 2011). All phenotypes were mapped using standard interval mapping, with the exception of amorphous sperm heads, which was mapped using Haley–Knott regression (Haley and Knott 1992). Multiple QTL models were fitted with the stepwiseqtl function of R/qtl (Manichaikul et al. 2009; Arends et al. 2010). In models that included the mitochondrion or Y chromosome, a covariate that accounted for the intercross direction was added. This covariate was used to capture the phenotypic effects from the mitochondrion or Y chromosome. A penalized LOD score derived from the 10,000 scantwo permutations was used to compare models. Genotype probabilities were calculated every 3 cM with a genotyping error rate of 0.001 and all phenotypes were mapped using Haley–Knott regression (Haley and Knott 1992).
Results
Parental and F1 hybrid fertility
Most phenotypes indicated that M. m. castaneusCAST males were less fertile than M. m. domesticusWSB males. There was no significant difference between M. m. castaneusCAST and M. m. domesticusWSB for proximal bent tail frequency or distal bent tail frequency, but M. m. castaneusCAST displayed significantly higher levels of infertility for all other phenotypes (Table 1). Furthermore, M. m. castaneusCAST displayed sperm heads with reduced apical hooks, consistent with subfertility (Figure 1A). To begin to determine whether these differences were strain-specific, we conducted a preliminary examination of another strain of M. m. castaneus (CIM). M. m. castaneusCIM showed relative right testis weights well below those of M. m. castaneusCAST (M. m. castaneusCAST, 3.11; M. m. castaneusCIM, 1.94; t-test: P < 0.001), indicating that the reduced fertility measures of M. m. castaneusCAST were not unique to this strain. Although M. m. castaneusCAST exhibited some degree of subfertility, males paired with M. m. domesticusWSB females produced offspring.
Table 1 . Mean fertility traits for parents and F1’s.
| Phenotype | M. m. casta | M. m. dom.a | cast. X dom.a | dom. X cast.a |
|---|---|---|---|---|
| Right testis weight (mg) | 43.15b (5; ±1.49) | 66.36c (19; ±11.51) | 63.40c (22; ±4.011) | 78.70d (13; ±6.47) |
| Relative right testis weight (mg/g) | 3.11b (5; ±0.19) | 3.95c (19; ±0.57) | 3.86c (22; ±0.29) | 3.45d (13; ±0.28) |
| Sperm density (millions/mL) | 3.089e (25; ±0.82) | 12.37f (17; ±4.08) | 15.24f (22; ±3.07) | 14.68f (11; ±4.70) |
| Seminiferous tubule area (µm2) | 25,690.47b (5; ±1725.04) | 34,583.45c (6; ±5328.86) | 24,917.02b (6; ±2269.43) | 26,496.08b (6; ±2460.66) |
| Proportion of apoptotic cellsg | 0e (5; ±0.000) | 0.002e (6; ±0.003) | 0.048f (6; ±0.016) | 0.065f (6; ±0.018) |
| Proximal bent tailh | 0.069e (8; ±0.030) | 0.042e (10; ±0.022) | 0.0090f (10; ±0.0074) | 0.0060f (10; 0.0070) |
| Distal bent tailh | 0.020e (8; ±0.011) | 0.011e,f (10; ±0.012) | 0.0020f (10; ±0.0042) | 0.0040f (10; ±0.0052) |
| Headless/tailless spermh | 0.18e (8; ±0.060) | 0.072f (10; ±0.036) | 0.092f (10; ±0.041) | 0.081f (10; ±0.037) |
| Amorphous sperm headh | 0.19e (8; ±0.086) | 0.013f (10; ±0.013) | 0.0090f (10; ±0.012) | 0.0050f (10; ±0.0097) |
| Total sperm abnormalitiesh | 0.46e (8; ±0.071) | 0.14f (10; ±0.052) | 0.11f (10; ±0.044) | 0.096f (10; ±0.039) |
| Unpaired X/Y chromosomesi | 0.066j (91) | 0.051j (99) | 0.375k (96) | 0.302k (63) |
Mean (N; SD). Unpaired X/Y chromosomes, proportion (total cells).
Groups significantly different by t-test, P < 0.05.
Groups significantly different by Mann-Whitney U Test, P < 0.05.
Proportion of seminiferous tubules with any apoptotic cells.
Proportion of abnormal sperm.
Proportion of meiocytes with unpaired X and Y chromosomes at late pachytene.
Groups significantly different by logistic regression, P < 0.001.
Figure 1 .
Epididymal sperm head morphologies. M. m. domesticusWSB, M. m. castaneusCAST, and the F1 hybrids (A). Epididymal sperm head morphology was characterized by two main principal components in the F2 males (B). The first principal component largely explained changes in the apical hook, whereas the second principal component characterized a change in sperm head width.
F1 hybrid male sterility between M. m. castaneusCAST and wild-derived strains from other subspecies of house mice has not been evaluated. We quantified sterility phenotypes in F1 hybrid males from reciprocal crosses between M. m. castaneusCAST and M. m. domesticusWSB. For most phenotypes examined, males from both directions of the cross had fertility measures that matched or exceeded those of M. m. domesticusWSB males (Table 1). Like the M. m. domesticusWSB parent, F1’s from both cross directions also showed sperm heads with pronounced apical hooks (Figure 1A), a shape consistent with fertility (White et al. 2011), and low frequencies of abnormal sperm types (Table 1). Although F1’s generally showed high levels of fertility, several potential signs of hybrid subfertility emerged. The (M. m. domesticusWSB × M. m. castaneusCAST) F1 had significantly reduced relative right testis weights compared to M. m. domesticusWSB and the reciprocal F1 (Table 1). In addition, F1’s from both cross directions had significantly reduced seminiferous tubule areas (indistinguishable from the M. m. castaneusCAST parent; Table 1). Both F1 directions also had a higher percentage of seminiferous tubules with apoptotic cells than either parent (Table 1). Hybrid defects in spermatogenesis were also apparent in spermatocytes in the late phase of meiosis. We detected significantly higher proportions of spermatocytes with unpaired X and Y chromosomes as compared to either parent strain (Table 1).
We set up intercrosses by pairing F1 males with F1 females from the same parental cross direction. Males from both F1 directions sired offspring in every pairing attempted [(M. m. domesticusWSB × M. m. castaneusCAST) F1 direction: 4 pairings; (M. m. castaneusCAST × M. m. domesticusWSB) F1 direction: 10 pairings], indicating that males in both cross directions were fertile. Although we detected a few F1 phenotypes that revealed defects during spermatogenesis, they had no strong effect on the ability to sire offspring.
F2 hybrid sterility
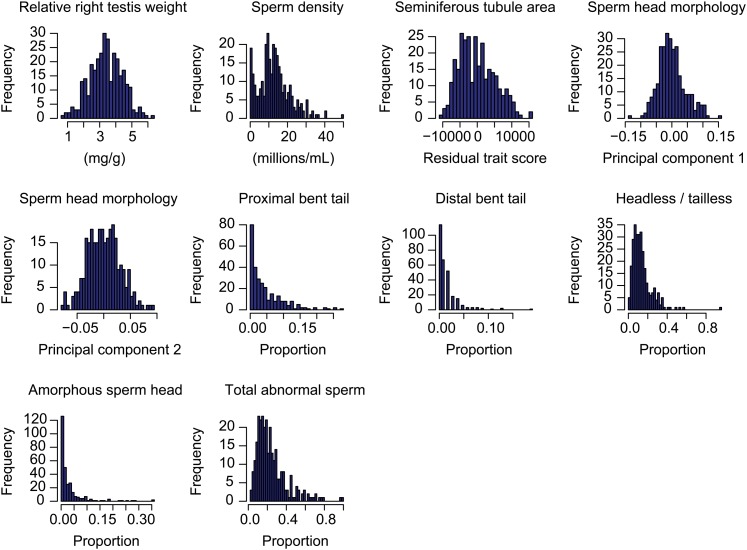
We examined hybrid male sterility in 313 F2 males (99 males originated from the direction with M. m. domesticusWSB as the female parent; 214 males originated from the direction with M. m. castaneusCAST as the female parent). There was an expansion of variance for all phenotypes examined in the F2 males (Figure 2), with a large proportion of the distributions falling in a low fertility range. We used phenotypic means from the sterile F1 hybrids between M. m. musculusPWD × M. m. domesticusWSB (White et al. 2011) to set thresholds for infertility in the F2 males. For several phenotypes, a percentage of males fell within the sterile range (relative right testis weight, 22%; sperm density, 9.3%; proximal bent tail, 9.9%; distal bent tail, 17.3%; and amorphous sperm head, 8.0%), highlighting the importance of recessive factors contributing to hybrid male sterility. We also found high broad-sense heritabilities for each of these phenotypes (relative right testis weight, 0.837; sperm density, 0.768; seminiferous tubule area, 0.590; proximal bent tail, 0.927; distal bent tail, 0.884; headless/tailless sperm, 0.866; amorphous sperm head, 0.951; and total sperm abnormalities, 0.921), verifying that a large proportion of the phenotypic variance was due to genetic differences. Broad-sense heritability was not calculated for the principal component scores of sperm head morphology because the variance from the F2’s is not directly comparable to the variance among the parents and F1’s.
Figure 2 .
F2 phenotype distributions from M. m. castaneusCAST × M. m. domesticusWSB intercross. Seminiferous tubule area is the residual trait score of tubule area regressed on testis weight.
The majority of variation in sperm head morphology among the F2 males was captured by two principal component scores. Principal component one (PC1) explained the curvature of the apical hook on the sperm head (PC1; 52.11% of the phenotypic variance; Figure 1B), whereas principal component two (PC2) accounted for an overall change in sperm head width (PC2; 25.91% of the phenotypic variance; Figure 1B). Similar to what was observed in sterile (M. m. musculusPWD × M. m. domesticusWSB) F1 male hybrids (White et al. 2011), lower values of PC1 were associated with large reductions of the apical sperm head hook.
Most phenotypes were significantly correlated (Table 2), suggesting the presence of common genetic factors. Two phenotypes captured specific abnormalities in spermatogenesis that were largely independent of other hybrid sterility traits. Sperm head morphology PC2 was only negatively correlated with headless/tailless sperm (Table 2). In this case, higher proportions of headless/tailless sperm were associated with thinner sperm heads. The residual trait score of seminiferous tubule area was only negatively correlated with amorphous sperm heads (Table 2).
Table 2 . Spearman’s rank correlation coefficients between hybrid sterility phenotypes.
| Relative right testis | Sperm density | Sperm head PC1 | Sperm head PC2 | Semin. tubule areaa | Prox. bent tail | Dist. bent tail | Headless/tailless | Amorph. head | Total abnormal sperm | |
|---|---|---|---|---|---|---|---|---|---|---|
| Rel. right testis weight | 0.51 | 0.24 | 0.01 | 0.02 | −0.31 | −0.30 | −0.29 | −0.35 | −0.42 | |
| Sperm density | <0.001 | 0.35 | −0.06 | 0.05 | −0.34 | −0.20 | −0.26 | −0.47 | −0.42 | |
| Sperm head PC1 | <0.001 | <0.001 | 0 | 0.05 | −0.26 | −0.15 | −0.28 | −0.38 | −0.38 | |
| Sperm head PC2 | 0.911 | 0.357 | 1 | −0.04 | −0.10 | −0.10 | −0.14 | −0.001 | −0.09 | |
| Semin. tubule areaa | 0.699 | 0.422 | 0.365 | 0.491 | 0.03 | −0.05 | 0.09 | −0.17 | 0.01 | |
| Prox. bent tail | <0.001 | <0.001 | <0.001 | 0.100 | 0.641 | 0.25 | 0.25 | 0.31 | 0.62 | |
| Dist. bent tail | <0.001 | 0.001 | 0.011 | 0.100 | 0.391 | <0.001 | 0.12 | 0.21 | 0.38 | |
| Headless/tailless | <0.001 | <0.001 | <0.001 | 0.018 | 0.156 | <0.001 | 0.050 | 0.32 | 0.81 | |
| Amorphous head | <0.001 | <0.001 | <0.001 | 0.992 | 0.004 | <0.001 | <0.001 | <0.001 | 0.58 | |
| Total abnormal sperm | <0.001 | <0.001 | <0.001 | 0.123 | 0.826 | <0.001 | <0.001 | <0.001 | <0.001 |
Upper diagonal, Spearman’s rho with significant correlations in bold; lower diagonal, P values.
Residual trait scores of seminiferous tubule area regressed on testis weight.
Single QTL interval mapping
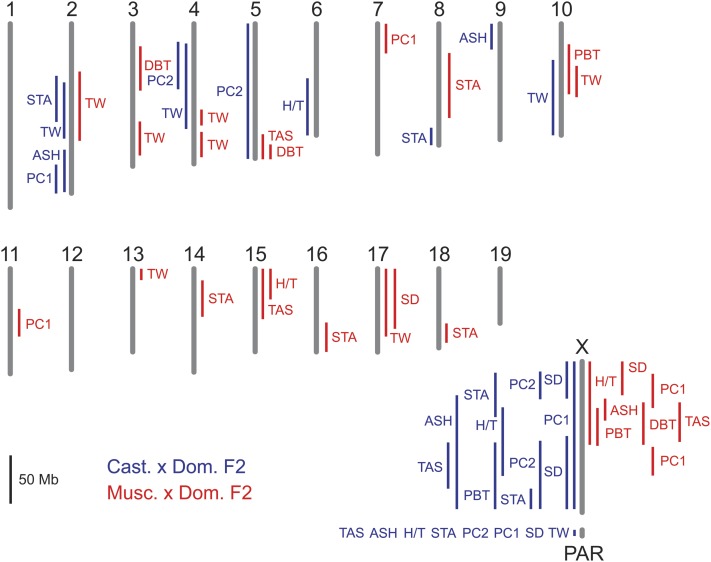
Using standard interval mapping, (Lander and Botstein 1989; Broman and Sen 2009), we found QTL associated with every phenotype except distal bent tail. For relative right testis weight, the strongest QTL was in the PAR, a narrow stretch of sequence homology on the distal end of the X and Y chromosomes (Figure 3; Tables 3 and 4; Supporting Information, Figure S1). We also detected testis weight QTL on chromosomes 2 and 4. The QTL in the PAR was consistent across multiple testis weight phenotypes (absolute right testis weight, relative right testis weight, and the residual trait scores of testis weight regressed on body weight); however, the QTL on chromosomes 2 and 4 dropped below a 5% significance threshold when mapped as absolute right testis weight and the residual trait scores of testis weight regressed on body weight (Table S1). For seminiferous tubule area, we found QTL on chromosomes 2 and 8, the distal end of the X chromosome, and within the PAR. The phenotypic distributions for other phenotypes exhibited strong skews from normality (Figure 2). We used transformations and alternative mapping methods for these phenotypes to assess the robustness of the results. Results were consistent across methodologies except for one phenotype (proximal bent tail), where the QTL dropped below a 5% significance threshold when mapped in a nonparametric framework (Table S2).
Figure 3 .
Single QTL scan for hybrid male sterility. The 1.5 LOD support intervals for QTL that exceed a genome-wide 5% significance threshold are shown (TW, relative right testis weight; SD, sperm density and sperm density binary; PC1, sperm head morphology PC1; STA, seminiferous tubule area; PBT, proximal bent tail; H/T, headless/tailless; ASH, amorphous sperm head; and TAS, total abnormal sperm). QTL mapping to the mitochondrion or Y chromosome are not shown.
Table 3 . Single QTL mapping.
| Phenotype | Chr. | Position (cM) | LOD scorea | Position (Mb) | 1.5 LOD Int (Mb) | DDb | DCb | CCb |
|---|---|---|---|---|---|---|---|---|
| Rel. right testis weight | 2 | 47.5 | 4.10 | 80.7 | 64.1−122.2 | 3.12 (±0.12) | 3.40 (±0.08) | 3.64 (±0.11) |
| 4 | 30 | 3.74 | 69.9 | 30.3−118.7 | 3.53 (±0.12) | 3.53 (±0.09) | 2.93 (±0.15) | |
| Sperm density | X | 58 | 3.75 | 141.7 | 87.3−162.9 | 14.68 (±0.68) | — | 10.643 (±0.792) |
| Sperm density (binary) | X | 6 | 3.13 | 21.8 | 10.2−48.6 | 0.98 (±0.03) | — | 0.86 (±0.03) |
| Sperm head PC1c | 2 | 96 | 4.35 | 177.5 | 149.1−179.0 | −0.01 (±0.01) | −0.01 (±0.004) | 0.02 (±0.01) |
| X | 56 | 3.79 | 137.9 | 10.2−162.9 | −0.01 (±0.004) | — | 0.01 (±0.004) | |
| Sperm head PC2c | M/Y | 0 | 11.07 | — | — | 0.02 (±0.003) | — | −0.01 (±0.002) |
| X | 16 | 10.85 | 41.3 | 21.8−100.7 | −0.01 (±0.002) | — | 0.01 (±0.003) | |
| Semin. tubule aread | 2 | 44 | 7.78 | 75.6 | 57.4−105.0 | 2401.97 (±661.27) | 268.68 (±442.59) | −2637.73 (±643.27) |
| 8 | 60.6 | 4.53 | 125.0 | 112.7−130.8 | 1373.74 (±568.94) | 266.27 (±404.28) | −2399.04 (±611.15) | |
| X | 69.1 | 4.70 | 162.9 | 141.7−162.9 | 1174.16 (±388.86) | — | −1675.42 (±457.60) | |
| Prox. bent taile | X | 60 | 3.33 | 145.5 | 93.9−162.9 | 0.19 (±0.01) | — | 0.24 (±0.01) |
| Headless/taillesse | X | 48 | 10.12 | 113.5 | 57.6−133.6 | 0.33 (±0.01) | — | 0.44 (±0.02) |
| Amorph. sperm heade | 2 | 86 | 4.95 | 169.1 | 133.7−177.5 | 0.13 (±0.01) | 0.16 (±0.01) | 0.21 (±0.01) |
| 9 | 2 | 8.13 | 14.4 | 3.1−29.8 | 0.16 (±0.01) | 0.14 (±0.01) | 0.23 (±0.01) | |
| X | 40 | 3.53 | 93.9 | 45.1−162.9 | 0.14 (±0.01) | — | 0.20 (±0.01) | |
| Total abnormal sperme | X | 50 | 9.60 | 120.2 | 93.9–141.7 | 0.44 (±0.02) | — | 0.59 (±0.01) |
All QTL are significant at a 5% significance threshold.
Phenotype means of each genotype (±SE). D, M. m. domesticusWSB; M, M. m. castaneusCAST.
Transformed to normal quantiles. Higher values of PC1 and PC2 are correlated with higher levels of sterility.
Residual trait scores of seminiferous tubule area regressed on testis weight. Lower values are correlated with higher levels of sterility.
Arcsine squareroot transformed.
Table 4 . QTL linked to the pseudoautosomal region.
| Cross direction | Phenotype | LOD scorea | DDb | DCb | CCb |
|---|---|---|---|---|---|
| Dom. × Cast. | Rel. right testis weight | 6.20 | 3.57 (±0.26) | 2.92 (±0.12) | 3.92 (±0.13) |
| Cast. × Dom. | Rel. right testis weight | 7.33 | 3.73 (±0.09) | 3.62 (±0.24) | 2.93 (±0.01) |
| Sperm density | 6.44 | 15.69 (±0.80) | 17.02 (±2.16) | 9.94 (±0.92) | |
| Sperm head PC1c | 4.12 | 0.01 (±0.004) | 0.02 (±0.01) | −0.02 (±0.01) | |
| Sperm head PC2c | 3.14 | 0.002 (±0.003) | 0.01 (±0.01) | 0.02 (±0.003) | |
| Semin. tubule aread | 10.64 | 1788.93 (±459.11) | 4039.19 (±1240.40) | −2897.30 (±532.58) | |
| Prox. bent taile | 3.74 | 0.19 (±0.01) | 0.19 (±0.03) | 0.25 (±0.01) | |
| Headless/taillesse | 5.26 | 0.34 (±0.01) | 0.33 (±0.03) | 0.42 (±0.01) | |
| Amorph. sperm heade | 4.73 | 0.14 (±0.01) | 0.12 (±0.03) | 0.21 (±0.01) | |
| Total abnormal sperme | 7.98 | 0.44 (±0.02) | 0.42 (±0.05) | 0.59 (±0.02) |
Only QTL with a LOD score >3 are shown.
Phenotype means of each genotype (±SE). D, M. m. domesticusWSB; M, M. m. castaneusCAST.
Transformed to normal quantiles. Lower values of PC1 and PC2 are correlated with higher levels of sterility.
Residual trait scores of seminiferous tubule area regressed on testis weight. Lower values are correlated with higher levels of sterility.
Arcsine squareroot transformed.
We detected several QTL associated with sperm density. When the entire phenotypic distribution was used for mapping, we detected a significant QTL on the distal end of the X chromosome and within the PAR (Figure 2; Tables 3 and 4). Sperm density was converted to a binary character to account for the large number of males with near-zero sperm densities (Figure 2). We split the distribution into two bins by setting a threshold (1.5 millions/mL) that maximized the LOD score of the QTL on the X chromosome. When treated as a binary character, we detected a significant QTL at the proximal end of the X chromosome. We also analyzed sperm density with a two-part procedure, which uses two separate models to map the trait (Broman 2003; Broman and Sen 2009): a binary trait above and below a threshold (1.5 millions/mL) and a normal, quantitative character above the threshold. Consistent with what was observed with the binary model, the QTL on the proximal end of the X chromosome largely controlled high vs. low sperm density, whereas the QTL on the distal end of the X chromosome affected variation within the normal range of the distribution. The QTL within the PAR affected high vs. low sperm density and variation within the normal range of the distribution (Figure 4).
Figure 4 .
Single QTL scans with two-part models. The models evaluate support for QTL associations from the presence/absence of the trait (blue line), the normal portion of the distribution (red line), and the combined distribution (black line). Genome-wide significance thresholds are indicated by the dashed lines and were derived independently for the autosomes and the X chromosome from 1000 permutations of the combined distribution model (black line) (α = 0.05).
All abnormal sperm types (proximal bent tail, headless/tailless sperm, amorphous sperm heads, and total abnormal sperm) had QTL linked to the distal end of the X chromosome and within the PAR (Figure 3; Tables 3 and 4). In addition, amorphous sperm head was associated with two autosomal QTL on chromosomes 2 and 9. Proximal bent tail and amorphous sperm head had spikes at zero in their distributions, so we also applied a two-part model to differentiate the binary and quantitative portions of the traits (as described above). The effects of the X-linked QTL varied among phenotypes (Figure 4). For proximal bent tail, the QTL affected both the presence and absence of the trait as well as variation within the quantitative portion of the distribution. For amorphous sperm head, the X-linked QTL only contributed to variation within the quantitative range. Autosomal QTL had mixed effects, contributing to the presence of amorphous heads and variation within the normal range. The PAR QTL only contributed to variation within the normal range of the distributions.
Sperm head morphology PC1 and PC2 were associated with autosomal, X-linked, and PAR-linked QTL. Sperm head morphology PC1 was linked to chromosome 2, the distal end of chromosome X, and the PAR, whereas sperm head morphology PC2 was linked to the proximal end of chromosome X and the PAR (Figure 3; Tables 3 and 4). For PC2, we also found a strongly supported QTL linked to the mitochondrion or Y chromosome.
With the exception of the relative right testis weight QTL on chromosome 2 and sperm head morphology PC2 on the mitochondrion or Y chromosome, sterility was consistently associated with M. m. castaneusCAST alleles (Table 3). On the autosomes, sterility connected to M. m. castaneusCAST alleles spanned a range of dominance effects. On chromosome 2, there was evidence for several distinct effects. At the proximal end of chromosome 2, sterility was associated with the M. m. domesticusWSB allele for relative right testis weight and with the M. m. castaneusCAST allele for seminiferous tubule area. At the distal end of chromosome 2, sterility was associated with the M. m. castaneusCAST allele for sperm head morphology PC1.
Linkage to the pseudoautosomal region
The M. m. castaneusCAST PAR is larger than the PAR in M. m. domesticusWSB (M. A. White and B. A. Payseur, unpublished results). Markers within this extended region can be genotyped from the M. m. castaneusCAST X chromosome and the PAR of the Y chromosome, but only from the M. m. domesticusWSB X chromosome. Therefore, markers within this region exhibit segregation patterns that are specific to the direction of the intercross (Figure 5). To account for these different segregation patterns, we treated cross-direction as an additive covariate and performed single QTL interval mapping within the PAR. We found a strong QTL for relative right testis weight associated with the PAR in both directions of the intercross (Table 4). In the M. m. domesticusWSB X chromosome × M. m. castaneusCAST Y chromosome direction, the greatest reduction in testis weight occurred when the region was heterozygous (DCsterile genotype in Table 4; Figure 5). In the M. m. castaneusCAST X chromosome × M. m. domesticusWSB Y chromosome direction, testis weight was only reduced when the PAR region was paired with a M. m. domesticus Y chromosome PAR (CCsterile genotype in Table 4; Figure 5).
Figure 5 .
Pseudoautosomal region (PAR) SNP markers in F1 and F2 animals. Two markers (denoted by horizontal lines) are present within the Y chromosome PAR of M. m. castaneusCAST but not within the Y chromosome PAR of M. m. domesticusWSB. All genotypes from both intercross directions are shown. M. m. domesticusWSB alleles are shown in blue and M. m. castaneusCAST alleles are shown in black. The red region of the Y chromosome denotes sequence that is nonhomologous with the X chromosome. Sterile genotypes in F2 males are shown in dashed boxes. Recombinant Y chromosomes have asterisks. We detected some recombinants between the M. m. castaneus X chromosome and the nonhomologous region of the M. m. domesticus Y chromosome, as indicated by the M. m. castaneus genotype present on the M. m. domesticus Y chromosome (lower cross).
The DCsterile and CCsterile genotypes (M. m. domesticusWSB X chromosome × M. m. castaneusCAST Y chromosome direction and M. m. castaneusCAST X chromosome × M. m. domesticusWSB Y chromosome direction, respectively) contain a mixture of recombinant and nonrecombinant Y chromosomes (Figure 5). Although we could not distinguish the two with available markers, we estimated the fraction of each type of chromosome by comparing to the number of recombinant Y chromosomes in the DD and DC genotypes (M. m. domesticusWSB X chromosome × M. m. castaneusCAST Y chromosome direction and M. m. castaneusCAST X chromosome × M. m. domesticusWSB Y chromosome direction, respectively) (Figure 5). We found 11 males with a DD genotype and 15 males with a DC genotype. Because roughly equal numbers of recombinant Y chromosomes should be present in the DCsterile and CCsterile genotypes, we estimated that 76.6% (36 of 47 males) of the DCsterile genotype was composed of a nonrecombinant M. m. castaneus Y chromosome paired with a M. m. domesticus X chromosome and 83.1% (74 of 89) of the CCsterile genotype was composed of a nonrecombinant M. m. domesticus Y chromosome paired with a M. m. castaneus X chromosome. Both directions of the cross indicated that sterility is a consequence of heterozygosity, suggesting that mispairing of structurally different PARs might be involved.
Although heterozygosity in the PAR caused sterility in both directions of the cross, the phenotypic effects appeared to differ between directions of the intercross. The remainder of the phenotypes (sperm density, sperm head morphology PC1, sperm head morphology PC2, seminiferous tubule area, proximal bent tail, headless/tailless sperm, amorphous sperm head, and total abnormal sperm; Table 4) were only linked to the PAR in the M. m. castaneusCAST × M. m. domesticusWSB cross direction. In every case, the phenotypic means of genotypic classes matched the pattern observed for relative right testis weight, with reduced fertility in heterozygotes with the M. m. domesticusWSB Y chromosome PAR.
Multiple QTL mapping
To look for additional QTL and epistatic interactions, we fit multiple QTL models. We first considered two-locus genotypic combinations. All mapping was conducted in a parametric framework. Using a genome-wide significance threshold of 5%, we detected pairs of QTL for relative right testis weight (2 and 4, 2 and PAR, 4 and PAR, M/Y and X, M/Y and PAR), seminiferous tubule area (2 and 2, 2 and 8, 2 and 10, 2 and X, 2 and PAR, 8 and 10, 8 and X, 8 and PAR, 10 and X, M/Y and X, X and PAR), sperm head morphology PC1 (2 and X, 2 and PAR, X and PAR), sperm head morphology PC2 (4 and X, 5 and PAR, M/Y and X, M/Y and PAR, X and X, X and PAR), and amorphous sperm head (2 and 9, 2 and X, 2 and PAR, 9 and X, 9 and PAR) (Table S3). Phenotypic effects for most QTL pairs followed an additive mode; however, some phenotypes exhibited strong epistatic interactions between QTL on the distal end of the X chromosome and the mitochondrion or Y chromosome (Table S3). In these cases, a reduction in fertility was observed when the X chromosome was paired with the mitochondrion or Y chromosome of the other subspecies. This pairwise epistatic interaction mirrored the phenotypic patterns we observed for genotypic classes in the PAR, but with a lower LOD score. These results suggest that linkage to the distal end of the X chromosome and linkage to the PAR were driven by the same QTL.
We fit models that could incorporate more than two QTL using a forward/backward stepwise search algorithm. The percentage of phenotypic variance explained by these models varied among phenotypes (relative right testis weight, 30.84%; sperm density, 8.12%; sperm head morphology PC1, 12.55%; sperm head morphology PC2, 46.72%; seminiferous tubule area, 28.64%; headless/tailless, 19.19%; amorphous sperm head, 24.18%; and total abnormal sperm, 13.90%). Most QTL identified from two-locus QTL mapping were recovered by these analyses (Table 5). Multiple QTL mapping detected autosomal QTL that were missed by single QTL analyses, including loci at which the M. m. domesticusWSB allele was associated with sterility (relative right testis weight, chromosome 10; sperm head morphology PC2, chromosomes 4 and 5; seminiferous tubule area, chromosome X; and headless/tailless, chromosome 6) (Table 5).
Table 5 . Multiple QTL Mapping.
| Phenotype | Chr. | Position (cM) | LOD scorea | Position (Mb) | 1.5 LOD interval (Mb) | % Phen. Varianceb | Additivec | Dominanced | Effecte |
|---|---|---|---|---|---|---|---|---|---|
| Rel. right testis weight | 2 | 47.5 | 6.91 | 80.7 | 68.4–107.8 | 7.40 | 0.38 (±0.07) | 0.17 (±0.10) | Dom. additive |
| 4 | 30 | 5.31 | 69.9 | 28.8–112.6 | 5.62 | −0.35 (±0.08) | 0.24 (±0.11) | Cast. recessive | |
| 10 | 51 | 3.53 | 106.4 | 49.7–127.7 | 3.68 | 0.31 (±0.08) | 0.02 (±0.11) | Dom. additive | |
| M/Y | 0 | 7.45 | — | — | 8.01 | −0.86 (±0.15) | — | Cast. | |
| PARf | 0 | 14.44 | — | — | 16.37 | — | — | — | |
| Sperm density | PARf | 0 | 5.59 | — | — | 8.12 | — | — | — |
| Sperm head PC1g | 2 | 97.7 | 4.28 | 179.0 | 142.3–179.0 | 6.23 | 0.01 (±0.003) | −0.007 (±0.005) | Cast. recessive |
| PARf | 3 | 4.00 | — | — | 5.81 | — | — | — | |
| Sperm head PC2g | 4 | 16.1 | 4.90 | 41.7 | 28.8–77.8 | 4.38 | −0.01 (±0.002) | 0.004 (±0.003) | Dom. additive |
| 5 | 12 | 3.80 | 32.5 | 9.01–148.4 | 3.37 | −0.01 (±0.002) | −0.004 (±0.003) | Dom. recessive | |
| M/Y | 0 | 17.31 | — | — | 17.13 | −0.03 (±0.003) | — | Dom. | |
| X | 12 | 6.33 | 33.5 | 10.2–49.5 | 5.72 | 0.01 (±0.002) | — | Cast. | |
| X | 48 | 8.25 | 113.5 | 92.3–162.7 | 7.57 | 0.01 (±0.002) | — | Cast. | |
| Semin. tubule areah | 2 | 45 | 9.58 | 77.0 | 64.1–107.8 | 11.38 | −2607.1 (±383.5) | 155.3 (±582.6) | Cast. additive |
| X | 21 | 4.81 | 47.7 | 16.0–67.7 | 5.50 | 1280.8 (±269.9) | — | Dom. | |
| PARf | 0 | 10.71 | — | — | 12.85 | — | — | — | |
| Headless/taillessi | 6 | 42 | 3.95 | 111.9 | 78.6–137.6 | 5.29 | −0.05 (±0.01) | −0.03 (±0.02) | Dom. recessive |
| X | 42 | 10.30 | 97.3 | 52.0–133.6 | 14.53 | 0.06 (±0.01) | — | Cast. | |
| Amorph. sperm headi | 2 | 87 | 4.73 | 169.9 | 142.3–179.0 | 5.98 | 0.04 (±0.01) | 0.001 (±0.01) | Cast. additive |
| 9 | 3 | 8.59 | 16.8 | 3.1–31.4 | 11.20 | 0.04 (±0.01) | −0.04 (±0.01) | Cast. recessive | |
| X | 60 | 4.63 | 145.5 | 84.0–162.9 | 5.85 | 0.03 (±0.01) | — | Cast. | |
| Total abnormal spermi | X | 48 | 9.33 | 113.5 | 92.3–151.3 | 13.90 | 0.08 (±0.01) | — | Cast. |
All QTL are significant at a 5% genome-wide significance threshold.
Percentage of phenotypic variance explained by the QTL.
Additive effect. Half the difference between the phenotype averages of the two homozygotes.
Dominance deviation. Difference between the phenotype average of the heterozygotes and the midpoint of the phenotype averages of the two homozygotes.
Subspecies allele associated with sterility and the effect of that allele. Dom., M. m. domesticusWSB; Cast., M. m. castaneusCAST.
Effects of QTL in the PAR are not reported as they combine the effects across both cross directions.
Transformed to normal quantiles.
Residual trait scores of seminiferous tubule area regressed on testis weight.
Arcsine squareroot transformed.
Shared sterility QTL with M. m. musculusPWD × M. m. domesticusWSB hybrids
Our previous identification of QTL for the same phenotypes in a similarly sized intercross between M. m. musculusPWD and M. m. domesticusWSB (White et al. 2011) allows a direct comparison of the genetic architecture of hybrid male sterility in two subspecies pairs. Statistical methods are available that map QTL shared in multiple crosses (Lyons et al. 2004), but they are not designed for epistatic QTL, including hybrid incompatibilities. To begin to characterize similarity in the genetic architecture of hybrid male sterility between subspecies pairs, we counted QTL with overlapping 1.5 LOD intervals. Three autosomal QTL intervals (chromosomes 2, 4, and 10) and several intervals on chromosome X overlapped between the two studies (Figure 6). The M. m. domesticusWSB allele at chromosomes 2 and 10 reduced testis weight in both crosses. All other shared QTL were associated with the M. m. musculusPWD or M. m. castaneusCAST alleles. To determine whether the number of overlapping autosomal QTL was more than expected by chance, we permuted the M. m. castaneusCAST × M. m. domesticusWSB QTL and counted QTL that overlapped with QTL found in the M. m. musculusPWD × M. m. domesticusWSB intercross. Three or more shared QTL were observed in a large fraction of the 10,000 permutations (P = 0.698), suggesting that the observed overlap was within expectations under chance alone. Although this test ignores variation in marker density across the genome, the results reinforce the notion that few QTL are shared between the two crosses.
Figure 6 .
Comparison of hybrid sterility QTL from two intersubspecific crosses of house mice. The 1.5 LOD support intervals for QTL that exceed a genome-wide 5% significance threshold are shown from single QTL interval mapping and multiple QTL mapping (TW, relative right testis weight; SD, sperm density and sperm density binary; PC1, sperm head morphology PC1; STA, seminiferous tubule area; PBT, proximal bent tail; DBT, distal bent tail; H/T, headless/tailless; ASH, amorphous sperm head; and TAS, total abnormal sperm). Blue QTL are from the intercross between M. m. castaneusCAST and M. m. domesticusWSB and red QTL are from the intercross between M. m. musculusPWD and M. m. domesticusWSB (White et al. 2011). QTL that mapped to the mitochondrion or Y chromosome are not shown.
Discussion
The role of differences in fertility between M. m. domesticusWSB and M. m. castaneusCAST
Loci on the autosomes, the X chromosome, and in the PAR confer hybrid male sterility between M. m. domesticusWSB and M. m. castaneusCAST. These QTL may fall into two broad categories. First, they could reflect hybrid incompatibilities that are not present in the parental strains. Second, QTL could be responsible for phenotypic differences between strains, but not hybrid dysfunction. QTL in the first category are connected to the speciation process; those belonging to the second category are not. The conflation of these QTL classes is a challenge faced by all mapping studies that involve crosses between reproductively isolated groups. In our study, the subfertility of M. m. castaneusCAST raises the likely possibility that some QTL cause differences in fertility between the parental strains, but are not responsible for reproductive barriers between M. m. castaneus and M. m. domesticus.
The designation of individual QTL as hybrid incompatibilities or loci responsible for fertility differences among these strains will require additional studies. The Dobzhansky–Muller model predicts that heterosubspecific combinations of alleles at multiple loci interact to reduce fertility. The epistatic interactions between the distal end of the X chromosome and the Y chromosome or mitochondrial genome fit this prediction, but most QTL showed little evidence for epistasis. Statistical power to identify interacting QTL is limited in crosses of this size because the number of mice with the sterile multilocus genotype is small (epistasis can still generate marginal effects, allowing these loci to be detected as individual QTL). QTL mapping in larger crosses would help determine whether the QTL we detected represent hybrid incompatibilities. To conserve power, such studies could involve targeted genomic scans for epistasis involving the QTL reported here. Still, several pieces of evidence indicate that reproductive variation among F2’s reflects hybrid incompatibilities segregating in our intercross. First, the proximal bent tail sterility phenotype, which was prominent among F2’s, was largely absent from both parental strains. The elevated frequency of this phenotype in the F2 generation (but not the F1) requires epistasis involving recessive alleles. Second, M. m. domesticusWSB alleles were associated with sterility at QTL for multiple phenotypes. Because M. m. domesticusWSB males exhibited higher levels of fertility for every phenotype, these QTL require interactions with the M. m. castaneusCAST genome to cause sterility in F2’s. Third, we detected significantly higher frequencies of apoptotic cells in the seminiferous tubules of F1 hybrids than in the parental strains and an increase of unpaired X and Y chromosomes during meiosis, indicating dominant interactions between the parental genomes. Finally, the QTL with the strongest and most widespread effects on sterility (in the PAR) only reduced fertility when heterozygous, limiting its effects to hybrid mice.
F1 hybrid male sterility
F1 hybrids produced large numbers of F2 progeny in both cross directions. F1 fertility has been repeatedly observed in crosses between classical inbred mouse strains (largely of M. m. domesticus origin) and wild-caught (Anunciado et al. 2000; Ishikawa et al. 2000) or wild-derived inbred strains of M. m. castaneus (e.g., Janaswami et al. 1997; Lyons et al. 2003, 2004; Yi et al. 2006), matching our observations. One contributor to this fertility could be introgression of small pieces of classical inbred strain genomes into the genome of M. m. castaneusCAST (Yang et al. 2011). Nevertheless, we recovered developmental signs of abnormal spermatogenesis in the F1 hybrids, including higher levels of apoptosis and a large frequency of spermatocytes carrying unpaired X and Y chromosomes at meiosis. One candidate for abnormal spermatogenesis in the F1 hybrids between M. m. castaneusCAST and M. m. domesticusWSB is the PAR. Sterility was associated with a heterozygous PAR in the F2 population, and the PAR was heterozygous in all F1 males. The PAR has been previously linked to meiotic arrest and F1 sterility in crosses between Mus spretus and C57BL/6J (Guénet et al. 1990; Matsuda et al. 1991, 1992; Hale et al. 1993).
Although F1 hybrids between M. m. castaneusCAST and M. m. domesticusWSB were largely fertile, this may not be representative of the entire M. m. castaneus lineage. Fertility in F1 hybrids has only been evaluated in a handful of inbred and wild representatives of M. m. castaneus, and F1 hybrid male sterility is known to be polymorphic among strains of M. m. musculus and M. m. domesticus (Vyskočilová et al. 2005, 2009; Good et al. 2008b; Piálek et al. 2008). Furthermore, inbreeding depression in the parental strains might mask reduced fertility in the F1’s.
The inbred strains used in this study exhibit similar testis weights to wild populations of M. m. castaneus (Matsuda et al. 1982) and M. m. domesticus (Turner et al. 2012), arguing against severe effects of inbreeding depression. However, the other fertility phenotypes will need to be examined in outbred parental controls to determine whether F1 hybrids between these subspecies are subfertile.
F2 hybrid male sterility
A substantial fraction of F2 males exhibited phenotypes that previously have been connected with sterility. High levels of abnormal sperm (Kawai et al. 2006) and strong reductions in the apical sperm hook (Immler et al. 2007; Firman and Simmons 2009) are negatively correlated with fertilization success in rodents; severely amorphous sperm heads are unable to fertilize ova (Krzanowska and Lorenc 1983; Oka et al. 2007; Styrna 2008). Furthermore, abnormal sperm head shapes often arise from aneuploidies during meiosis (Prisant et al. 2007; Perrin et al. 2008). All sterility phenotypes observed in F1 and F2 hybrids between M. m. musculusPWD and M. m. domesticusWSB except distal bent tail (White et al. 2011) were also observed in F2 hybrids between M. m. castaneusCAST and M. m. domesticusWSB, indicating that these measures capture disruptions in spermatogenesis across multiple subspecies of house mice.
Several QTL for hybrid male sterility overlapped with genomic regions known to affect male reproductive traits in house mice. The testis weight QTL found on chromosomes 4 and 10 colocalize with QTL mapped in crosses between two classical inbred strains (Le Roy et al. 2001; Bolor et al. 2006) and in crosses between M. m. musculusPWD and M. m. domesticusWSB (White et al. 2011). The identification of this QTL in multiple intra- and intersubspecific crosses suggests that it controls normal variation in testis weight in house mice. Sperm density (treated as a binary trait) maps to the proximal end of the X chromosome; this region reduces sperm density when introgressed from M. m. musculus into M. m. domesticus (Good et al. 2008a). The possibility that sperm head morphology PC2 and relative right testis weight map to the Y chromosome (they might instead be linked to mtDNA) agrees with previous results from crosses between classical inbred strains (Krzanowska 1969; Styrna et al. 1991a,b and 2002). Additionally, chromosome substitution strains that carried the middle or distal regions of chromosome 2 or the middle region of chromosome 6 from M. m. castaneusCAST on the genomic background of a classical inbred strain suffered severe drops in fecundity (Davis et al. 2007).
We did not detect linkage to the region on chromosome 17 that harbors Prdm9, the only gene known to cause hybrid sterility in vertebrates (identified in crosses between M. m. musculus and a classical inbred strain (Forejt and Iványi 1974; Forejt 1996; Mihola et al. 2009). Divergence in the number of zinc fingers in the PRDM9 protein (Mihola et al. 2009) has been proposed as a mechanism for hybrid sterility; this divergence may disrupt the protein’s ability to methylate histones (Oliver et al. 2009). However, heterozygosity in this region is not sufficient to cause sterility and requires additional interacting factors (Forejt 1996). Both M. m. musculusPWD × M. m. domesticusWSB and M. m. castaneusCAST × M. m. domesticusWSB crosses produce mice that are heterozygous for the number of zinc finger repeats (Parvanov et al. 2010), but only the M. m. musculusPWD × M. m. domesticusWSB cross yielded QTL in the Prdm9 region (White et al. 2011). The role of Prdm9 in house mouse speciation might be limited to hybrid male sterility between M. m. musculus and M. m. domesticus. A more detailed examination of abnormal spermatogenesis in M. m. castaneus/M. m. domesticus F2 males will reveal whether spermatogenesis arrests during late pachytene, where Prdm9 has its primary effect on hybrid sterility (Forejt and Iványi 1974; Yoshiki et al. 1993).
Comparing the genetic architecture of hybrid male sterility in two subspecies pairs
Our genetic studies of hybrid male sterility in M. m. domesticusWSB - M. m. musculusPWD (White et al. 2011) and M. m. domesticusWSB - M. m. castaneusCAST revealed a subset of QTL that overlap between the two subspecies pairs. Some of these QTL may correspond with those identified in previous studies of hybrid male sterility in house mice. As in our study, sperm density and abnormal sperm morphology have been linked to the proximal end of the X chromosome in crosses between M. m. molossinus and M. m. domesticus (Oka et al. 2004) and in crosses between M. m. musculus and M. m. domesticus (Storchová et al. 2004; Good et al. 2008a; White et al. 2011). These QTL may have a common evolutionary origin. Fine-scale mapping will be necessary to distinguish whether these QTL reflect the same underlying mutation(s).
Combining results from single and multiple QTL analyses (and counting loci that contribute to multiple phenotypes once), we found eight autosomal QTL that contribute to hybrid male sterility between M. m. castaneusCAST × M. m. domesticusWSB. In comparison, we detected 16 autosomal QTL in crosses between M. m. domesticusWSB and M. m. musculusPWD (White et al. 2011). Because our two studies used a common strain (M. m. domesticusWSB), measured the same phenotypes, genotyped a shared set of SNP markers, and featured similar sample sizes, these studies offered comparable power to detect QTL. As a result, we conclude that the larger number of autosomal QTL in M. m. domesticusWSB × M. m. musculusPWD reflects greater genetic complexity of hybrid male sterility between M. m. musculus and M. m. domesticus than between M. m. castaneus and M. m. domesticus, despite similar divergence times.
Most of the autosomal QTL we identified in either study (18 out of 21) were not shared among subspecies pairs. This result agrees with the phylogenetic history of house mice. High levels of phylogenetic discordance among gene trees indicate that the ancestor of M. m. musculus and M. m. castaneus diverged rapidly from the M. m. domesticus lineage (Tucker et al. 1989; Prager et al. 1996, 1998; Lundrigan et al. 2002; Goios et al. 2007; Geraldes et al. 2008; Liu et al. 2008; White et al. 2009; Keane et al. 2011). The short internal branch leading from the root to the most recent common ancestor of M. m. musculus and M. m. castaneus provided little time for derived-derived incompatibilities (Orr 1995) to evolve. As a result, shared incompatibilities are more likely to have arisen along the M. m. domesticus lineage as ancestral-derived incompatibilities. Therefore, most incompatibilities should be unshared between these two subspecies pairs because unshared mutations could have accumulated on both the M. m. musculus and M. m. castaneus branches. Genetic mapping of hybrid male sterility between the third subspecies pair, M. m. castaneus and M. m. musculus, will assist with assigning incompatibilities to the phylogeny.
Hybrid male sterility and the PAR
Heterozygosity in the PAR was strongly associated with most hybrid sterility measures. The PAR is a small region of sequence homology between the X and Y chromosomes, which is restricted to the distal 700 kb of the X chromosome in house mice (Burgoyne 1982; Palmer et al. 1997; Perry et al. 2001). During each male meiosis, an obligate crossover in this small region helps ensure accurate pairing and segregation of the sex chromosomes (Keitges et al. 1985; Rouyer et al. 1986; Soriano et al. 1987). Disruptions in pairing (Burgoyne et al. 1992; Mohandas et al. 1992) or reduced recombination (Shi et al. 2001) can result in aneuploidies or complete meiotic arrest. Consequently, subspecies divergence within the PAR could directly reduce male fertility.
Our results highlight several novel patterns of PAR-linked hybrid male sterility. This is the first example of PAR-linked hybrid sterility among very recently diverged lineages. Hybrid male sterility between more divergent species, Mus spretus and M. m. domesticus (C57BL/6J) (She et al. 1990; Suzuki et al. 2004), has been mapped to the PAR (Guénet et al. 1990). In this species pair, sterility was also limited to heterozygotes (Matsuda et al. 1991; Hale et al. 1993), in which high levels of dissociation among the X and Y chromosomes triggered meiotic arrest (Matsuda et al. 1992; Oka et al. 2010). We observed high frequencies of spermatocytes carrying unpaired X and Y chromosomes at late pachytene in F1 hybrids between M. m. castaneusCAST and M. m. domesticusWSB, an effect likely caused by intersubspecific differences in the PAR region. The increased sterility in F2 hybrids may be attributed to epistatic interactions between a heterozygous PAR and homozygous (recessive) loci elsewhere in the genome.
Other crosses implicate the M. m. castaneusCAST X chromosome as a source of incompatibilities. The M. m. castaneusCAST X chromosome was underrepresented in the Collaborative Cross, a set of recombinant inbred lines resulting from crosses between eight strains (Collaborative Cross Consortium 2012). Because the founder strains were biased toward M. m. domesticus ancestry, selection against the M. m. castaneusCAST X chromosome might have been driven by the PAR-associated hybrid sterility we document here.
Genetic characterization of PAR-linked hybrid male sterility
The high level of variation in hybrid male sterility we observed among heterozygous genotypes indicates that additional genetic factors contribute to PAR-linked hybrid sterility. Although the detailed mechanisms underlying X and Y pairing in the PAR remain unknown, evidence is beginning to indicate that unique mechanisms regulate recombination in this region. In house mice, the PAR harbors an exceptionally high number of double-strand break repair hotspots (Smagulova et al. 2011) and uses machinery to repair double-strand breaks that is distinct from the rest of the genome (Kauppi et al. 2011). A more detailed quantification of meiotic defects between M. m. castaneusCAST and M. m. domesticusWSB will enable mapping of loci that modify PAR-linked hybrid sterility between these subspecies, leading to the discovery of genes specific to X and Y pairing, recombination, and segregation during meiosis.
Several genetic tools exist in these subspecies to aid in fine mapping of genes associated with these traits. Numerous mutant mouse strains have been generated that harbor abnormalities in male reproductive phenotypes (reviewed in Matzuk and Lamb 2002, 2008). These resources provide an extensive list of candidate genes underlying QTL. Furthermore, the eight founder strains of the collaborative cross include M. m. domesticusWSB and M. m. castaneusCAST (Churchill et al. 2004). This inbred panel of mice should accelerate fine mapping of the hybrid sterility QTL identified here and our results will aid the interpretation of sterility patterns segregating in this larger cross.
Supplementary Material
Acknowledgments
We thank Jenny Wagner and John Bak for help measuring male sterility traits and Denise Schwahn for characterizing abnormal spermatogenesis in the testis histology samples. In addition, we thank Brian Steffy and Tim Wiltshire for conducting the genotyping, Karl Broman for assistance in the QTL mapping, Leslie Turner for useful discussions, Francois Bonhomme and Annie Orth for providing the CIM strain, and Michael Nachman and one anonymous reviewer for helpful comments on the manuscript. This research was funded by National Science Foundation grant DEB 0918000. M.A.W. was supported by an National Library of Medicine (NLM) training grant in Computation and Informatics in Biology and Medicine to the University of Wisconsin (NLM 2T15LM007359).
Footnotes
Communicating editor: D. Begun
Literature Cited
- Anunciado R. V., Imamura T., Ohno T., Horio F., Namikawa T., 2000. Developing a new model for non-insulin dependent diabetes mellitus (NIDDM) by using the Philippine wild mouse, Mus musculus castaneus. Exp. Anim. 49: 1–8 [DOI] [PubMed] [Google Scholar]
- Arends D., Prins P., Jansen R. C., Broman K. W., 2010. R/qtl: high-throughput multiple QTL mapping. Bioinformatics 26: 2990–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbash D. A., 2011. Comment on “A test of the snowball theory for the rate of evolution of hybrid incompatibilities”. Science 333: 1576. [DOI] [PubMed] [Google Scholar]
- Barbash D. A., Siino D. F., Tarone A. M., Roote J., 2003. A rapidly evolving MYB-related protein causes species isolation in Drosophila. Proc. Natl. Acad. Sci. USA 100: 5302–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes J. J., Malik H. S., 2009. Altered heterochromatin binding by a hybrid sterility protein in Drosophila sibling species. Science 326: 1538–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. A., Lloyd S., Hafezparast M., Lennon-Pierce M., Eppig J. T., et al. , 2000. Genealogies of mouse inbred strains. Nat. Genet. 24: 23–25 [DOI] [PubMed] [Google Scholar]
- Bolor H., Wakasui N., Zhao W., Ishikawa A., 2006. Detection of quantitative trait loci causing abnormal spermatogenesis and reduced testis weight in the small testis (Smt) mutant mouse. Exp Anim Tokyo 55: 97–108 [DOI] [PubMed] [Google Scholar]
- Bomblies K., Lempe J., Epple P., Warthmann N., Lanz C., et al. , 2007. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 5: e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursot P., Auffray J.-C., Britton-Davidian J., Bonhomme F., 1993. The evolution of house mice. Annu. Rev. Ecol. Syst. 24: 119–152 [Google Scholar]
- Boursot P., Din W., Anand R., Darviche D., Dod B., et al. , 1996. Origin and radiation of the house mouse: mitochondrial DNA phylogeny. J. Evol. Biol. 9: 391–415 [Google Scholar]
- Brideau N. J., Flores H. A., Wang J., Maheshwari S., Wang X., et al. , 2006. Two Dobzhansky-Muller genes interact to cause hybrid lethality in Drosophila. Science 314: 1292–1295 [DOI] [PubMed] [Google Scholar]
- Britton-Davidian J., Fel-Clair F., Lopez J., Alibert P., Boursot P., 2005. Postzygotic isolation between the two European subspecies of the house mouse: estimates from fertility patterns in wild and laboratory-bred hybrids. Biol. J. Linn. Soc. Lond. 84: 379–393 [Google Scholar]
- Broman K., Sen S., 2009. A Guide to QTL Mapping with R/qtl, Springer-Verlag, New York [Google Scholar]
- Broman K. W., 2003. Mapping quantitative trait loci in the case of a spike in the phenotype distribution. Genetics 163: 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman K. W., Rowe L. B., Churchill G. A., Paigen K., 2002. Crossover interference in the mouse. Genetics 160: 1123–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman K. W., Wu H., Sen S., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890 [DOI] [PubMed] [Google Scholar]
- Burgoyne P. S., 1982. Genetic homology and crossing over in the X and Y chromosomes of Mammals. Hum. Genet. 61: 85–90 [DOI] [PubMed] [Google Scholar]
- Burgoyne P. S., Mahadevaiah S. K., Sutcliffe M. J., Palmer S. J., 1992. Fertility in mice requires X-Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell 71: 391–398 [DOI] [PubMed] [Google Scholar]
- Butlin R. K., 2005. Recombination and speciation. Mol. Ecol. 14: 2621–2635 [DOI] [PubMed] [Google Scholar]
- Carter T. C., Falconer D. S., 1951. Stocks for detecting linkage in the mouse, and the theory of their design. J. Genet. 50: 307–323 [DOI] [PubMed] [Google Scholar]
- Cattani M. V., Presgraves D. C., 2009. Genetics and lineage-specific evolution of a lethal hybrid incompatibility between Drosophila mauritiana and its sibling species. Genetics 181: 1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ding J., Ouyang Y., Du H., Yang J., et al. , 2008. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proc. Natl. Acad. Sci. USA 105: 11436–11441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G. A., Airey D. C., Allayee H., Angel J. M., Attie A. D., et al. , 2004. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 36: 1133–1137 [DOI] [PubMed] [Google Scholar]
- Collaborative Cross Consortium, 2012. The genome architecture of the collaborative cross mouse genetic reference population. Genetics 190: 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A., 1989. Patterns of speciation in Drosophila. Evolution 43: 362–381 [DOI] [PubMed] [Google Scholar]
- Davis R. C., Jin A., Rosales M., Yu S., Xia X., et al. , 2007. Genome-wide set of congenic mouse strains derived from CAST/Ei on a C57BL/6 background. Genomics 90: 306–313 [DOI] [PubMed] [Google Scholar]
- Dean M. D., Nachman M. W., 2009. Faster fertilization rate in conspecific vs. heterospecific matings in house mice. Evolution 63: 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dod B., Jermin L. S., Boursot P., Chapman V. H., Nielsen J. T., et al. , 1993. Counterselection on sex-chromosomes in the mus-musculus european hybrid zone. J. Evol. Biol. 6: 529–546 [Google Scholar]
- Dod B., Smadja C., Karn R., Boursot P., 2005. Testing for selection on the androgen-binding protein in the Danish mouse hybrid zone. Biol. J. Linn. Soc. Lond. 84: 447–459 [Google Scholar]
- Dumont B. L., Payseur B. A., 2011. Genetic analysis of genome-scale recombination rate evolution in house mice. PLoS Genet. 7: e1002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont B. L., White M. A., Steffy B., Wiltshire T., Payseur B. A., 2011. Extensive recombination rate variation in the house mouse species complex inferred from genetic linkage maps. Genome Res. 21: 114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvaux L., Belkhir K., Boulesteix M., Boursot P., 2011. Isolation and gene flow: inferring the speciation history of European house mice. Mol. Ecol. 20: 5248–5264 [DOI] [PubMed] [Google Scholar]
- Feenstra B., Skovgaard I. M., Broman K. W., 2006. Mapping quantitative trait loci by an extension of the Haley-Knott regression method using estimating equations. Genetics 173: 2269–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree P. M., Barbash D. A., 2009. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol. 7: e1000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firman R. C., Simmons L. W., 2009. Sperm competition and the evolution of the sperm hook in house mice. J. Evol. Biol. 22: 2505–2511 [DOI] [PubMed] [Google Scholar]
- Forejt J., 1996. Hybrid sterility in the mouse. Trends Genet. 12: 412–417 [DOI] [PubMed] [Google Scholar]
- Forejt J., Iványi P., 1974. Genetic studies on male sterility of hybrids between laboratory and wild mice (Mus musculus L.). Genet. Res. 24: 189–206 [DOI] [PubMed] [Google Scholar]
- Frazer K. A., Eskin E., Kang H. M., Bogue M. A., Hinds D. A., et al. , 2007. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448: 1050–1053 [DOI] [PubMed] [Google Scholar]
- Geraldes A., Basset P., Gibson B., Smith K. L., Harr B., et al. , 2008. Inferring the history of speciation in house mice from autosomal, X-linked, Y-linked and mitochondrial genes. Mol. Ecol. 17: 5349–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes A., Basset P., Smith K. L., Nachman M. W., 2011. Higher differentiation among subspecies of the house mouse (Mus musculus) in genomic regions with low recombination. Mol. Ecol. 20: 4722–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goios A., Pereira L., Bogue M., Macaulay V., Amorim A., 2007. mtDNA phylogeny and evolution of laboratory mouse strains. Genome Res. 17: 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Dean M. D., Nachman M. W., 2008a A complex genetic basis to X-linked hybrid male sterility between two species of house mice. Genetics 179: 2213–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Handel M. A., Nachman M. W., 2008b Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution 62: 50–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J. M., Giger T., Dean M. D., Nachman M. W., 2010. Widespread over-expression of the X chromosome in sterile F1 hybrid mice. PLoS Genet. 6: e1001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorová S., Divina P., Storchova R., Trachtulec Z., Fotopulosova V., et al. , 2008. Mouse consomic strains: exploiting genetic divergence between Mus m. musculus and Mus m. domesticus subspecies. Genome Res. 18: 509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guénet J. L., Nagamine C., Simon-Chazottes D., Montagutelli X., Bonhomme F., 1990. Hst-3: an X-linked hybrid sterility gene. Genet. Res. 56: 163–165 [DOI] [PubMed] [Google Scholar]
- Hale D. W., Washburn L. L., Eicher E., 1993. Meiotic abnormalities in hybrid mice of the C57BL/6J x Mus spretus cross suggest a cytogenetic basis for Haldane’s rule of hybrid sterility. Cytogenet. Cell Genet. 63: 221–234 [DOI] [PubMed] [Google Scholar]
- Haley C. S., Knott S. A., 1992. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity 69: 315–324 [DOI] [PubMed] [Google Scholar]
- Hollocher H., Wu C.-I., 1996. The genetics of reproductive isolation in the Drosophila simulans clade: X vs. autosomal effects and male vs. female effects. Genetics 143: 1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immler S., Moore H. D. M., Breed W. G., Birkhead T. R., 2007. By hook or by crook? Morphometry, competition and cooperation in rodent sperm. PLoS ONE 2: e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A., Matsuda Y., Namikawa T., 2000. Detection of quantitative trait loci for body weight at 10 weeks from Philippine wild mice. Mamm. Genome 11: 824–830 [DOI] [PubMed] [Google Scholar]
- Iványi P., Vojtísková M., Démant P., Micková M., 1969. Genetic factors in the ninth linkage group influencing reproductive performance in male mice. Folia Biol. (Praha) 15: 401–421 [PubMed] [Google Scholar]
- Janaswami P. M., Birkenmeier E. H., Cook S. A., Rowe L. B., Bronson R. T., et al. , 1997. Identification and genetic mapping of a new polycystic kidney disease on mouse chromosome 8. Genomics 40: 101–107 [DOI] [PubMed] [Google Scholar]
- Kao K. C., Schwartz K., Sherlock G., 2010. A genome-wide analysis reveals no nuclear Dobzhansky-Muller pairs of determinants of speciation between S. cerevisiae and S. paradoxus, but suggests more complex incompatibilities. PLoS Genet. 6: e1001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppi L., Barchi M., Baudat F., Romanienko P. J., Keeney S., et al. , 2011. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science 331: 916–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y., Hata T., Suzuki O., Matsuda J., 2006. The relationship between sperm morphology and in vitro fertilization ability in mice. J. Reprod. Dev. 52: 561–568 [DOI] [PubMed] [Google Scholar]
- Keane T. M., Goodstadt L., Danecek P., White M. A., Wong K., et al. , 2011. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477: 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitges E., Rivest M., Siniscalco M., Gartler S. M., 1985. X-linkage of steroid sulphatase in the mouse is evidence for a functional Y-linked allele. Nature 315: 226–227 [DOI] [PubMed] [Google Scholar]
- Krzanowska H., 1969. Factor responsible for spermatozoan abnormality located on the Y chromosome in mice. Genet. Res. 13: 17–24 [DOI] [PubMed] [Google Scholar]
- Krzanowska H., Lorenc E., 1983. Influence of egg investments on in-vitro penetration of mouse eggs by misshapen spermatozoa. J. Reprod. Fertil. 68: 57–62 [DOI] [PubMed] [Google Scholar]
- Lander E. S., Botstein D., 1989. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121: 185–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukaitis C., Critser E., Karn R., 1997. Salivary androgen-binding protein (ABP) mediates sexual isolation in Mus musculus. Evolution 51: 2000–2005 [DOI] [PubMed] [Google Scholar]
- Le Roy I., Tordjman S., Migliore-Samour D., Degrelle H., Roubertoux P., 2001. Genetic architecture of testis and seminal vesicle weights in mice. Genetics 158: 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-Y., Chou J.-Y., Cheong L., Chang N.-H., Yang S.-Y., et al. , 2008. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell 135: 1065–1073 [DOI] [PubMed] [Google Scholar]
- Liu Y.-H., Takahashi A., Kitano T., Koide T., Shiroishi T., et al. , 2008. Mosaic genealogy of the Mus musculus genome revealed by 21 nuclear genes from its three subspecies. Genes Genet. Syst. 83: 77–88 [DOI] [PubMed] [Google Scholar]
- Long Y., Zhao L., Niu B., Su J., Wu H., et al. , 2008. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl. Acad. Sci. USA 105: 18871–18876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundrigan B. L., Jansa S. A., Tucker P. K., 2002. Phylogenetic relationships in the genus mus, based on paternally, maternally, and biparentally inherited characters. Syst. Biol. 51: 410–431 [DOI] [PubMed] [Google Scholar]
- Lyons M. A., Wittenburg H., Li R., Walsh K. A., Churchill G. A., et al. , 2003. Quantitative trait loci that determine lipoprotein cholesterol levels in DBA/2J and CAST/Ei inbred mice. J. Lipid Res. 44: 953–967 [DOI] [PubMed] [Google Scholar]
- Lyons M. A., Wittenburg H., Li R., Walsh K. A., Korstanje R., et al. , 2004. Quantitative trait loci that determine lipoprotein cholesterol levels in an intercross of 129S1/SvImJ and CAST/Ei inbred mice. Physiol. Genomics 17: 60–68 [DOI] [PubMed] [Google Scholar]
- Macholán M., Munclinger P., Sugerková M., Dufková P., Bímová B., et al. , 2007. Genetic analysis of autosomal and X-linked markers across a mouse hybrid zone. Evolution 61: 746–771 [DOI] [PubMed] [Google Scholar]
- Macholán M., Baird S. J. E., Munclinger P., Dufková P., Bímová B., et al. , 2008. Genetic conflict outweighs heterogametic incompatibility in the mouse hybrid zone? BMC Evol. Biol. 8: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichaikul A., Moon J. Y., Sen S., Yandell B. S., Broman K. W., 2009. A model selection approach for the identification of quantitative trait loci in experimental crosses, allowing epistasis. Genetics 181: 1077–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. H., Willis J. H., 2010. Geographical variation in postzygotic isolation and its genetic basis within and between two Mimulus species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 2469–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masly J. P., Presgraves D. C., 2007. High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biol. 5: e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Imai H. T., Moriwaki K., Kondo K., Bonhomme F., 1982. X-Y chromosome dissociation in wild derived Mus musculus subspecies, laboratory mice, and their F1 hybrids. Cytogenet. Cell Genet. 34: 241–252 [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Hirobe T., Chapman V. M., 1991. Genetic basis of X-Y chromosome dissociation and male sterility in interspecific hybrids. Proc. Natl. Acad. Sci. USA 88: 4850–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Moens P. B., Chapman V. M., 1992. Deficiency of X and Y chromosomal pairing at meiotic prophase in spermatocytes of sterile interspecific hybrids between laboratory mice (Mus domesticus) and Mus spretus. Chromosoma 101: 483–492 [DOI] [PubMed] [Google Scholar]
- Matute D. R., Butler I. A., Turissini D. A., Coyne J. A., 2010. A test of the snowball theory for the rate of evolution of hybrid incompatibilities. Science 329: 1518–1521 [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Lamb D. J., 2002. Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. 4: s41–s49 [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Lamb D. J., 2008. The biology of infertility: research advances and clinical challenges. Nat. Med. 14: 1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihola O., Trachtulec Z., Vlcek C., Schimenti J. C., Forejt J., 2009. A mouse speciation gene encodes a meiotic histone h3 methyltransferase. Science 323: 373–375 [DOI] [PubMed] [Google Scholar]
- Mohandas T. K., Speed R. M., Passage M. B., Yen P. H., Chandley A. C., et al. , 1992. Role of the pseudoautosomal region in sex-chromosome pairing during male meiosis: meiotic studies in a man with a deletion of distal Xp. Am. J. Hum. Genet. 51: 526–533 [PMC free article] [PubMed] [Google Scholar]
- Moulia C., Aussel J., Bonhomme F., 1991. Wormy mice in a hybrid zone: a genetic control of susceptibility to parasite infection. J. Evol. Biol. 4: 679–687 [Google Scholar]
- Mouse Genome Sequencing Consortium, 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420: 520–562 [DOI] [PubMed] [Google Scholar]
- Moyle L. C., 2007. Comparative genetics of potential prezygotic and postzygotic isolating barriers in a Lycopersicon species cross. J. Hered. 98: 123–135 [DOI] [PubMed] [Google Scholar]
- Moyle L. C., Graham E. B., 2005. Genetics of hybrid incompatibility between Lycopersicon esculentum and L. hirsutum. Genetics 169: 355–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle L. C., Nakazato T., 2008. Comparative genetics of hybrid incompatibility: sterility in two Solanum species crosses. Genetics 179: 1437–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle L. C., Nakazato T., 2010. Hybrid incompatibility “snowballs” between Solanum species. Science 329: 1521–1523 [DOI] [PubMed] [Google Scholar]
- Moyle L. C., Payseur B. A., 2009. Reproductive isolation grows on trees. Trends Ecol. Evol. 24: 591–598 [DOI] [PubMed] [Google Scholar]
- Muller H. J., 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6: 71–125 [Google Scholar]
- Munclinger P., Bozikova E., Sugerkova M., Pialek J., Macholan M., 2002. Genetic variation in house mice (Mus, muridae, rodentia) from the Czech and Slovak republics. Folia Zool. (Brno) 51: 81–92 [Google Scholar]
- Nachman M. W., Payseur B. A., 2012. Recombination rate variation and speciation: theoretical predictions and empirical results from rabbits and mice. Philos. Trans. R Soc. B. Biol. Sci. 367: 409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor M., Grams K., Bertucci L., Reiland J., 2001. Chromosomal inversions and the reproductive isolation of species. Proc. Natl. Acad. Sci. USA 98: 12084–12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Mita A., Sakurai-Yamatani N., Yamamoto H., Takagi N., et al. , 2004. Hybrid breakdown caused by substitution of the X chromosome between two mouse subspecies. Genetics 166: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Aoto T., Totsuka Y., Takahashi R., Ueda M., et al. , 2007. Disruption of genetic interaction between two autosomal regions and the X chromosome causes reproductive isolation between mouse strains derived from different subspecies. Genetics 175: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Mita A., Takada Y., Koseki H., Shiroishi T., 2010. Reproductive isolation in hybrid mice due to spermatogenesis defects at three meiotic stages. Genetics 186: 339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver P. L., Goodstadt L., Bayes J. J., Birtle Z., Roach K. C., et al. , 2009. Accelerated evolution of the Prdm9 speciation gene across diverse metazoan taxa. PLoS Genet. 5: e1000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. A., 1995. The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics 139: 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. A., Turelli M., 2001. The evolution of postzygotic isolation: accumulating Dobzhansky-Muller incompatibilities. Evolution 55: 1085–1094 [DOI] [PubMed] [Google Scholar]
- Orth A., Adama T., Din W., Bonhomme F., 1998. Natural hybridization of two subspecies of house mice, Musculus domesticus and Mus musculus castaneus, near Lake Casitas (California). Genome 41: 104–110 [DOI] [PubMed] [Google Scholar]
- Palmer S., Perry J., Kipling D., Ashworth A., 1997. A gene spans the pseudoautosomal boundary in mice. Proc. Natl. Acad. Sci. USA 94: 12030–12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvanov E. D., Petkov P. M., Paigen K., 2010. Prdm9 controls activation of mammalian recombination hotspots. Science 327: 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payseur B., Nachman M., 2005. The genomics of speciation: investigating the molecular correlates of X chromosome introgression across the hybrid zone between Mus domesticus and Mus musculus. Biol. J. Linn. Soc. Lond. 84: 523–534 [Google Scholar]
- Payseur B. A., 2010. Using differential introgression in hybrid zones to identify genomic regions involved in speciation. Mol Ecol Resour 10: 806–820 [DOI] [PubMed] [Google Scholar]
- Payseur B. A., Krenz J. G., Nachman M. W., 2004. Differential patterns of introgression across the X chromosome in a hybrid zone between two species of house mice. Evolution 58: 2064–2078 [DOI] [PubMed] [Google Scholar]
- Perrin A., Morel F., Moy L., Colleu D., Amice V., et al. , 2008. Study of aneuploidy in large-headed, multiple-tailed spermatozoa: case report and review of the literature. Fertil. Steril. 90: 1201.e1–1201.e17 [DOI] [PubMed] [Google Scholar]
- Perry J., Palmer S., Gabriel A., Ashworth A., 2001. A short pseudoautosomal region in laboratory mice. Genome Res. 11: 1826–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis N., Orr H. A., 2009. A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science 323: 376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piálek J., Vyskočilová M., Bímová B., Havelková D., Piálková J., et al. , 2008. Development of unique house mouse resources suitable for evolutionary studies of speciation. J. Hered. 99: 34–44 [DOI] [PubMed] [Google Scholar]
- Prager E. M., Tichy H., Sage R. D., 1996. Mitochondrial DNA sequence variation in the eastern house mouse, Mus musculus: comparison with other house mice and report of a 75-bp tandem repeat. Genetics 143: 427–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E. M., Orrego C., Sage R. D., 1998. Genetic variation and phylogeography of central Asian and other house mice, including a major new mitochondrial lineage in Yemen. Genetics 150: 835–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves D. C., 2003. A fine-scale genetic analysis of hybrid incompatibilities in Drosophila. Genetics 163: 955–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves D. C., Balagopalan L., Abmayr S. M., Orr H. A., 2003. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature 423: 715–719 [DOI] [PubMed] [Google Scholar]
- Prisant N., Escalier D., Soufir J.-C., Morillon M., Schoevaert D., et al. , 2007. Ultrastructural nuclear defects and increased chromosome aneuploidies in spermatozoa with elongated heads. Hum. Reprod. 22: 1052–1059 [DOI] [PubMed] [Google Scholar]
- Raufaste N., Orth A., Belkhir K., Senet D., Smadja C., et al. , 2005. Inferences of selection and migration in the Danish house mouse hybrid zone. Biol. J. Linn. Soc. Lond. 84: 593–616 [Google Scholar]
- Rieseberg L., 2001. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 16: 351–358 [DOI] [PubMed] [Google Scholar]
- Rouyer F., Simmler M. C., Johnsson C., Vergnaud G., Cooke H. J., et al. , 1986. A gradient of sex linkage in the pseudoautosomal region of the human sex chromosomes. Nature 319: 291–295 [DOI] [PubMed] [Google Scholar]
- Russell L. D., Ettlin R., Sinha Hikin A., Clegg E., 1990. Histological and Histopathological Evaluation of the Testis, Cache River Press, Clearwater, Florida [Google Scholar]
- Sage R. D., Atchley W., Capanna E., 1993. House mice as models in systematic biology. Syst. Biol. 42: 523–561 [Google Scholar]
- Sage R. D., Heyneman D., Lim K. C., Wilson A. C., 1986. Wormy mice in a hybrid zone. Nature 324: 60–63 [DOI] [PubMed] [Google Scholar]
- Salcedo T., Geraldes A., Nachman M. W., 2007. Nucleotide variation in wild and inbred mice. Genetics 177: 2277–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura K., Yamamoto M., 1997. Characterization of a reproductive isolation gene, zygotic hybrid rescue, of Drosophila melanogaster by using minichromosomes. Heredity 79: 97–103 [Google Scholar]
- Searle A. G., Beechey C. V., 1974. Sperm-count, egg-fertilization and dominant lethality after X-irradiation of mice. Mutat. Res. 22: 63–72 [DOI] [PubMed] [Google Scholar]
- She J. X., Bonhomme F., Boursot P., Thaler L., Catzeflis F., 1990. Molecular phylogenies in the genus Mus: comparative analysis of electrophoretic, scnDNA hybridization, and mtDNA RFLP data. Biol. J. Linn. Soc. Lond. 41: 83–103 [Google Scholar]
- Shi Q., Spriggs E., Field L. L., Ko E., Barclay L., et al. , 2001. Single sperm typing demonstrates that reduced recombination is associated with the production of aneuploid 24,XY human sperm. Am. J. Med. Genet. 99: 34–38 [DOI] [PubMed] [Google Scholar]
- Smagulova F., Gregoretti I. V., Brick K., Khil P., Camerini-Otero R. D., et al. , 2011. Genome-wide analysis reveals novel molecular features of mouse recombination hotspots. Nature 472: 375–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Keitges E. A., Schorderet D. F., Harbers K., Gartler S. M., et al. , 1987. High rate of recombination and double crossovers in the mouse pseudoautosomal region during male meiosis. Proc. Natl. Acad. Sci. USA 84: 7218–7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchová R., Gregorová S., Buckiová D., Kyselová V., Divina P., et al. , 2004. Genetic analysis of X-linked hybrid sterility in the house mouse. Mamm. Genome 15: 515–524 [DOI] [PubMed] [Google Scholar]
- Styrna J., 2008. Genetic control of gamete quality in the mouse–a tribute to Halina Krzanowska. Int. J. Dev. Biol. 52: 195–199 [DOI] [PubMed] [Google Scholar]
- Styrna J., Imai H. T., Moriwaki K., 1991a An increased level of sperm abnormalities in mice with a partial deletion of the Y chromosome. Genet. Res. 57: 195–199 [DOI] [PubMed] [Google Scholar]
- Styrna J., Klag J., Moriwaki K., 1991b Influence of partial deletion of the Y chromosome on mouse sperm phenotype. J. Reprod. Fertil. 92: 187–195 [DOI] [PubMed] [Google Scholar]
- Styrna J., Bilińska B., Krzanowskaa H., 2002. The effect of a partial Y chromosome deletion in B10.BR-Ydel mice on testis morphology, sperm quality and efficiency of fertilization. Reprod. Fertil. Dev. 14: 101–108 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Shimada T., Terashima M., Tsuchiya K., Aplin K., 2004. Temporal, spatial, and ecological modes of evolution of Eurasian Mus based on mitochondrial and nuclear gene sequences. Mol. Phylogenet. Evol. 33: 626–646 [DOI] [PubMed] [Google Scholar]
- Sweigart A. L., Fishman L., Willis J. H., 2006. A simple genetic incompatibility causes hybrid male sterility in mimulus. Genetics 172: 2465–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Presgraves D. C., 2009. Evolution of the Drosophila nuclear pore complex results in multiple hybrid incompatibilities. Science 323: 779–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Chen S., Hartl D. L., Laurie C. C., 2003. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. I. Differential accumulation of hybrid male sterility effects on the X and autosomes. Genetics 164: 1383–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter K. C., Payseur B. A., Harris L. W., Bakewell M. A., Thibodeau L. M., et al. , 2008. Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Res. 18: 67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter K. C., Thibodeau L. M., Gompert Z., Buerkle C. A., Nachman M. W., et al. , 2010. The variable genomic architecture of isolation between hybridizing species of house mice. Evolution 64: 472–485 [DOI] [PubMed] [Google Scholar]
- Ting C. T., Tsaur S. C., Wu M. L., Wu C.-I., 1998. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282: 1501–1504 [DOI] [PubMed] [Google Scholar]
- True J. R., Weir B. S., Laurie C. C., 1996. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans. Genetics 142: 819–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. K.Lee B. Eicher E. 1989. Y-chromosome evolution in the subgenus Mus (Genus Mus). Genetics 122: 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M., Orr H. A., 2000. Dominance, epistasis and the genetics of postzygotic isolation. Genetics 154: 1663–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L. M., Schwahn D. J., Harr B., 2012. Reduced male fertility is common but highly variable in form and severity in a natural house mouse hybrid zone. Evolution 66: 443–458 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe F., Dod B., Boursot P., Bellis M., Bonhomme F., 1986. Absence of Y-chromosome introgression across the hybrid zone between Mus musculus domesticus and Mus musculus musculus. Genet. Res. 48: 191–197 [DOI] [PubMed] [Google Scholar]
- Vyskočilová M., Pražanová G., Piálek J., 2009. Polymorphism in hybrid male sterility in wild-derived Mus musculus musculus strains on proximal chromosome 17. Mamm. Genome 20: 83–91 [DOI] [PubMed] [Google Scholar]
- Vyskočilová M., Trachtulec Z., Forejt J., Pialek J., 2005. Does geography matter in hybrid sterility in house mice? Biol. J. Linn. Soc. Lond. 84: 663–674 [Google Scholar]
- White M. A., Ané C., Dewey C. N., Larget B. R., Payseur B. A., 2009. Fine-scale phylogenetic discordance across the house mouse genome. PLoS Genet. 5: e1000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. A., Steffy B., Wiltshire T., Payseur B. A., 2011. Genetic dissection of a key reproductive barrier between nascent species of house mice. Genetics 189: 289–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Bell T. A., Churchill G. A., Pardo-Manuel De Villena F., 2007. On the subspecific origin of the laboratory mouse. Nat. Genet. 39: 1100–1107 [DOI] [PubMed] [Google Scholar]
- Yang H., Wang J. R., Didion J. P., Buus R. J., Bell T. A., et al. , 2011. Subspecific origin and haplotype diversity in the laboratory mouse. Nat. Genet. 43: 648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi N., Zinniel D. K., Kim K., Eisen E. J., Bartolucci A., et al. , 2006. Bayesian analyses of multiple epistatic QTL models for body weight and body composition in mice. Genet. Res. 87: 45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki A., Moriwaki K., Sakakura T., Kusakabe M., 1993. Histological studies on male-sterility of hybrids between laboratory and wild mouse strains. Dev. Growth Differ. 35: 271–281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.