Abstract
Purpose.
Conjunctivochalasis (CCh) is an age-related inflammatory ocular surface disease manifesting redundant, loose conjunctiva folds. The pathogenic role of Pentraxin 3 (PTX3) in controlling upregulation of matrix metalloproteinase 1 (MMP-1) and MMP-3 in CCh remains undefined.
Methods.
Cytolocation of PTX3 and apoptosis were compared by immunostaining and terminal deoxyribonucleotidyl transferase-mediated FITC-linked dUTP nick-end DNA labeling (TUNEL) assay between normal and CCh specimens containing the conjunctiva and the Tenon. Second to third cultures of normal and CCh fibroblasts were treated with or without Aprotinin, Batimastat, or N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid (NNGH), followed by transfection with or without PTX3 siRNA, and TNF-α or IL-1β. Cell lysates and culture media were collected to assess apoptosis measured by the Cell Death Detection ELISA and expression of PTX3, MMP-1, and MMP-3 transcripts and proteins by quantitative RT-PCR and Western blot, respectively.
Results.
PTX3 immunostaining was negative in normal specimens, but strongly positive in the subconjunctival stroma of CCh specimens. More apoptotic cells were found in CCh samples than in normal specimens. Expression of PTX3 transcripts and protein was not constitutive in resting normal fibroblasts but was in resting CCh fibroblasts and was upregulated by IL-1β in both cell lysates and culture media of both fibroblasts. PTX3 siRNA further upregulated MMP-1 and MMP-3 transcripts in resting normal fibroblasts, but synergistically with IL-1β upregulated the expression of MMP-1 and MMP-3 transcripts only in CCh fibroblasts, with activation of MMP-1 more so than MMP-3. PTX3 siRNA knockdown also promoted cell death characterized by apoptosis and necrosis, and such cell death could be rescued by inhibitors against serine proteinase, MMP1, or MMP3.
Conclusions.
Perturbation of PTX3 expression might partake in apoptosis and pathogenesis of CCh by upregulating expression of MMP-1 and MMP-3, and activation of MMP-1 and MMP-3.
This work represents a major advance in the field in conjunctivochalasis (CCh) to link the pathogenic role of PTX3 in controlling transcription and activation of MMP-1 and MMP-3 in the conjunctival tissue.
Introduction
Conjunctivochalasis (CCh), defined as a loose, redundant, and nonedematous bulbar conjunctiva interposed between the globe and the eyelid, is a frequently overlooked ocular surface disease in the aging population (for review see Ref. 1). Although initially asymptomatic, the consequence of CCh is dryness, tearing, subconjunctival hemorrhage, and incomplete lid closure.2–4 The pro-inflammatory cytokines such as TNF-α and IL-1β are elevated in tears of CCh patients.5–7 The coexisting ocular surface inflammation might further be aggravated by delayed tear clearance, which is frequently related to CCh.8–10 It remains unclear whether ocular surface inflammation might be causatively linked to CCh.
We have long suspected that excessive proteolytic degradation by matrix metalloproteinases (MMPs) contributes to CCh. Previously, we have reported that cultured CCh fibroblasts produce more MMP-1 and MMP-3 transcripts and proteins than normal conjunctival fibroblasts,11 and such overexpression of MMP-1 and MMP-3 is further enhanced by TNF-α or IL-1β.12 As reported, a significantly higher number of conjunctival epithelial and stromal cells express MMP-3 and MMP-9 in CCh patients.7 Hence, it is plausible that overexpression of MMPs is a hallmark of CCh, which may be causatively linked to ocular surface inflammation.
Under the stimulation of TNF-α, cultured human fibroblasts secrete TNF-stimulated gene 6 (TSG-6) and gene 14 (TSG-14),13 of which the latter is also known as Pentraxin 3 (PTX3).14 Neither TSG-6 nor PTX3 is expressed in most normal cells, but both are rapidly upregulated in the presence of the pro-inflammatory TNF-α or IL-1β.13,15–17 We have recently discovered that TSG-6 is constitutively expressed by human normal conjunctival epithelial tissue, is overexpressed in normal conjunctival fibroblasts under stimulation of TNF-α or IL-1β, and exerts an anti-inflammatory function by halting activation of MMP-1 and MMP-3 and apoptosis of CCh conjunctival fibroblasts.18 Because in PTX3 null mice disclose that PTX3 has a cardioprotective role in acute myocardial infarction19 and an atheroprotective effect,20 we wondered whether PTX3 might also play an anti-inflammatory role similar to TSG-6 in CCh. Herein, we provide strong experimental evidence supporting the notion that PTX3 suppresses inflammation and apoptosis of conjunctival fibroblasts by inhibiting gene transcription and activation of MMP-1 and MMP-3 to combat the development of CCh.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium (DMEM), Ham's F-12 medium, amphotericin B, gentamicin, fetal bovine serum, L-glutamine, human epidermal growth factor, β-mercaptoethanol, 0.25% trypsin/1mM EDTA (T/E), Hank's balanced salt solution (HBSS), PBS pH 7.4, Dispase II, collagenase A, and insulin-transferrin-sodium selenite supplement were obtained from Roche (Indianapolis, IN). Hydrocortisone, dimethyl sulfoxide, cholera toxin, BSA, Triton X-100, Hoechst 33,342, Aprotinin (a serine proteinase inhibitor), β-actin, IL-1β, TNF-α, and anti-rat IgG-FITC were purchased from Sigma-Aldrich (St. Louis, MO). HiPerFect siRNA transfection reagent was obtained from Qiagen (Valencia, CA).
Rat anti-human PTX3 IgG, and mouse anti-human pro/actMMP-1 and pro/actMMP-321 were obtained from R&D Systems (Minneapolis, MN). Polyclonal rabbit anti-mouse immunoglobulins/horseradish peroxidase (HRP) and polyclonal swine anti-rat immunoglobulins/HRP were purchased from Dako (Carpinteria, CA). Batimastat, an inhibitor of both MMP1 and MMP3, and centrifugal filter units were purchased from EMD Millipore (Billerica, MA). N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid (NNGH), a specific MMP-3 inhibitor, 22–24 was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY). DeadEnd Fluorometric TUNEL System and RQ1 RNase-Free DNase were purchased from Promega (Madison, WI).
Cultures of Human Conjunctival Fibroblasts
All human subjects were treated in accordance with the Declaration of Helsinki. Under the Protocol No. 06-013 approved by the Institutional Review Board of Baptist Hospital of Miami/South Miami Hospital (Miami, FL), CCh specimens were obtained after surgical removal from 12 patients between the ages of 59 and 82 . Normal conjunctival specimens were obtained from 10 corneoscleral rims of human cadaveric donors (aged between 60 and 81) provided by the Florida Lions Eye Bank (Miami, FL), with the cause of death being accidental. To remove epithelial sheets,25 both conjunctival specimens were digested with 10 mg/mL Dispase II at 4°C for 12 hours under 95% humidified 5% CO2 in plastic dishes containing supplemented hormonal epithelial medium (SHEM), which was made of an equal volume of HEPES buffered DMEM and Ham's F-12 containing bicarbonate, 0.5% dimethyl sulfoxide, 2 ng/mL mouse epidermal growth factor (EGF), 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL sodium selenite, 0.5 μg/mL hydrocortisone, 30 ng/mL cholera toxin A subunit, 5% FBS, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B. The remaining subconjunctival stromal tissue was minced and digested with 1 mg/mL collagenase A in SHEM at 37°C for 12 hours under 95% humidified 5% CO2. After centrifugation at 3000 rpm for 5 minutes, cells released by collagenase A were collected from the pellet and seeded at a density of 4.0 × 104 cells per 12-well plate in SHEM. Upon 80% to 90% confluence, fibroblasts were released by T/E and split at 1:4 ratio to another 12-well plate in the same medium. The second to third passage cultures were used in this study.
Cell Treatments
Cultures of second or third passages at 90% confluence were switched to DMEM with 0.5% FBS for 48 hours and added with or without 20 ng/mL of TNF-α or IL-1β for 4 or 24 hours before being harvested for total RNAs or proteins, respectively. Other cultures were pretreated with or without 100 nM Aprotinin, 0.2 μg/mL Batimastat, or 60 μM NNGH for 1 hour before transfection by 100 nM of PTX3 siRNA (SR3039150B) or scrambled RNA (scRNA) (SR30004) (OriGene Technologies, Rockville, MD) for 48 hours. During the last 24 hours, some cultures were treated with or without 20 ng/mL IL-1β.
Immunostaining
The conjunctival tissue and the underlying Tenon's capsule from both normal and CCh specimens were separated, cryosectioned to 6-μm thickness, and dried for 5 minutes before being fixed with 4% paraformaldehyde for 15 minutes. The sections were permeabilized with 0.2% Triton X-100 in PBS for 15 to 30 minutes and blocked with 0.2% BSA in PBS for 1 hour at room temperature before being incubated in the primary antibody against PTX3 (1:100) overnight at 4°C. After three washes with PBS, the samples were incubated with corresponding AlexaFluor–conjugated secondary IgG (1:100) for 60 minutes. The samples were then counterstained with Hoechst 33342 and analyzed with a Zeiss LSM 700 confocal microscope (Thornhood, NY). Corresponding animal sera were used as the negative controls.
TUNEL Assay
For the terminal deoxyribonucleotidyl transferase (TdT)-mediated FITC-linked dUTP nick-end DNA labeling (TUNEL) assay, cryosectioned tissues were dried for 5 minutes before being fixed with 4% paraformaldehyde for 20 minutes at room temperature and permeabilized with 1% Triton X-100. The samples were then incubated for 60 minutes at 37°C with exogenous TdT and green fluorescein-conjugated dUTP for repair of nicked 30-hydroxyl DNA ends. Cells treated with DNase I were used as the positive control, while the negative control was incubated with the buffer lacking rTdT enzyme. As the result, the apoptotic nuclei were labeled with green fluorescence. After counterstaining by Hoechst 33342, sections were analyzed with a Zeiss LSM 700 confocal microscope. TUNEL-positive (apoptotic) cells were counted as the percentage of total cells counted (count, 2000 cells for each experiment).
Cell Death Detection ELISA
Culture media and cell lysates equivalent to 104 cells were collected and subjected to the Cell Death Detection ELISAplus (Roche, Indianapolis, IN), which is a photometric enzyme immunoassay for in vitro qualitative and quantitative determination of cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes), generated by apoptotic cell death using mouse monoclonal anti-histone and anti-DNA antibodies, respectively. Positive and negative controls were provided by the manufacturer. The activity was determined by absorbance measured at 405 nm using the Fusion Universal Microplate Analyzer (Packard, Indianapolis, IN).
Quantitative RT-PCR (qRT-PCR)
Total RNAs were extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse-transcribed using High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). cDNA of each sample was amplified by real-time RT-PCR using specific primer–probe mixtures and DNA polymerase in 7000 Real-Time PCR System (Applied Biosystems). The real-time PCR profile consisted of 10 minutes of initial activation at 95°C followed by 40 cycles of 15-second denaturation at 95°C and 1 minute annealing and extension at 60°C. All assays were performed four times; the results were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The relative gene expression data were analyzed by the comparative CT method. All gene expression assays used are listed in Table 1.
Table 1. .
Assay ID and Probes Sequence Use for Real-Time PCR
|
Gene Name |
Assay ID (Taqman Expression Assay) |
UniGene |
Product Length |
| GADPH | Hs02758891_g1 | Hs.4279728 | 93 |
| PTX3 | Hs00173615_m1 | Hs.591286 | 96 |
| MMP-1 | Hs00899658_m1 | Hs.83169 | 64 |
| MMP-3 | Hs00968305_m1 | Hs.375129 | 126 |
ID, identification.
Western Blot Analysis
To identify the different expressions for PTX3, MMP-1, and MMP-3 proteins, Western blot analysis was performed using their specific antibodies. A total of 20 μg (for PTX3) or 35 μg (for MMP-1 and MMP-3) proteins from cell lysates or 20 to 25 μL concentrated culture media from different fibroblast cultures were separated by electrophoresis on 4% to 15% (wt/vol) gradient acrylamide-ready gels under denaturing and reducing conditions. Protein bands were transferred to a nitrocellulose membrane, which was then blocked with 5% (wt/vol) fat-free milk in TBST (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% [vol/vol] Tween-20) followed by sequential incubation with specific primary antibodies to PTX3 (1:1000), pro/active MMP-1 and pro/active MMP-3 (1:500), and their respective secondary antibody using β-actin as the loading control. Immunoreactive proteins were detected with Western Lighting Chemiluminescence Reagent (PerkinElmer, Inc., Waltham, MA). The protein levels were determined by densitometry with Image J1.43 (National Institutes of Health, Bethesda, MD).
Statistical Analysis
All summary data were reported as means ± SD for each group and compared using Student's unpaired t-test by Microsoft Excel (Microsoft, Redmond, WA). Test results were reported as 2-tailed P values, where P < 0.05 was considered statistically significant.
Results
Overexpression of PTX3 in CCh Subconjunctival Tissue and Tenon's Capsule
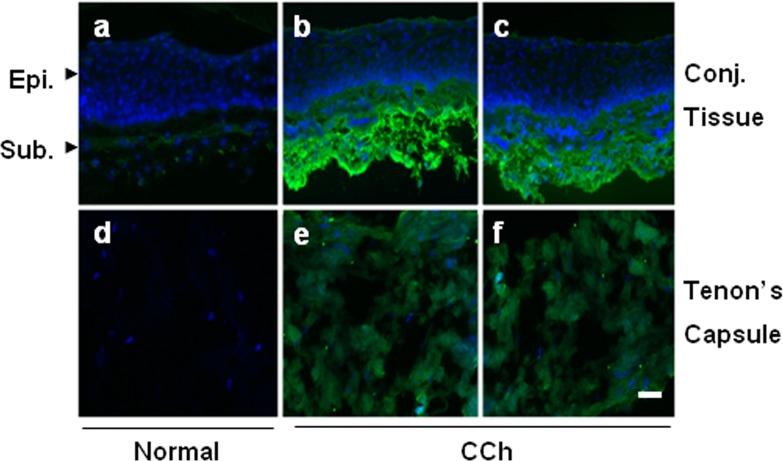
As a first step in exploring the role of PTX3 in CCh, we performed immunostaining to PTX3 in both normal and CCh specimens. The results showed that PTX3 staining was negative in the entire conjunctival epithelium for both normal and CCh specimens (Fig. 1). PTX3 staining was also negative in the normal conjunctival stroma and Tenon's capsule. In contrast, intensive positive PTX3 staining was present in the subconjunctival tissue, and weak positive staining was present in the Tenon's capsule of two representative CCh specimens. The above findings were consistently noted in four cadavers and four CCh specimens. These results suggest that PTX3 protein was not constitutively expressed by in vivo normal conjunctiva epithelial and stromal tissues and Tenon's capsule but was overexpressed in the CCh subconjunctival tissue and Tenon's capsule in vivo.
Figure 1. .
Immunofluorescence staining of PTX3 in normal and CCh specimens. One representative normal (a, d) and two representative CCh (b, c, e, f) conjunctival tissue specimens (a–c) and Tenon's capsule (d–f) were subjected to immunofluorescence staining to PTX3. Strong positive immunostaining to PTX3 was noted in the CCh conjunctival stroma (b, c), while weak positive staining was present in the CCh Tenon's capsule (e, f). Negative staining was noted in the normal tissue specimens (a, d). Nuclei were counterstained by Hoechst 33342. Scale bar = 50 μm.
More Apoptotic Cells in CCh Subconjunctival Tissue and Tenon's Capsule
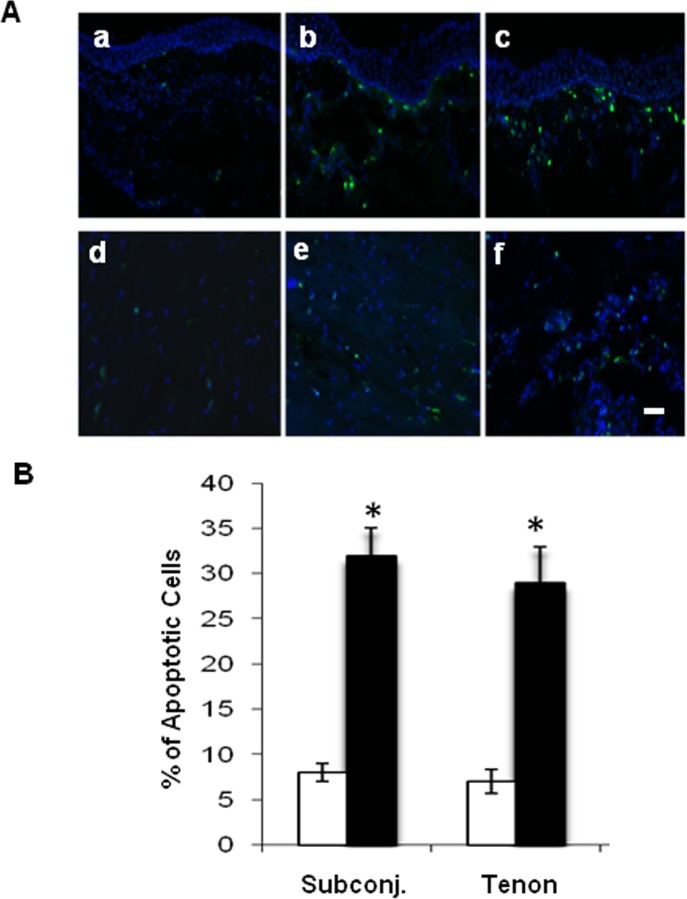
TUNEL assay showed that there were more scattered FITC-positive nuclei-staining apoptotic cells in CCh subconjunctival tissue (Fig. 2A; b and c) and Tenon (Fig. 2A; e and f) than in normal subconjunctival tissue (Fig. 2A; a) and Tenon (Fig. 2A; d). Such a difference was confirmed by calculating the percentage of TUNEL-positive cells in the total cells counted (Fig. 2B). In fact, the number of apoptotic cells was four times higher in CCh subconjunctival tissue and Tenon's capsule than in the normal counterparts (Fig. 2B; P < 0.05, n = 4). These results show that CCh subconjunctival and Tenon tissues exhibited more apoptotic cells than their normal counterparts, suggesting that apoptosis might be involved in the pathogenesis of CCh.
Figure 2. .
Apoptosis detection by TUNEL in normal and CCh conjunctiva and Tenon's capsule. (A) TUNEL assay revealed more apoptotic cells (green) in two representative CCh subconjunctival tissue specimens (b, c) and Tenon's capsule (e, f) than in one representative normal subconjunctival tissue specimen (a) and Tenon's capsule (d). Nuclei were counterstained by Hoechst 33342. All were taken at the same magnification. Scale bar = 100 μm. (B) The above difference was supported by measuring the average percentage of the apoptotic cells in the total cells counted (total counts, 2000 for each experiment) within the normal (□) and CCh (▪) subconjunctival tissue and Tenon's capsule (*P < 0.05 between normal and CCh groups, n = 4).
Upregulation of PTX3 Transcripts and Protein by TNF-α or IL-1β in Both Normal and CCh Fibroblasts
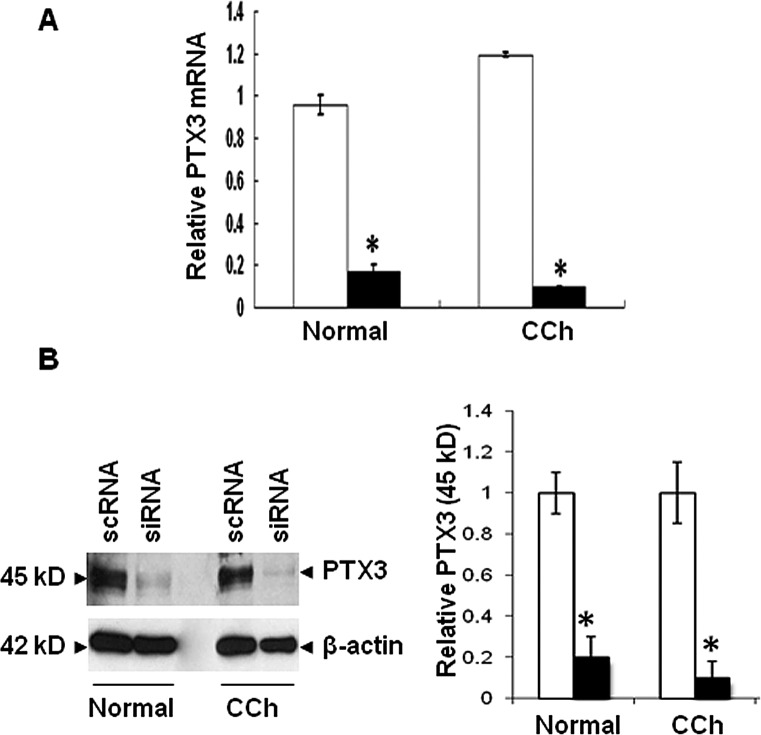
To explore the aforementioned overexpression of PTX3 in the subconjunctival stroma of CCh specimens, we isolated both normal and CCh conjunctival fibroblasts. qRT-PCR confirmed that expression of PTX3 transcripts was negligibly low in resting normal conjunctival fibroblasts, but significantly upregulated 50- and 46-fold by TNF-α and IL-1β, respectively (Fig. 3A; both P < 0.01, n = 4). In contrast, expression of PTX3 transcripts by resting CCh conjunctival fibroblasts was 4-fold that expressed by resting normal conjunctival fibroblasts (P < 0.01, n = 4) and was also upregulated 14- and 15-fold by TNF-α and IL-1β, respectively (both P < 0.05, n = 4). Western blot confirmed that PTX3 was undetectable in both cell lysates (Fig. 3B) and culture media (Fig. 3C) of resting normal fibroblasts. However, a 90-kDa dimer of PTX3 was found in both cell lysates and culture media of resting CCh fibroblasts. The 45-kDa monomer in cell lysates was increased 3- and 2-fold in normal fibroblasts, but 5- and 6-fold in CCh fibroblasts, by TNF-α and IL-1β, respectively. The 90-kDa dimer was barely detected in cell lysates in normal fibroblasts but was upregulated by 2-fold in cell lysates of CCh fibroblasts by TNF-α and IL-1β. Both 45-kDa monomer and 90-kDa dimer of PTX3 in culture media were markedly increased in both normal and CCh fibroblasts (4- and 2-fold of increase, respectively), with the 45-kDa monomer more promoted by IL-1β in CCh fibroblasts (6-fold increase). These results suggest that expressions of PTX3 transcripts and protein were not constitutive in resting normal fibroblasts but were constitutive in resting CCh fibroblasts. However, addition of TNF-α or IL-1β markedly upregulated expression of PTX3 transcripts and 45-kDa monomer and 90-kDa dimer in cell lysates and culture media of both fibroblasts.
Figure 3. .
Upregulation of PTX3 transcripts and proteins by TNF-α or IL-1β in normal and CCh fibroblasts. Both normal (□) and CCh (▪) conjunctival fibroblasts were cultured in DMEM with 0.5% FBS for 48 hours before being treated with PBS, TNF-α, or IL-1β for 4 hours. (A) Expression of PTX3 transcripts was determined by qRT-PCR using GAPDH as the internal control (#P < 0.05 between normal and CCh groups; *P < 0.05 between PBS and TNF-α or IL-1β groups; n = 4). (B) After treatment for 24 hours, proteins in cell lysates were analyzed for expression of PTX3 protein by Western blot using β-actin as the loading control. Densitometry was used to quantify both PTX3 doublet bands at 45 kDa and the single band at 90 kDa (#P < 0.05 between normal and CCh groups; *P < 0.05 between PBS and TNF-α or IL-1β groups; n = 3). (C) After treatment for 24 hours, proteins in culture media were also analyzed for expression of PTX3 proteins by Western blot and densitometry analysis. (#P < 0.05 between normal and CCh groups; *P < 0.05 between PBS and TNF-α or IL-1β groups; n = 3).
PTX3 Knockdown Upregulates MMP-1 and MMP-3 Transcripts in Both Fibroblasts but Activates MMP-1 Only in CCh Fibroblasts
Previously, we have reported overexpression MMP-1 and MMP-3 transcripts and proteins,11 and such overexpression was further promoted by TNF-α or IL-1β12 in CCh fibroblasts. Because expression of PTX3 transcripts and protein was also upregulated by TNF-α or IL-1β in CCh fibroblasts (Fig. 3), we wanted to determine whether there was any causative relationship between the expression of PTX3 and that of MMP-1 and MMP-3. To do so, we decided to use PTX3 siRNA to downregulate PTX3 transcript and protein expression. qRT-PCR verified that the chosen PTX3 siRNA effectively downregulated 82% and 92% of the PTX3 transcripts expressed by normal and CCh fibroblasts, respectively (Fig. 4A; P < 0.05, n = 4). Western blot analysis also confirmed that this siRNA also downregulated 80% and 90% of the PTX3 protein level in normal and CCh fibroblasts, respectively (Fig. 4B; P < 0.05, n = 3).
Figure 4. .
Knockdown efficiency of PTX3 siRNA. Both normal and CCh fibroblasts were cultured in DMEM with 0.5% FBS for 48 hours before being transfected with 100 nM of PTX3 siRNA (▪) or scRNA (□) for another 48 hours, with 20 ng/mL IL-1β added for the last 24 hours. (A) qRT-PCR showed that expression of PTX3 transcripts was markedly downregulated by PTX3 siRNA treatment using GAPDH as the internal control (*P < 0.05 between scRNA and PTX3 siRNA groups, n = 4). (B) Western blot showed that expression of PTX3 protein was also markedly downregulated by PTX3 siRNA treatment using β-actin as the loading control (*P < 0.05 between scRNA and PTX3 siRNA groups, n = 3).
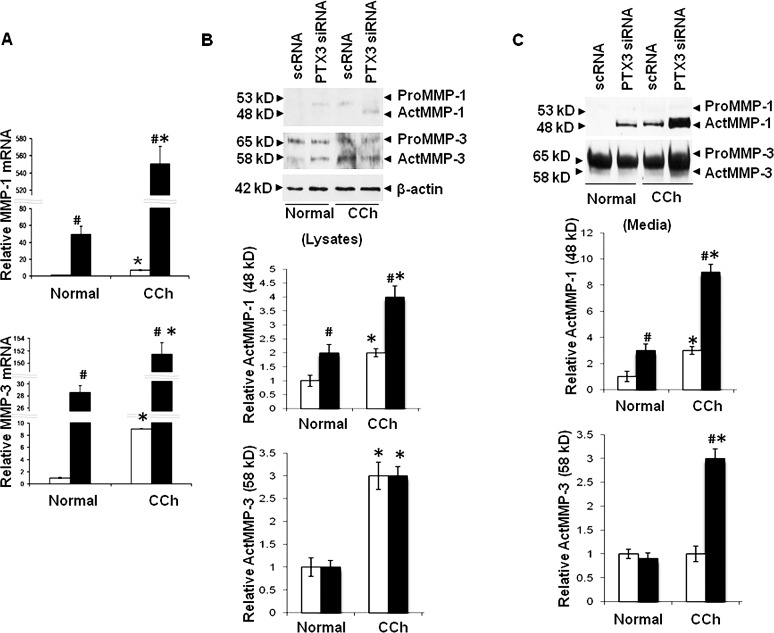
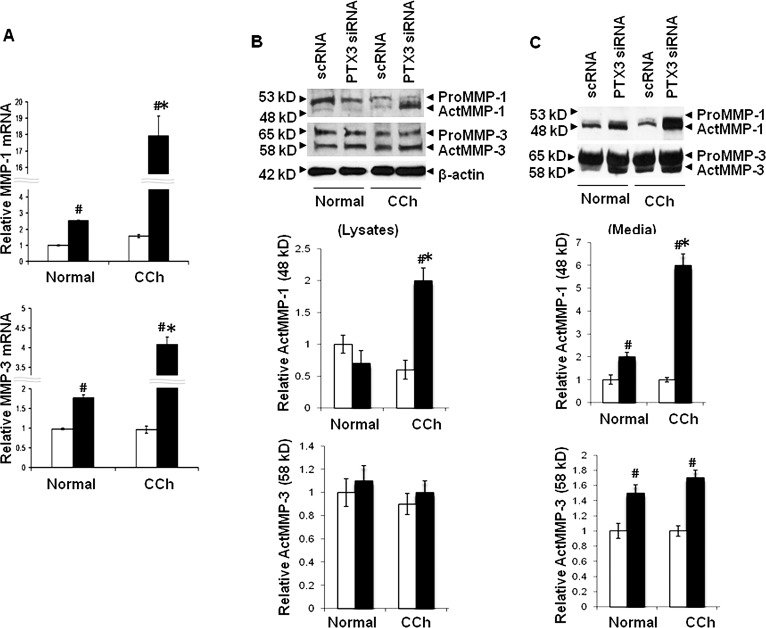
Consistent with our previous study,11 expression of MMP-1 and MMP-3 transcripts by resting CCh fibroblasts treated with scRNA was 7- and 9-fold more than resting normal fibroblasts (Fig. 5A; both P < 0.05, n = 4). PTX3 siRNA significantly upregulated MMP-1 and MMP-3 transcripts by 46- and 29-fold, in resting normal fibroblasts, and by 76- and 16-fold, in resting CCh fibroblasts (Fig. 5A; all P < 0.05, n = 4). The overall level of MMP-1 and MMP-3 transcripts in resting CCh fibroblasts upregulated by PTX3 siRNA was more pronounced than in resting normal fibroblasts (Fig. 5A; all P < 0.05, n = 4). Consistent with our previous study,11 both MMP-1 and MMP-3 proteins in cell lysates (Fig. 5B) and culture media (Fig. 5C) expressed by resting CCh fibroblasts were more than in resting normal fibroblasts. Specifically, both 53-kDa proMMP-1 and 48-kDa actMMP-1 proteins were absent in cell lysates and culture media of resting normal fibroblasts. In contrast, 53-kDa proMMP-1 was found in cell lysates and 48-kDa actMMP-1 was found in culture media of resting CCh fibroblasts. PTX3 knockdown upregulated actMMP-1 by 2- and 3-fold in cell lysates and culture media of resting normal fibroblasts (Figs. 5B, 5C; P < 0.05, n = 3), but not proMMP-1 (not shown). By comparison, PTX3 knockdown induced 2- and 3-fold increase of actMMP-1 in cell lysates and media, respectively, of resting CCh fibroblasts (Fig. 5B; P < 0.05, n = 3), but not proMMP-1 (not shown). In comparison, both 65-kDa proMMP-3 and 58-kDa actMMP-3 proteins were found in cell lysates of both resting normal and CCh fibroblasts (Fig. 5B). PTX3 siRNA only induced a 3-fold increase of actMMP-3 in resting CCh fibroblasts (Fig. 5C; P < 0.05, n = 3), but not in resting normal fibroblasts (not shown). Thus, resting CCh fibroblasts uniquely expressed actMMP-1 and actMMP-3.
Figure 5. .
Upregulation of MMP-1 and MMP-3 transcripts and proteins in normal and CCh fibroblasts by PTX3 knockdown without IL-1β stimulation. Both normal and CCh fibroblasts were cultured in DMEM with 0.5% FBS for 48 hours and transfected with scRNA (□) or PTX3 siRNA (▪) for another 48 hours without IL-1β. (A) Cell lysates were collected for quantitation of MMP-1 and MMP-3 mRNAs by qRT-PCR (#P < 0.05 between normal and CCh groups; *P < 0.05 between scRNA and PTX3 siRNA groups; n = 4). (B) Cell lysates were collected for quantitation of pro and active forms of MMP-1 and MMP-3 proteins by Western blot using β-actin as the loading control and densitometry quantification (#P < 0.05 between normal and CCh groups; *P < 0.05 between scRNA and PTX3 siRNA groups; n = 3). (C). Culture media were also collected for quantitation of pro and active forms of MMP-1 and MMP-3 proteins by Western blot and densitometry (#P < 0.05 between normal and CCh groups; *P < 0.05 between scRNA and PTX3 siRNA groups; n = 3).
Consistent with our previous report,12 expression of MMP-1 and MMP-3 transcripts was upregulated by IL-1β in both normal and CCh fibroblasts (Fig. 6 versus Fig. 5). PTX3 siRNA further promoted 3- and 2-fold increases of both MMP-1 and MMP-3 transcripts in IL-1β–treated normal fibroblasts, respectively (Fig. 6A; both P < 0.05, n = 4), but 7- and 2-fold in IL-1β-treated CCh fibroblasts (Fig. 6A; both P < 0.05, n = 4). Compared with the control treated with scRNA, IL-1β upregulated actMMP-1 protein by 2-fold only in culture media in normal fibroblasts (Fig. 6B). The levels of actMMP-1 in cell lysates and culture media were notably upregulated in CCh fibroblasts by PTX3 siRNAs (Figs. 6B, 6C; 3- and 3-fold, respectively, P < 0.05, n = 3). IL-1β also moderately upregulated actMMP-3 in culture media in both normal and CCh fibroblasts treated with scRNA or PTX3 siRNA (cf. Fig. 5 and Fig. 6C; both P < 0.05, n = 3). However, the change of actMMP-3 protein levels after PTX3 knockdown between normal and CCh fibroblasts was not as noticeable. Taken together, the above findings indicate that knockdown by PTX3 siRNA further upregulated transcripts of MMP-1 more than that of MMP-3 in both normal and CCh fibroblasts. Unique expression of actMMP-1 in culture media in resting CCh fibroblasts (Fig. 5) could be reproduced in normal fibroblasts by PTX3 knockdown and augmented by IL-1β.
Figure 6. .
Upregulation of MMP-1 and MMP-3 mRNAs and proteins in normal and CCh fibroblasts by PTX3 knockdown with IL-1β stimulation. In the same cultures described in Figure 4 (scRNA [□] and PTX3 siRNA [▪]) with IL-1β added for the last 24 hours. (A) Cell lysates were collected for quantitation of MMP-1 and MMP-3 mRNAs by qRT-PCR (#P < 0.05 between normal and CCh groups; *P < 0.05 between scRNA and PTX3 siRNA groups; n = 4). (B) Cell lysates were collected for quantitation of pro and active forms of MMP-1 and MMP-3 proteins by Western blot using β-actin as the loading control, and densitometry quantification (#P < 0.05 between normal and CCh groups; *P < 0.05 between scRNA and PTX3 siRNA groups; n = 3). (C) Culture media were also collected for quantitation of pro and active forms of MMP-1 and MMP-3 proteins by Western blot and densitometry (#P < 0.05 between normal and CCh groups; *P < 0.05 between scRNA and PTX3 siRNA groups; n = 3).
Rescue of Cell Apoptosis by Protease Inhibitors Caused by PTX3 Knockdown
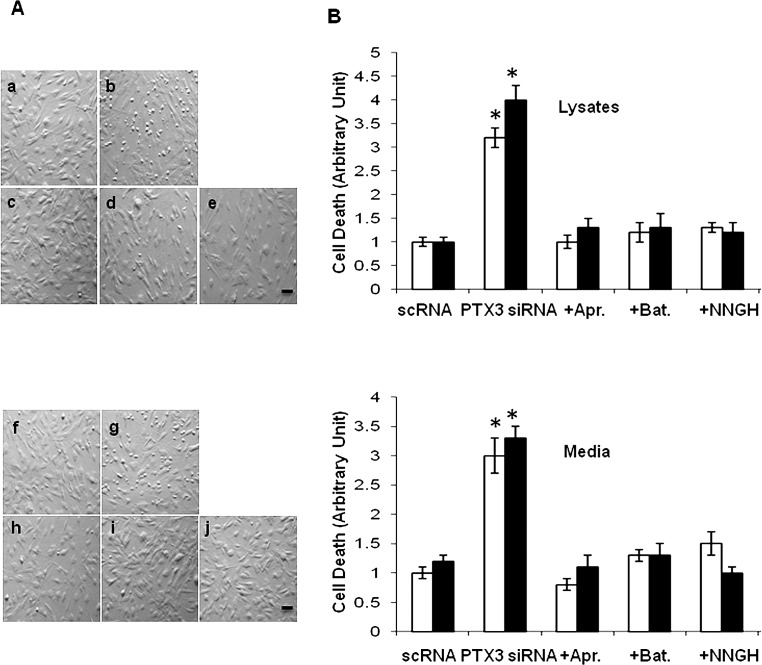
Because apoptosis was promoted in CCh specimen in vivo (Fig. 2), we thus investigated whether there was any difference in apoptosis of these fibroblasts by PTX3 knockdown in vitro. No morphologic difference was noted for both fibroblasts when treated with scRNA in vitro. Of interest, many small round and detached cells appeared as early as 24 hours and became apparent about 36 hours following transfection by PTX3 siRNA in both normal (Fig. 7A; b) and CCh (Fig. 7A; g) fibroblast cultures. This morphologic change was correlated with an increase of apoptosis as judged by the Cell Death Detection ELISA in cell lysates (Fig. 7B; top) and necrosis in culture media (Fig. 7B; bottom). In short, the extent of cell apoptosis was low in both normal and CCh fibroblasts treated with scRNA (Fig. 7B) but increased 3- and 4-fold by PTX3 siRNA in cell lysates of normal and CCh fibroblasts, respectively (Fig. 7B; both P < 0.05, n = 3). A similar result was noted in culture media (Fig. 7B). The result indicates that PTX siRNA further promoted the extent of cell necrosis by 3-fold in culture media of both normal and CCh fibroblasts (Fig. 7B; P < 0.05, n = 3). These results indicate that CCh fibroblasts exhibited similar apoptosis to that in normal fibroblasts and that such apoptosis was further promoted by PTX3 knockdown.
Figure 7. .
Rescue of apoptosis by protease inhibitors in normal and CCh fibroblasts promoted by PTX3 knockdown. Both normal and CCh fibroblasts were cultured in DMEM with 0.5% FBS for 48 hours and transfected with PTX3 siRNA for another 48 hours, while IL-1β was added for the last 24 hours. (A) Phase contrast microscopy of normal (a–e) and CCh (f–j) fibroblasts showed that PTX3 siRNA (b, g) caused more detached round cells than scRNA (a, f). However, such changes were abolished by pretreatment with three protease inhibitors for 1 hour (Aprotinin [c, h]; Batimastat, [d, i]; and NNGH, [e, j], respectively). Scale bar = 100 μm. (B) The extent of cell apoptosis in cell lysates (top) and necrosis in culture media (bottom) were also significantly increased by PTX3 siRNA treatment (count percentage of apoptosis or necrosis cell numbers in 2000 total cells each experiment, *P < 0.05 between scRNA and PTX3 siRNA groups, n = 4) but were rescued by these three protease inhibitors in both normal (□) and CCh (▪) fibroblasts (P > 0.05 between scRNA and PTX3 siRNA + inhibitor groups, n = 4).
To determine whether overexpression of MMP-1 and MMP-3 was responsible for such cell apoptosis, we pretreated normal and CCh fibroblasts with Aprotinin (a serine protease inhibitor; Fig. 7A; c and h), Batimastat (an MMP-1 and MMP-3 inhibitor; Fig. 7A; d and i), or NNGH (a specific MMP-3 inhibitor; Fig. 7A; e and j) for 1 hour, followed by PTX3 siRNA knockdown in the presence of IL-1β stimulation. The results show that the aforementioned morphologic changes caused by PTX3 siRNA knockdown were completely abolished by these three protease inhibitors in both normal and CCh fibroblasts (Fig. 7). These results indicate that the apoptosis caused by PTX3 knockdown was strongly correlated with overexpression of MMPs in cell lysates and culture media. Because rescue could be achieved by Aprotinin to the same extent as Batimastat and NNGH, we speculate that serine proteinases that are involved in activation of MMP-1 might have played an important role.
Discussion
Together with C-reaction protein (CRP) and serum amyloid P (SAP), PTX3 (TSG-14), a 45-kDa glycoprotein, belongs to the superfamily of acute phase proteins.26 Like CRP and SAP, PTX3 forms multimeric structures, but unlike CRP and SAP, whose sequence and regulation have diverged from mouse to man, PTX3 is highly conserved in evolution among many species.27 Moreover, in response to infections or injuries, both CRP and SAP are produced primarily in the liver and play a protective role by interacting with the complement system and promoting clearance of foreign material from the circulation.28,29 In contrast, PTX3 is produced by a variety of tissues and cells and in particular by innate immunity cells, such as mononuclear phagocytes, dendritic cells, fibroblasts, and endothelial cells14 in response to primary inflammatory signals (e.g., TNF-α, IL-1β) and Toll-like receptor (TLR) engagement.30 This explains why the blood level of PTX3 is low in normal conditions (<2 ng/mL in humans) but increases rapidly in patients with sepsis,31 severe meningococcal disease,32 dengue virus infection,33 leptospirosis,34 bacteremia,35 acute myocardial infarction,36 and rheumatoid arthritis.37 Recent studies in PTX3 null mice suggest that PTX3 has a cardioprotective role in acute myocardial infarction19 and an atheroprotective effect.20 Thus, the finding of intense positive immunostaining of PTX3 in CCh subconjunctival stroma and Tenon's capsule (Fig. 1) may also suggest that PTX3 might exert a similar anti-inflammatory role in CCh. Such PTX3 overexpression in vivo was supported by overexpression of PTX3 transcripts and protein by resting CCh fibroblasts (Fig. 3). Because upregulation of PTX3 transcripts and protein could further be promoted by TNF-α and IL-1β (Fig. 3), one likely reason to explain PTX3 overexpression in CCh is the presence of a higher level of pro-inflammatory cytokines in CCh patients' tear meniscus.5,7 The presence of the dimeric PTX3 protein in culture media of CCh fibroblasts suggests that PTX3 was secreted and might exert anti-inflammatory function extracellularly.
We have long speculated that excessive degradation of the underlying Tenon's capsule in CCh38,39 is caused by overexpression of MMP-1 and MMP-3.11,12 Herein, we noted that actMMP-1 was uniquely found in cell lysates and culture media (Fig. 5) of resting CCh fibroblasts, and such expression was further augmented by IL-1β in CCh fibroblasts (Fig. 6). These data suggest that proteolytic degradation of matrix might be caused by actMMP-1 rather than actMMP-3. We also noted that expression of TSG-6,18 PTX3, MMP-1, and MMP-3 transcripts was all upregulated by TNF-α and IL-1β in a manner similar to overexpression of CCAAT/enhancer binding protein delta (CEBPD) in U373MG cells .40 To resolve their interrelationship, we used PTX3 knockdown to demonstrate further upregulation transcription and activation of MMP-1 and MMP-3 in normal and CCh fibroblasts (Fig. 5). The results suggest that PTX3, similar to TSG-6,18 also played a causative role in expression and activation of MMP-1 and MMP-3. In the absence of IL-1β, PTX3 siRNA dramatically upregulated expression of MMP-1 transcripts more than that of MMP-3 transcripts (Fig. 5A) and significantly induced expression of actMMP-1 in lysates and culture media of resting normal fibroblasts (Fig. 5) in a manner similar to that found in resting CCh fibroblasts. In the presence of IL-1β, the aforementioned changes were more accentuated (Fig. 6). In contrast, PTX3 siRNA resulted in little difference in proMMP-1 and proMMP-3 in cell lysates and culture media without (Fig. 5) or with IL-1β (Fig. 6). Thus, similar to TSG-6,18 perturbation of PTX3 expression might be involved in pathogenesis of CCh in reference to activation of MMP-1. As to whether PTX-3 might be involved in regulating pathogenesis of CCH through other MMPs, it is a subject of current investigation in our lab.
Notwithstanding how PTX3 was actually perturbed in CCh fibroblasts, our studies further disclose that PTX3 knockdown could lead to cell death associated with apoptosis or necrosis in both normal and CCh fibroblasts (Fig. 7). Because apoptosis of conjunctival fibroblasts was another hallmark of CCh,18 the above finding also suggested the role of PTX3 in controlling the homeostasis of the conjunctival stroma. Because Aprotinin, Batimastat, and NNGH all helped rescue both fibroblasts from such cell death caused by PTX3 knockdown, overexpression of actMMP-1 and actMMP3 likely played an important causative role. A high level of extracellular actMMP-1 and/or actMMP-3 might not only dissolve the extracellular matrix leading to CCh but also contribute to anoikis, which is a form of apoptosis because of interruption of cell–matrix adhesion.41 Nonetheless, we could not ignore the role of intracellular actMMP-1 and actMMP-3, which have also been implicated to lead to cell death of cultured neurons and myocyte cells,42,43 as well as cell DArgic neurons and hepatic myofibroblasts,21,23,44 respectively. Because Aprotinin (an inhibitor of serine proteinase that is known to activate MMPs)45 achieved the same extent of rescue as Batimastat (a competitive, reversible, and broad-spectrum inhibitor of MMPs, especially MMP-1)46 and NNGH (an MMP-3-specific inhibitor),23,24 we suspect that the action of PTX3 in controlling activation of MMP-1 and MMP-3 might reside not only at the transcriptional and translational levels but also post translational activation of MMPs.
Footnotes
Supported by Grant 201102187 from Shenzhen Science and Technology Bureau, Guangdong, China; Grants EY017497 and EY021045 from the National Eye Institute of the National Institutes of Health, Bethesda, Maryland; a research grant from TissueTech, Inc., Miami, Florida; and an unrestricted grant from the Ocular Surface Research & Education Foundation, Miami, Florida.
Disclosure: P. Guo, TissueTech, Inc. (F, E); S.-Z. Zhang, TissueTech, Inc. (F, E); H. He, TissueTech, Inc. (F, E); Y.-T. Zhu, TissueTech, Inc. (F, E); S.C.G. Tseng, TissueTech, Inc. (F, E)
References
- 1. Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998;43:225–232 [DOI] [PubMed] [Google Scholar]
- 2. Di Pascuale MA, Espana EM, Kawakita T, Tseng SC. Clinical characteristics of conjunctivochalasis with or without aqueous tear deficiency. Br J Ophthalmol. 2004;88:388–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yokoi N, Komuro A, Nishii M, et al. Clinical impact of conjunctivochalasis on the ocular surface. Cornea. 2005;24:S24–S31 [DOI] [PubMed] [Google Scholar]
- 4. Mimura T, Usui T, Yamagami S, et al. Subconjunctival hemorrhage and conjunctivochalasis. Ophthalmology. 2009;116:1880–1886 [DOI] [PubMed] [Google Scholar]
- 5. Acera A, Rocha G, Vecino E, Lema I, Duran JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40:315–321 [DOI] [PubMed] [Google Scholar]
- 6. Erdogan-Poyraz C, Mocan MC, Bozkurt B, Gariboglu S, Irkec M, Orhan M. Elevated tear interleukin-6 and interleukin-8 levels in patients with conjunctivochalasis. Cornea. 2009;28:189–193 [DOI] [PubMed] [Google Scholar]
- 7. Ward SK, Wakamatsu TH, Dogru M, et al. The role of oxidative stress and inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2010;51:1994–2002 [DOI] [PubMed] [Google Scholar]
- 8. Prabhasawat P, Tseng SCG. Frequent association of delayed tear clearance in ocular irritation. Br J Ophthalmol. 1998;82:666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Dogru M, Matsumoto Y, et al. The impact of nasal conjunctivochalasis on tear functions and ocular surface findings. Am J Ophthalmol. 2007;144:930–937 [DOI] [PubMed] [Google Scholar]
- 10. Maskin SL. Effect of ocular surface reconstruction by using amniotic membrane transplant for symptomatic conjunctivochalasis on fluorescein clearance test results. Cornea. 2008;27:644–649 [DOI] [PubMed] [Google Scholar]
- 11. Li D-Q, Meller D, Tseng SCG. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by cultured conjunctivochalasis fibroblasts. Invest Ophthalmol Vis Sci. 2000;41:404–410 [PubMed] [Google Scholar]
- 12. Meller D, Li D-Q, Tseng SCG. Regulation of collagenase, stromelysin, and gelatinase B in human conjunctival and conjunctivochalasis fibroblasts by interleukin-1b and tumor necrosis factor-a. Invest Ophthalmol Vis Sci. 2000;41:2922–2929 [PubMed] [Google Scholar]
- 13. Lee TH, Lee GW, Ziff EB, Vilcek J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol Cell Biol. 1990;10:1982–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366 [DOI] [PubMed] [Google Scholar]
- 15. Lee TH, Wisniewski H-G, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding protein, closely related to the adhesion receptor CD44. J Cell Biol. 1992;116:545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997;8:143–156 [DOI] [PubMed] [Google Scholar]
- 17. Breviario F, d'Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells: cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197 [PubMed] [Google Scholar]
- 18. Guo P, Zhang SZ, He H, Zhu YT, Tseng SC. TSG-6 controls transcription and activation of matrix metalloproteinase 1 in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2012;53:1372–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salio M, Chimenti S, De AN, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064 [DOI] [PubMed] [Google Scholar]
- 20. Norata GD, Marchesi P, Pulakazhi VV, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708 [DOI] [PubMed] [Google Scholar]
- 21. Choi DH, Kim EM, Son HJ, et al. A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J Neurochem. 2008;106:405–415 [DOI] [PubMed] [Google Scholar]
- 22. Kim YS, Kim SS, Cho JJ, et al. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25:3701–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim EM, Shin EJ, Choi JH, et al. Matrix metalloproteinase-3 is increased and participates in neuronal apoptotic signaling downstream of caspase-12 during endoplasmic reticulum stress. J Biol Chem. 2010;285:16444–16452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leong DJ, Gu XI, Li Y, et al. Matrix metalloproteinase-3 in articular cartilage is upregulated by joint immobilization and suppressed by passive joint motion. Matrix Biol. 2010;29:420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Espana EM, Romano AC, Kawakita T, Di Pascuale M, Smiddy R, Tseng SC. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281 [DOI] [PubMed] [Google Scholar]
- 26. Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13 [DOI] [PubMed] [Google Scholar]
- 27. Bottazzi B, Garlanda C, Cotena A, et al. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol Rev. 2009;227:9–18 [DOI] [PubMed] [Google Scholar]
- 28. Jiang HX, Siegel JN, Gewurz H. Binding and complement activation by C-reactive protein via the collagen-like region of C1q and inhibition of these reactions by monoclonal antibodies to C-reactive protein and C1q. J Immunol. 1991;146:2324–2330 [PubMed] [Google Scholar]
- 29. Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–2147 [PubMed] [Google Scholar]
- 30. Garlanda C, Bottazzi B, Moalli F, et al. Pentraxins and atherosclerosis: the role of PTX3. Curr Pharm Des. 2011;17:38–46 [DOI] [PubMed] [Google Scholar]
- 31. Mauri T, Bellani G, Patroniti N, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36:621–629 [DOI] [PubMed] [Google Scholar]
- 32. Sprong T, Peri G, Neeleman C, et al. Pentraxin 3 and C-reactive protein in severe meningococcal disease. Shock. 2009;31:28–32 [DOI] [PubMed] [Google Scholar]
- 33. Mairuhu AT, Peri G, Setiati TE, et al. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. 2005;76:547–552 [DOI] [PubMed] [Google Scholar]
- 34. Wagenaar JF, Goris MG, Gasem MH, et al. Long pentraxin PTX3 is associated with mortality and disease severity in severe Leptospirosis. J Infect. 2009;58:425–432 [DOI] [PubMed] [Google Scholar]
- 35. Huttunen R, Hurme M, Aittoniemi J, et al. High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PLoS One. 2011;6:e17653 Available at: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0017653. Accessed 20 January 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peri G, Introna M, Corradi D, et al. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641 [DOI] [PubMed] [Google Scholar]
- 37. Luchetti MM, Piccinini G, Mantovani A, et al. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA). Clin Exp Immunol. 2000;119:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kheirkhah A, Casas V, Blanco G, et al. Amniotic membrane transplantation with fibrin glue for conjunctivochalasis. Am J Ophthalmol. 2007;144:311–313 [DOI] [PubMed] [Google Scholar]
- 39. Kheirkhah A, Casas V, Esquenazi S, et al. New surgical approach for superior conjunctivochalasis. Cornea. 2007;26:685–691 [DOI] [PubMed] [Google Scholar]
- 40. Ko CY, Chang LH, Lee YC, et al. CCAAT/enhancer binding protein delta (CEBPD) elevating PTX3 expression inhibits macrophage-mediated phagocytosis of dying neuron cells. Neurobiol Aging. 2012;33:422.e11–422.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364 [DOI] [PubMed] [Google Scholar]
- 42. Vos CM, Sjulson L, Nath A, et al. Cytotoxicity by matrix metalloprotease-1 in organotypic spinal cord and dissociated neuronal cultures. Exp Neurol. 2000;163:324–330 [DOI] [PubMed] [Google Scholar]
- 43. Chen H, Li D, Saldeen T, Mehta JL. TGF-beta 1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. Am J Physiol Heart Circ Physiol. 2003;284:H1612–H1617 [DOI] [PubMed] [Google Scholar]
- 44. Si-Tayeb K, Monvoisin A, Mazzocco C, et al. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am J Pathol. 2006;169:1390–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149 [DOI] [PubMed] [Google Scholar]