Abstract
Purpose.
To investigate the therapeutic effects of metformin, a commonly used antidiabetic drug, in preventing endotoxin-induced uveitis (EIU) in rats.
Methods.
EIU in Lewis rats was developed by subcutaneous injection of lipopolysaccharide (LPS; 150 μg). Metformin (300 mg/kg body weight, intraperitoneally) or its carrier was injected either 12 hours before or 2 hours after LPS induction. Three and 24 hours after EIU, eyes were enucleated and aqueous humor (AqH) was collected. The MILLIPLEX-MAG Rat cytokine-chemokine magnetic bead array was used to determine inflammatory cytokines. The expression of Cox-2, phosphorylation of AMPK, and NF-κB (p65) were determined immunohistochemically. Primary human nonpigmented ciliary epithelial cells (HNPECs) were used to determine the in vitro efficacy of metformin.
Results.
Compared with controls, the EIU rat AqH had significantly increased number of infiltrating cells and increased levels of various cytokines and chemokines (TNF-α, MCP-1, IL-1β, MIP-1α, IL-6, Leptin, and IL-18) and metformin significantly prevented the increase. Metformin also prevented the expression of Cox-2 and phosphorylation of p65, and increased the activation of AMPK in the ciliary bodies and retinal tissues. Moreover, metformin prevented the expression of Cox-2, iNOS, and activation of NF-kB in the HNPECs and decreased the levels of NO and PGE2 in cell culture media.
Conclusions.
Our results for the first time demonstrate a novel role of the antidiabetic drug, metformin, in suppressing uveitis in rats and suggest that this drug could be developed to prevent uveitis complications.
Our studies found that metformin, a commonly used drug for diabetes, prevents endotoxin-induced uveitis in rats via activating AMPK and suppressing NF-kB-mediated ocular inflammatory response. Thus, our studies indicate that metformin could be developed as a potential agent to prevent uveitis.
Introduction
Uveitis, caused by various factors, such as autoimmune disorders, infections, exposure to toxins, and many other unknown factors, is an ocular inflammatory condition that could lead to total blindness1,2; however, the exact etiology of the disease progression is not yet well understood. The levels of various cytokines, as well as chemokines, in uveal tissues are found to be significantly increased because of ocular inflammation.2 Activation of intracellular signaling cascades and alterations of the expression pattern of various inflammatory proteins in ocular tissues are common characteristics of uveitis.3,4 Activation of redox-sensitive transcription factors, such as nuclear factor (NF)-κB, has been shown to be involved in a number of inflammatory diseases, including uveitis.5,6 NF-κB is known to regulate the expression of a number of genes responsible for inflammatory markers and various other cytokines and chemokines.7 Because activation of NF-κB is a prominent feature of uveitis, therapeutic agents targeted toward suppression of NF-κB could potentially help in curbing ocular inflammation. Further, a number of antioxidants and plant products that prevent the expression of NF-κB–dependent inflammatory markers have been shown to prevent uveitis complications, both in animal and human studies8; however, because of a lack of specific toxicity studies, safe delivery methods, and unwanted side effects, some of these agents have never reached to clinical settings. Therefore, it is useful to identify potential therapeutic drugs that are already found to be safe for human use to ameliorate ocular inflammatory complications, such as uveitis.
Metformin (Glucophage, Glumteza, Riomet) is an oral medication used either alone or in combination with other medications to treat patients with type 2 diabetes (Fig. 1).9–11 Although suppression of glucose production in liver is a well-established function of metformin, it can also show beneficial effects in other tissues, such as adipose, skeletal muscle, and vascular endothelium, during hyperglycemia. Metformin not only ameliorates insulin resistance and hyperinsulinemia but is also known to improve ovulation and regulate the menstrual cycle in women after long-term use.12 Recently, metformin has been reported to hold potential for cancer treatment.13–15 The anticarcinogenic affects appear to be associated with the regulation of NF-κB, matrix metalloproteinase (MMP)-2/9, AKT (also known as protein kinase B), and extracellular-signal-related kinase (ERK)1/2 signaling pathways that are known to be important mediators of inflammation, tumor invasion and metastasis.16 Recently, Quaile et al.17 reported that the no observable adverse effect level of metformin was 200 mg/kg/d (mean area under the curve 0–24 = 41.1 μg h/mL; mean Cmax = 10.3 μg/mL based on sex average week 13 values) in rats. Their study also revealed that metformin when used as monotherapy could also reduce toxicities such as body weight loss, necrosis and inflammation of parotid salivary gland, metabolic acidosis, morbidity, and mortality in rats. Moreover, metformin has been known to activate adenosine-monophosphate-activated protein kinase (AMPK), which can suppress NF-κB activation and thereby NF-κB–dependent inflammatory pathologies.18,19 Further, metformin improved survival in a mouse model of lethal endotoxemia by inhibiting high-mobility group protein B1 release.20 Collectively, recent literature revealed that metformin could be an important agent for the suppression of inflammatory complications and this drug could be safe for long-term use in humans. Therefore, metformin, which is already in the market to treat patients with diabetic complications, could be a potential therapeutic agent for preventing ocular inflammatory complications, such as uveitis. Hence, in the present study, we investigated the efficacy of metformin in preventing ocular inflammatory response in endotoxin-induced uveitis (EIU) in rats as well as examined its anti-inflammatory effects in cultured human nonpigmented ciliary epithelial cells (HNPECs). Our results indicate that metformin could prevent endotoxin-induced ocular inflammation leading to uveitis in rats and suggest that this drug could also be used as a novel therapeutic agent in preventing uveitis complications.
Figure 1. .
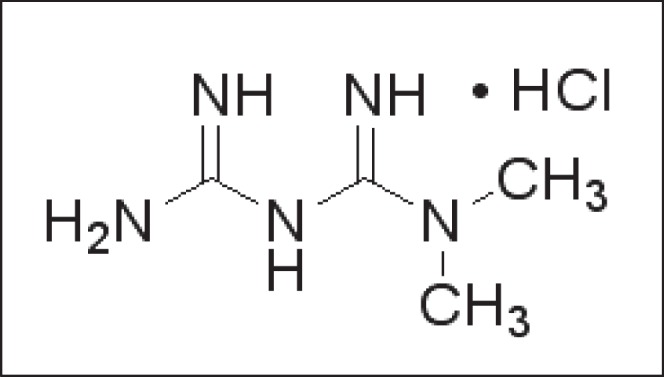
Chemical structure of metformin.
Materials and Methods
Materials
Primary HNPECs and culture media were obtained from ScienCell Research Laboratories (Carlsbad, CA). Metformin and lipopolysaccharide (LPS) (Escherichia coli 0111:B4 strain) were purchased from Sigma-Aldrich (St. Louis, MO). Nitrate/Nitrite and prostaglandin E2 (PGE2) ELISA kits were purchased from Cayman Chemical (Ann Arbor, MI) and Assay Designs (Farmingdale, NY) respectively. The MILLIPLEX MAG rat cytokine/chemokine magnetic bead panel along with Luminex xMAP detection method was purchased from Millipore Corporation (Billerica, MA). Rabbit monoclonal phospho-AMPK antibodies were obtained from Cell Signalling (Danvers, MA). Rabbit polyclonal inducible nitric oxide synthase (iNOS), goat polyclonal cyclooxygenase (Cox)-2, rabbit polyclonal phospho-p65 (Ser 536), mouse monoclonal p65 (F-6), and mouse monoclonal glyceraldehyde 3-phosphate dehydrogenase (GAPDH; A-3) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). All other reagents obtained from Sigma-Aldrich were of highest purity grade.
Animals
Adult (8–10 weeks) male Lewis rats (150–200 g; Harlan Laboratories, Houston, TX) were kept in 12-hour light/12-hour dark cycles for 3 days to acclimatize in the animal house facility at the University of Texas Medical Branch, Galveston, TX. The handling, treatment, and procedures on animals were carried out according to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research. Animals were randomly divided into four groups (n = 8). LPS (150 μg/100 μL PBS) was injected subcutaneously at two locations in the thigh of the animals to induce uveitis. In the experimental groups, intraperitoneal injection of metformin (300 mg/kg body weight) was given either 12 hours before (Pre-metformin group) or 2 hours after (Post-metformin group) LPS injection in respective animals. Animals in the control group were injected with vehicle (dimethyl sulfoxide + normal saline, dilution 1:20). The animals were euthanized at 3 hours to determine phosphorylation of AMPK as well as NF-κB in immunostained serial sections of the rat eyes. For all other parameters, the animals were euthanized at 24 hours after LPS injection. The aqueous humor (AqH) was collected from the eyes by an anterior chamber puncture with a 30-gauge needle under a surgical microscope. After determination of the number of infiltrating cells, as well as protein concentration in AqH, the samples were stored at −80°C until further use. Some of the eyes were transferred immediately to 4% paraformaldehyde for immunohistochemical studies.
Pathological Assessment
Using slit lamp microscope, the pathological severity of inflammation in EIU at the end of the experiment was scored by a masked investigator as described earlier.21 The intensity of clinical ocular inflammation was scored on a scale of 0 to 5.
Determination of Infiltrating Cells and Total Proteins in AqH
The AqH samples were diluted in an equal amount of Trypan-blue solution and number of infiltrating cells was counted using a hemocytometer under the light microscope (Nikon, Tokyo, Japan). The total protein concentration in the AqH samples was measured with a protein assay kit (Bio-Rad, Hercules, CA).
Determination of Cytokines/Chemokines in AqH
The levels of cytokines and chemokines in the AqH were determined by the MILLIPLEX MAG rat cytokine/chemokine magnetic bead array panel along with Luminex xMAP detection method as per manufacturer's protocol using a Millipore Multiplex system. The results are expressed as picograms per milliliter.
Paraffin Embedding of Eyes
The enucleated eyes from the rats were fixed in 4% paraformaldehyde for 24 hours. After fixing, the eyes were washed in ice-cold PBS (3 times) and immediately transferred in 70%, 90%, and 100% reagent alcohol for 24 hours each followed by embedding in paraffin. Sagittal sections of 5 μm were cut.
Histopathological and Immunofluorescence Studies
Rat eye sections were stained with hematoxylin and eosin (H&E) for histopathologic analysis of uveitis symptoms. For immunofluorescence studies, the eye sections were warmed at 60°C for 1 hour in the oven, deparaffinized in xylene, rehydrated by passing through 100%, 95%, 80%, and 70% ethanol, and washed with deionized water. Heat-induced epitope recovery was used as sections were submerged in 250 mL of 1× antigen retrieval citrate buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0) and steam-heated in a standard steamer for 15 minutes. After the antigen retrieval, the sections were rinsed in PBS twice and incubated with blocking buffer (2% BSA, 0.1% Triton X-100, 2% normal rabbit Ig G or 2% normal goat serum) overnight at 4°C. The sections were incubated with primary antibodies against phospho-AMPK or phospho-p65 antibodies (dilution 1:200) for overnight and washed with PBS (3 × 5 minutes each). The sections were incubated in respective Alexa Fluor-488 or Alexa Fluor-594 secondary antibodies (dilution 1:200) for 1 hour at room temperature followed by washing with PBS. The sections were mounted with Vectashield mounting media (Vector Labs, Burlingame, CA) containing 4′,6-diamidino-2-phenylindole and covered with a cover slip. The expression of p65, Cox-2, and AMPK in the ocular cells in the tissue sections was determined by fluorescent microscopy (EPI-800 microscope; Nikon, Tokyo, Japan) and photographed with a digital camera (Olympus, Center Valley, PA) fitted to the microscope.
In Vitro Cell Culture Study
HNPECs were cultured as per supplier's protocol (ScienCell Research Laboratories) using epithelial cell medium containing basal medium, antibiotics, epithelial cell growth supplement, and fetal bovine serum. The growth media was hydrocortisone and endotoxin free. The cells were grown in a humidified incubator at 37°C and 5% CO2. All incubations were performed in serum-free medium. The cells were pretreated with 40 μM of metformin for 1 hour and subsequently stimulated with 1 μg/mL LPS for various time intervals as stated in the figure legends. For in vitro experiments, the optimal effective dose was determined by initial experiments using 0 to 100 μM of metformin. The efficacy of dosages less than 40 μM metformin did not evoke significant response, whereas dosages of 40 μM or more metformin evoked a significant response as measured by nitric oxide (NO) and PGE2 levels in the culture media.
Western Blot Analysis
After the treatment, HNPECs were washed twice with ice cold PBS and lysed in radioimmunoprecipitation assay buffer containing 1 mM phenylmethylsulfonyl fluoride and 1:100 dilution of protease inhibitor cocktail (Sigma-Aldrich). The protein levels were measured from the supernatant and aliquots were diluted with 2× SDS sample buffer and boiled for 5 minutes. The cell lysates were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). The membranes were then incubated in blocking solution containing 5% weight per volume dried fat-free milk and 0.1% vol/vol Tween-20 in Tris-buffered saline. Subsequently, the membranes were incubated with anti-iNOS, -Cox-2, -phospho-p65, and total P-65 antibodies. The membranes were then probed with horseradish peroxidase–conjugated secondary antibody (GE Health Care, Piscataway, NJ) and visualized by chemiluminescence (Pierce Biotechnology, Rockford, IL). To determine translocation of NF-κB from the cytoplasm to the nucleus, HNPECs were treated with LPS for different time intervals followed by extraction of nuclear proteins as per manufacturer's protocol using a nuclear extraction kit (Cayman Chemicals).
Measurement of NO and PGE2
After the treatment, HNPECs culture media were retrieved, centrifuged to remove cell debris, and stored at −80°C until further use. The total levels of nitrate/nitrite and PGE2 in the culture media were measured by using a total nitrite colorimetric assay and an enzyme immunoassay kits, respectively. All assays were performed according to the manufacturer's instructions (Cayman Chemicals).
Statistical Analysis
Data are expressed as the mean ± SD. One-way ANOVA was used to compare inflammatory markers; P less than 0.05 was considered statistically significant. Wilcoxon–Mann-Whitney tests were used for the pairwise comparisons across groups. Analyses were stratified by side (left and right). All computations were performed with the SAS system (version 9; SAS Institute, Cary, NC).
Results
Metformin Prevents Severity Associated with EIU
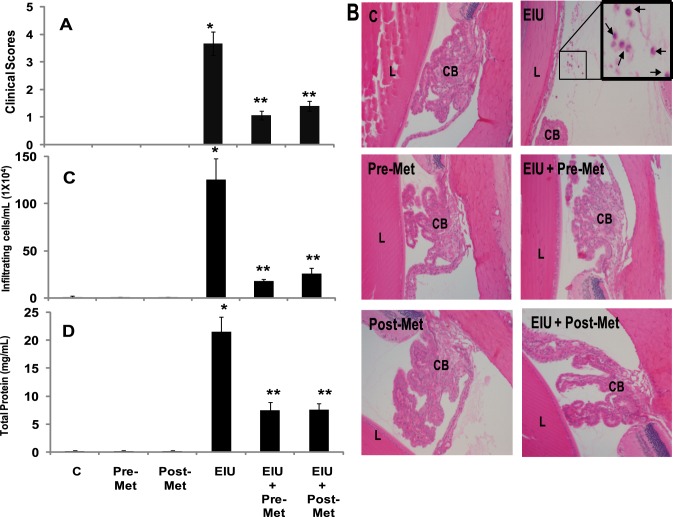
At first, we performed a dose response study to determine the effective dose (100 mg/kg, 300 mg/kg, and 500 mg/kg body weight) of metformin in preventing EIU in rats (n = 2). Our results suggest that a 100 mg/kg dose of metformin partially (∼30%) prevented the EIU-induced cellular infiltration, whereas 300 mg/kg and 500 mg/kg doses showed 80% and 85% protection, respectively against EIU-induced cellular infiltration in rat AqH (data not shown). Therefore, in the rest of our experiments, we used 300 mg/kg bd wt. We next examined the efficacy of metformin in the prevention of EIU symptoms in rats. Our results indicate that pathological scores for various experimental groups were as follows: EIU alone 3.6 ± 0.4, EIU+metformin-pretreated 1.0 ± 0.1, EIU+metformin-posttreated 1.4 ± 0.1, and controls 0.003 ± 0.005. Histopathological examination of the H&E-stained sagittal sections of rat eyes revealed LPS-induced infiltration of inflammatory cells in the AqH (Fig. 2A). Pretreatment as well as posttreatment of EIU rats with metformin significantly inhibited infiltration of cells in the AqH as compared with untreated or metformin-alone treated groups (Fig. 2B). The manual counting of the infiltrated cells in the AqH revealed a significant (>100-fold) increase in the infiltration of the cells in EIU rats, which significantly (80%) declined in EIU + metformin-treated rats (Fig. 2C). Similarly, total protein level in the AqH of the LPS group increased significantly (>20-fold) as compared with control or metformin-alone treated groups; however, pretreatment as well as posttreatment with metformin significantly (∼64% and ∼65%, respectively) suppressed the LPS-induced increase in the protein levels in AqH (Fig. 2D).
Figure 2. .
Metformin prevents LPS-induced infiltration of inflammatory cells and increase in protein levels in AqH. (A) The pathologic score of EIU in Lewis rat eyes injected with LPS in the absence and presence of metformin was determined at 24 hours with a slit lamp microscope. Results are given as mean ± SD (n = 6). #P < 0.001 versus control. **P < 0.001 versus EIU (Wilcoxon–Mann-Whitney test). (B) Histopathological results of paraffin-embedded sections showing infiltrated cells (inset) in the anterior chamber of EIU rat eyes without or with metformin injected 12 hours before or 2 hours after LPS administration. H&E-stained serial sections of rat eyes were photographed under a light microscope. Magnification, ×200. (C) The infiltrated inflammatory cells were determined by trypan blue exclusion cell counting and (D) total protein levels in the AqH. Results are expressed as the mean ± SD (n = 5); *P < 0.001 versus the control group; **P < 0.05 versus the EIU group. C, control; Pre-Met, pretreatment with metformin; Post-Met, posttreatment with metformin; EIU, endotoxin-induced uveitis; EIU + Pre-Met, endotoxin-induced uveitis + pretreatment with metformin; EIU + Post-Met, endotoxin-induced uveitis + post treatment with metformin; CB, ciliary body; L, lens.
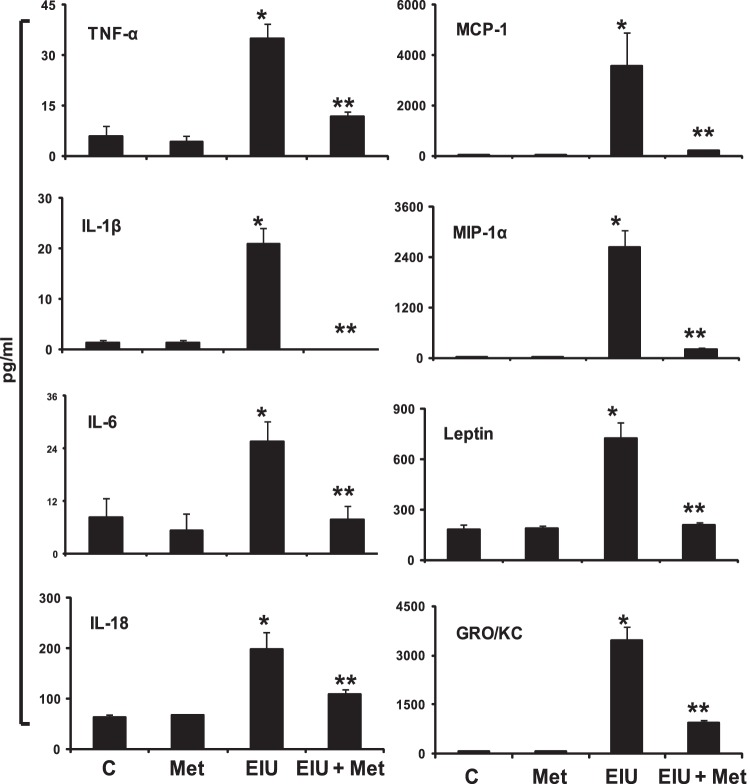
Metformin Decreases the Levels of Inflammatory Cytokines, Chemokines, and Growth Factors in AqH of EIU Rat Eyes
Endotoxin-induced increase in various cytokines, chemokines, and growth factors in the AqH is a hallmark of uveitis. Therefore, we next determined the levels of various cytokines, chemokines, and growth factors in AqH by using a magnetic bead array–based rat-specific inflammation kit (Millipore, Billerica, MA) that measures 23 inflammatory marker proteins from a single sample. The results were analyzed by Luminex xPONENT software (Luminex, Austin, TX) and expressed as pg/mL. As shown in Figure 3, a significant (P < 0.01) increase in the levels of inflammatory proteins, such as TNF-α, MCP-1, IL-1β, MIP-1α, IL-6, Leptin, IL-18, and GRO/KC, were observed in the EIU group. Posttreatment with metformin significantly (P < 0.05) suppressed the LPS-induced secretion of cytokines and chemokines in AqH (Fig. 3). Further, metformin also partially reduced (statistically nonsignificant) the levels of granulocyte macrophage–colony-stimulating factor, Eotaxin, RANTES, VEGF, granulocyte colony-stimulating factor, IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, IL-17, and IP-10 in EIU rats (data not shown).
Figure 3. .
Posttreatment with metformin suppressed LPS-induced increase in cytokines and chemokines in AqH. The MILLIPLEX MAG Rat cytokine/chemokine magnetic bead panel along with Luminex xMAP detection method was used to determine cytokines and chemokines. Results are expressed as the mean ± SD (n = 3; AqH was pooled from two rats for each data point); *P < 0.01 versus the control group; **P < 0.05 versus the EIU group. The results are expressed as pg/mL. C, control; Met, metformin; EIU, endotoxin-induced uveitis; EIU + Met, endotoxin-induced uveitis + metformin.
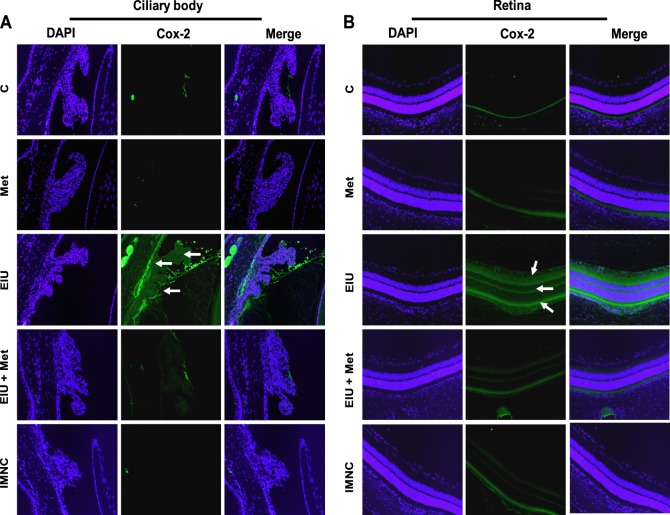
Metformin Inhibits Expression of Cox-2 in Ocular Tissues
The inhibitory effects of metformin on LPS-induced elevation in the levels of various inflammatory markers were also confirmed by immunohistochemical studies. We determined the effect of metformin on the expression of major proinflammatory protein, Cox-2, in ocular tissues of EIU rat eyes. As shown in Figure 4, the EIU rat ocular tissues revealed an increased expression of Cox-2 in the ciliary epithelial cells of ciliary bodies and retina (possibly in the retinal pigment epithelial cells) of EIU rat eyes, which was suppressed by metformin. Further, in addition to ocular cells, some of the infiltrated cells were also positive for the Cox-2 expression.
Figure 4. .
Posttreatment with metformin prevents expression of Cox-2. Serial sections of paraformaldehyde-fixed rat eyes enucleated 24 hours after EIU induction were immunostained with antibodies against Cox-2 and pictures were taken using a Nikon epifluorescence microscope. Arrows indicate Cox-2 expression in (A) ciliary bodies as well as (B) retinal tissues. A representative image is shown (n = 4). C, control; Met, metformin; EIU, endotoxin-induced uveitis; EIU + Met, endotoxin-induced uveitis + metformin; IMNC, isotype-matched negative control. Magnification ×200.
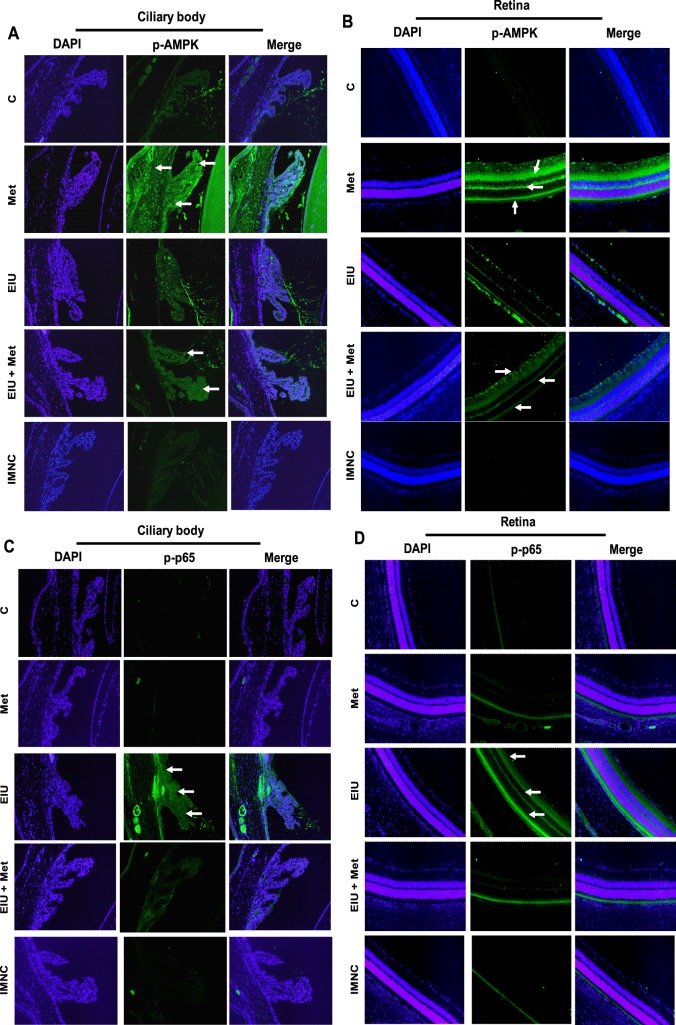
Metformin Triggers AMPK Activation and Inhibits NF-κB in Ocular Tissues
Because NF-κB is known to transcribe the genes responsible for various inflammatory markers, we next investigated the effect of metformin on LPS-induced activation of NF-κB (phospho-p65) in rat eye tissue sections immunohistochemically. The phosphorylation of p-65 in the ocular cells of ciliary bodies as well as retina was observed in LPS-treated rat eyes at 3 hours but not in the EIU + metformin group (Figs. 5C, 5D), indicating that metformin prevents LPS-induced p65 activation. Because AMPK activation by metformin has been shown to suppress the activation of p65, we next determined the effect of metformin on endotoxin-induced activation of AMPK in the rat eye tissues. Our results, shown in Figures 5A and 5B, indicate that EIU + metformin rat tissues had an elevated expression of AMPK as compared with EIU rat tissues. Further, as compared with control rat eye tissues, there was a marked activation of AMPK observed in metformin-alone treated rats. These results suggest that by activating AMPK, metformin could prevent endotoxin-induced NF-κB–dependent expression of inflammatory markers.
Figure 5. .
Posttreatment with metformin triggers phosphorylation of AMPK (A, B) and suppresses p-65 phosphorylation (C, D) in ocular tissues of LPS-induced EIU. Serial sections of paraformaldehyde-fixed rat eyes enucleated 3 hours after EIU induction were immunostained with antibodies against phospho-AMPK (A, B) and phospho-p65 (C, D). Nuclei were stained with DAPI and pictures were taken by Nikon epifluorescence microscope. Representative images are shown (n = 4). Arrows indicate phosphorylated AMPK (A, B) and phospho-p65 (C, D) in ciliary body (A, C), as well as retinal tissues (B, D). C, control; Met, metformin; EIU, endotoxin-induced uveitis; EIU + Met, endotoxin-induced uveitis + metformin; IMNC, isotype-matched negative control. Magnification ×200.
Metformin Suppresses LPS-Induced Inflammatory Response in HNPECs
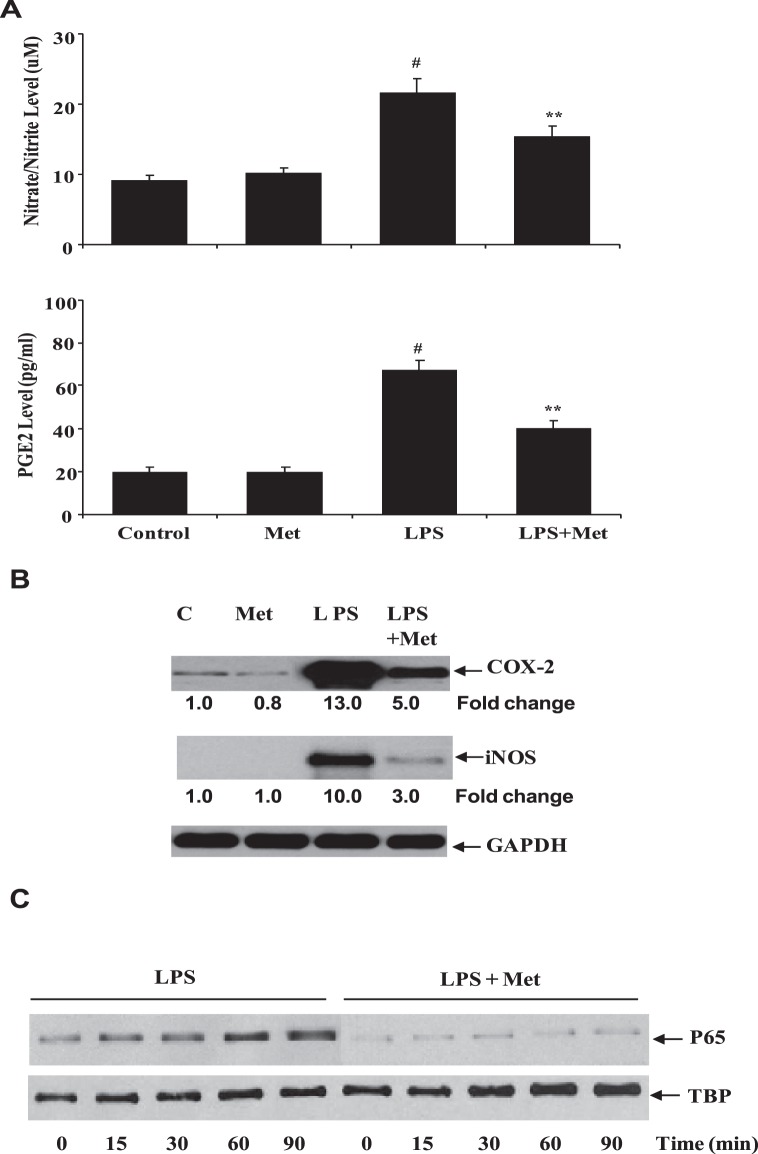
Because ciliary epithelial cells are known to play a crucial role in AqH homeostasis and it is disrupted during EIU, we used HNPECs as our in vitro model to understand the effects of metformin on intracellular inflammatory events. Therefore, we first examined the effect of metformin on LPS-induced inflammatory markers, such as NO and PGE2. As shown in Figure 6A, LPS significantly (>80%) increased the levels of nitrate/nitrite as well as PGE2 in culture media of HNEPCs as compared with the untreated cells or metformin-alone treated cells. The LPS-induced increase in the levels of nitrate/nitrite and PGE2 were significantly (>60%) prevented by the metformin. Because Cox-2 and iNOS enzymes catalyze the formation of PGE2 and NO, we next examined the effect of metformin on LPS-induced Cox-2 and iNOS protein expression in HNPECs. Our results, shown in the Figure 6B, indicate that LPS caused an approximately 13- and 10-fold increase in the expression Cox-2 and iNOS proteins, respectively in HNPECs. In the presence of metformin, however, the Cox-2 and iNOS protein expression was significantly suppressed to approximately 5- and 3-fold, respectively. Because NF-κB is known to transcribe the Cox-2 and iNOS genes, we next examined the effect of metformin on LPS-induced nuclear translocation of NF-κB. As shown in Figure 6C, metformin significantly prevented the nuclear translocation and activation of NF-κB. These results suggest that the metformin prevents LPS-induced inflammatory response by preventing the NF-κB–dependent expression of inflammatory markers.
Figure 6. .
Metformin prevents inflammatory response in HNPECs stimulated with LPS. (A) The levels of nitrate/nitrite and PGE2 in the culture media were determined with ELISA kit. Data are expressed as mean ± SD (n = 6). *P < 0.001 versus the control group; **P < 0.05 versus the LPS group. (B) The expression of Cox-2 and iNOS from cell lysates was determined by Western blot using specific antibodies. (C) The nuclear translocation of p65 was determined in the nuclear extract by Western blot using specific antibodies against p65. TBP, TATA binding protein (loading control); C, control; Met, metformin.
Discussion
This study for the first time reports that the antidiabetic drug metformin could suppress ocular inflammation leading to uveitis in rats. Our data indicate that infiltration of leukocytes and secretion of various cytokines and chemokines in AqH were significantly reduced by either pretreatment or posttreatment with metformin in EIU rats. Further, the expressions of inflammatory marker proteins, such as Cox-2, as well as transcription factors, such as NF-κB, in the uveal and retinal tissues were also inhibited by metformin.
The pivotal role of NF-κB in triggering various proinflammatory genes in a number of inflammatory diseases, including endotoxin- as well as autoimmune-induced uveitis has been well documented.22–24 Consistent with previous studies,25–29 our study also indicates that metformin prevents activation of NF-κB. In the present study, we have shown that the expression of LPS-induced NF-κB in ciliary bodies as well as retinal tissues was suppressed in metfomin-treated rats. Moreover, our in vitro cell culture studies also indicate that metformin inhibits LPS-induced nuclear translocation of NF-κB in HNPECs. One of the mechanisms of metformin-induced inhibition of NF-κB is through the activation of AMPK.18,19 A plethora of studies had demonstrated that the activation of AMPK signaling downregulates the NF-κB pathway.30–34 In this study, we observed that metformin triggers the activation of AMPK in the ciliary body complex as well as retinal cells. These results are in correlation with the inhibition of NF-κB in the respective ocular tissues (Fig. 5). Our results are also in accordance with a recent study by Suzuki et al.,35 who demonstrated that aminoimidazole carboxamide ribonucleotide, an activator of AMPK, prevents the inflammatory response to EIU. Further, previous studies also demonstrate that by activating AMPK, metformin suppresses NF-κB in a number of nonocular cells, such as macrophages and vascular endothelial cells.30–34 Although, in our studies, it appears that activation of AMPK by metformin suppresses NF-κB activation, we do not rule out the other possibilities by which metformin could regulate NF-κB in ocular tissues.
Even though the therapeutic use of metformin for hyperglycemia and type-2 diabetes was initiated in the 1950s, still it currently remains as one of the most commonly prescribed drugs with nearly 120 million prescriptions filled yearly worldwide.36–39 Recent investigations in the past decade or so indicate a profound efficacy of metformin in the treatment of polycystic ovary syndrome and cancer.39–41 The insulin-lowering effects of metformin have been considered as an integral to its anticancer properties, which has been associated with decreased cancer incidence and mortality in diabetic patients.39,42–44 A number of observational studies found a significantly declined number of cancer incidences and cancer-related mortality in diabetic patients who received standard doses (1500 to 2250 mg/d in adults) of metformin.42,45–49 Moreover, interim analyses of ongoing studies in breast cancer patients demonstrated that metformin is safe and well tolerated, and exhibits therapeutic effects on insulin metabolism, tumor cell proliferation, and apoptosis.50,51 Besides humans, metformin also displays significant growth inhibitory effects in numerous in vitro and in vivo animal models of carcinogenesis. In an in vitro cell culture model, metformin inhibited the proliferation of a variety of cancer cells, such as breast, prostate, colon, endometrial, ovarian, and glioma.52–58 A number of molecular mechanisms have been proposed to elucidate the efficacy of metformin as an anticancer agent. These include AMPK activation, reduced mammalian target of rapamycin signaling and protein synthesis, as well as a variety of other responses, including decreased epidermal growth factor receptor, Src, and mitogen-activated protein kinase activation, decreased expression of cyclins, and increased expression of p27. Furthermore, Tan et al.16 reported that metformin can exert anti-invasive and anti-metastatic effects in human endometrial carcinoma cells through NF-κB, MMP-2/9, as well as AKT and ERK1/2 pathways.
The results of a study undertaken by Kim et al.25 suggest that metformin activates AMPK and suppresses MDR1 expression in MCF-7/adr cells by inhibiting the activation of NF-κB and CREB. These studies so far have revealed a novel function of metformin as an anticancer agent in addition to already established antidiabetic function. Further, recent studies also demonstrate the anti-inflammatory role of metformin as well. Dandona et al.59 observed that metformin could reduce plasma migration inhibitor factor concentration in the obese individuals. This finding is suggestive of an anti-inflammatory and anti-atherogenic effect of metformin, which may have implications for the reduced cardiovascular mortality observed with metformin therapy in type-2 diabetes. Moreover, a study by Isoda et al.29 demonstrated that metformin can exert a direct vascular anti-inflammatory effect by inhibiting NF-κB through the blockade of the PI3K–AKT pathway. Our current study indicates the potential use of metformin in uveitis, one of the major causes of blindness in patients worldwide.
Like metformin, various therapeutic agents have been reported in the past to inhibit the inflammatory response in the EIU model.8 However, prescription-based use of many of these agents, such as guggulsterone,60 ethyl pyruvate,61 lutein,62 and astazanthin,63 in humans for the treatment of uveitis has not materialized for various reasons. Prominent reasons that limit their use in humans are lack of toxicity studies, lack of knowledge on safe dosage range, potential toxicity on higher therapeutic dosage, and lack of studies to determine safe delivery methods. Metformin is one such drug that could overcome these issues, as it has been extensively studied and found to be safe for human use. The clinical safety, well-characterized pharmacodynamic profile, and low cost of metformin could make it an ideal candidate for the development of potential therapeutic agent for ocular inflammatory diseases such as uveitis.
Footnotes
Supported by National Institutes of Health Grants EY015891 (KVR) and DK36118 (SKS).
Disclosure: N.M. Kalariya, None; M. Shoeb, None; N.H. Ansari, None; S.K. Srivastava, None; K.V. Ramana, None
References
- 1. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308 [DOI] [PubMed] [Google Scholar]
- 2. Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613 [DOI] [PubMed] [Google Scholar]
- 3. Sijssens KM, Rijkers GT, Rothova A, et al. Distinct cytokine patterns in the aqueous humor of children, adolescents and adults with uveitis. Ocul Immunol Inflamm. 2008;16:211–216 [DOI] [PubMed] [Google Scholar]
- 4. Curnow SJ, Murray PI. Inflammatory mediators of uveitis: cytokines and chemokines. Curr Opin Ophthalmol. 2006;17:532–537 [DOI] [PubMed] [Google Scholar]
- 5. Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Srivastava SK, Ramana KV. Focus on molecules: nuclear factor kappa B. Exp Eye Res. 2009;88:2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yadav UC, Kalariya NM, Ramana KV. Emerging role of antioxidants in the protection of uveitis complications. Curr Med Chem. 2011;18:931–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Svacina S. Metformin as the first line antidiabetic agent. Vnitr Lek. 2010;56:1225–1237 [PubMed] [Google Scholar]
- 10. Bosi E. Metformin—the gold standard in type 2 diabetes: what does the evidence tell us?. Diabetes Obes Metab. 2009;11:3–8 [DOI] [PubMed] [Google Scholar]
- 11. Shomali M. Add-on therapies to metformin for type 2 diabetes. Expert Opin Pharmacother. 2011;1:47–62 [DOI] [PubMed] [Google Scholar]
- 12. Diamanti-Kandarakis E, Christakou CD, Kandaraki E, et al. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162:193–212 [DOI] [PubMed] [Google Scholar]
- 13. Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clements A, Gao B, Yeap SH, et al. Metformin in prostate cancer: two for the price of one. Ann Oncol. 2011;22:2556–2560 [DOI] [PubMed] [Google Scholar]
- 15. Li D. Metformin as an antitumor agent in cancer prevention and treatment. J Diabetes. 2011;3:320–327 [DOI] [PubMed] [Google Scholar]
- 16. Tan BK, Adya R, Chen J, et al. Metformin treatment exerts antiinvasive and antimetastatic effects in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2011;96:808–816 [DOI] [PubMed] [Google Scholar]
- 17. Quaile MP, Melich DH, Jordan HL, et al. Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol. 2010;243:340–347 [DOI] [PubMed] [Google Scholar]
- 18. Hattori Y, Suzuki K, Hattori S, et al. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188 [DOI] [PubMed] [Google Scholar]
- 19. Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89:667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsoyi K, Jang HJ, Nizamutdinova IT, et al. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011;162:1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Kozak Y, Omri B, Smith JR, Naud MC, Thillaye-Goldenberg B, Crisanti P. Protein kinase Czeta (PKCzeta) regulates ocular inflammation and apoptosis in endotoxin-induced uveitis (EIU): signaling molecules involved in EIU resolution by PKCzeta inhibitor and interleukin-13. Am J Pathol. 2007;170:1241–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keino H. Therapeutic effect of the low molecular weight inhibitor of the NF-kappaB signaling pathway on experimental autoimmune uveoretinitis. Nihon Ganka Gakkai Zasshi. 2010;114:944–954 [PubMed] [Google Scholar]
- 24. Yadav UC, Srivastava SK, Ramana KV. Understanding the role of aldose reductase in ocular inflammation. Curr Mol Med. 2010;10:540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HG, Hien TT, Han EH, et al. Metformin inhibits P-glycoprotein expression via the NF-κB pathway and CRE transcriptional activity through AMPK activation. Br J Pharmacol. 2011;162:1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li SN, Wang X, Zeng QT, et al. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels. 2009;24:446–453 [DOI] [PubMed] [Google Scholar]
- 27. Tan BK, Adya R, Chen J, et al. Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res. 2009;83:566–574 [DOI] [PubMed] [Google Scholar]
- 28. Huang NL, Chiang SH, Hsueh CH, et al. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol. 2009;134:169–175 [DOI] [PubMed] [Google Scholar]
- 29. Isoda K, Young JL, Zirlik A, et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617 [DOI] [PubMed] [Google Scholar]
- 30. Bai A, Ma AG, Yong M, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708–1717 [DOI] [PubMed] [Google Scholar]
- 31. Yang Z, Kahn BB, Shi H, et al. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ko HJ, Zhang Z, Jung DY, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Zhang M, Liang B, et al. AMPKα2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo. Role of 26S proteasomes. Circ Res. 2010;106:1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu X, Mahadev K, Fuchsel L, et al. Adiponectin suppresses IκB kinase activation induced by tumor necrosis factor-α or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293:E1836–E1844 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki J, Manola A, Murakami Y, et al. Inhibitory effect of aminoimidazole carboxamide ribonucleotide (AICAR) on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52:6565–6571 [DOI] [PubMed] [Google Scholar]
- 36. Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108:1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bailey CJ, Day C. Metformin: its botanical background. Pract Diab Int. 2004;21:115–117 [Google Scholar]
- 38. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579 [DOI] [PubMed] [Google Scholar]
- 39. Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, et al. Metformin in cancer therapy: a new perspective for an old antidiabetic drug?. Mol Cancer Ther. 2010;9:1092–1099 [DOI] [PubMed] [Google Scholar]
- 40. Diamanti-Kandarakis E, Economou F, Palimeri S, et al. Metformin in polycystic ovary syndrome. Ann N Y Acad Sci. 2010;1205:192–198 [DOI] [PubMed] [Google Scholar]
- 41. Rotella CM, Monami M, Mannucci E. Metformin beyond diabetes: new life for an old drug. Curr Diabetes Rev. 2006;2:307–315 [DOI] [PubMed] [Google Scholar]
- 42. Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goodwin PJ, Pritchard KI, Ennis M, et al. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501–505 [DOI] [PubMed] [Google Scholar]
- 44. Algire C, Zakikhani M, Blouin MJ, et al. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–839 [DOI] [PubMed] [Google Scholar]
- 45. Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res. 2010;3:1451–1461 [DOI] [PubMed] [Google Scholar]
- 47. Landman GW, Kleefstra N, van Hateren KJ, et al. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Monami M, Lamanna C, Balzi D, et al. Sulphonylureas and cancer: a case-control study. Acta Diabetol. 2009;46:279–284 [DOI] [PubMed] [Google Scholar]
- 49. Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258 [DOI] [PubMed] [Google Scholar]
- 50. Niraula S, Stambolic V, Dowling RJO, et al. Clinical and biologic effects of metformin in early stage breast cancer. Cancer Res. 2010;70;104s. Abs No. PD03-06 [Google Scholar]
- 51. Hadad SM, Dewar JA, Elseedawy E, et al. Gene signature of metformin actions on primary breast cancer within a window of opportunity randomized clinical trial. J Clin Oncol. 2010;28 ; 560 [Google Scholar]
- 52. Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812 [DOI] [PubMed] [Google Scholar]
- 53. Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273 [DOI] [PubMed] [Google Scholar]
- 54. Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, et al. The antidiabetic drug Metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586 [DOI] [PubMed] [Google Scholar]
- 55. Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752 [DOI] [PubMed] [Google Scholar]
- 56. Cantrell LA, Zhou C, Mendivil A, et al. Metformin is a potent inhibitor of endometrial cancer cell proliferation—implications for a novel treatment strategy. Gynecol Oncol. 2010;116:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Isakovic A, Harhaji L, Stevanovic D, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gotlieb WH, Saumet J, Beauchamp MC, et al. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250 [DOI] [PubMed] [Google Scholar]
- 59. Dandona P, Aljada A, Ghanim H, et al. Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab. 2004;89:5043–5047 [DOI] [PubMed] [Google Scholar]
- 60. Kalariya NM, Reddy AB, Ansari NH, et al. Preventive effects of ethyl pyruvate on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52:5144–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kalariya NM, Shoeb M, Reddy AB, et al. Prevention of endotoxin-induced uveitis in rats by plant sterol guggulsterone. Invest Ophthalmol Vis Sci. 2010;51:5105–5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jin XH, Ohgami K, Shiratori K, et al. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2006;47:2562–2568 [DOI] [PubMed] [Google Scholar]
- 63. Suzuki Y, Ohgami K, Shiratori K, et al. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp Eye Res. 2006;82:275–281 [DOI] [PubMed] [Google Scholar]