Abstract
Aqueous film-forming foams (AFFFs) are a vital tool to fight large hydrocarbon fires and can be used by public, commercial, and military firefighting organizations. In order to possess these superior firefighting capabilities, AFFFs contain fluorochemical surfactants, of which many of the chemical identities are listed as proprietary. Large-scale controlled (e.g. training activities) and uncontrolled releases of AFFF have resulted in contamination of groundwater. Information on the composition of AFFF formulations is needed to fully define the extent of groundwater contamination and the first step is to fully define the fluorochemical composition of AFFFs used by the US military. Fast atom bombardment mass spectrometry (FAB-MS) and high resolution quadrupole-time-of-flight mass spectrometry (QTOF-MS) were combined to elucidate chemical formulas for the fluorochemicals in AFFF mixtures and, along with patent-based information, structures were assigned. Sample collection and analysis was focused on AFFFs that have been designated as certified for US military use. Ten different fluorochemical classes were identified in the seven military-certified AFFF formulations, and include anionic, cationic, and zwitterionic surfactants with perfluoroalkyl chain lengths ranging from 4-12. The environmental implications are discussed and research needs are identified.
Introduction
Aqueous film-forming foams (AFFF) formulations are chemical mixtures that are used to effectively extinguish hydrocarbon fuel-based fires and have a secondary benefit of preventing re-ignition.1 Due to their surface-tension lowering properties, AFFF containing fluorinated surfactants have superior firefighting capabilities compared to non-fluorinated fire extinguishing methods.2 Fluorinated surfactants have other unique properties that cause some of these compounds to be classified as persistent, bioaccumulative, and toxic.3 Historical reports of uncontrolled spills and the repeated use of AFFF during fire training and for AFFF performance testing have been correlated to higher concentrations of fluorochemicals, including perfluoroalkyl carboxylates, perfluoroalkyl sulfonates and fluorotelomer sulfonates, in biota, surface water or groundwater.4–8 These studies did not report the fluorochemical composition of the AFFF released and therefore there is no direct connection between the AFFF product spilled and the resulting contamination.
The US military possesses the largest stockpile (almost 11 million liters) of AFFF in the United States, accounting for approximately 29% of all AFFF in the US in 2004.9 Unlike general commercial AFFF formulations, AFFF sold to the US military must conform to military-specific performance and quality control requirements as prescribed by the military specification (Mil-Spec) MIL-F-24385, which specifies characteristics such as extinguishment time, corrosion rate, environmental impact as indicated by short term toxicity (LC50 (Fundulus herteroclitus)), biological oxygen demand (BOD), and chemical oxygen demand (COD)), and total fluorine content (no specific methodology is required).10 Non-military AFFF must comply with other performance standards. Once an AFFF product has been shown to perform to MIL-F-24385 requirements, the product is listed on the US military's AFFF Qualified Products Listing (QPL).
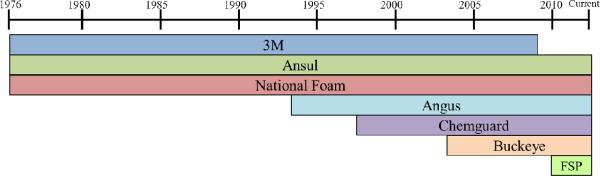
Since the initial development of AFFF materials in 1966, seven different manufacturers have developed AFFF that have passed military specifications and a subset were purchased on contract in large quantities by the military (Figure 1).1 The fluorochemicals contained in the AFFF formulations can be the result of electrochemical fluorination or telomerization processes. These AFFF formulations sold by 3M containing fluorochemicals synthesized by electrochemical fluorination accounted for 75% of the total AFFF stockpiled on military bases.9 The remaining stockpiled AFFF contain telomerization-based fluorochemicals,9 which are structurally distinct from those made by electrochemical fluorination, a process dominated by 3M.11, 12 Telomerization-based fluorochemicals possess carbon chains that are not fully fluorinated and typically have homologues of varying −C2F4– units, while electrofluorination-based fluorochemicals possess fully fluorinated carbon chains with homologues of varying −CF2– units.13 Although 3M voluntarily removed their AFFF products from manufacture due to the rising concern about PFOA/PFOS-based products in 2002,13, 14 currently there is no restriction by the US government on the use of stockpiled 3M AFFF.14 However, both the European Union and Canada have set forth regulations to cease use of and remove PFOS-based AFFF stockpiles.15, 16 Other fluorochemical and AFFF manufacturers have agreed to comply with the EPA PFOA/PFOS Stewardship program to cease production of all C8-based fluorinated compounds before 2015.17
Figure 1.
Timeline of AFFF product addition to the Department of Defense Qualified Products Listing (QPL) that were certified to MIL-F-24385 specifications. While the US military used AFFF since the development in 1963, the records of AFFF on the US military QPL are only available up to 1976. Although 3M remained on the QPL until 2010, the company ceased production of their AFFF product in 2002. “FSP” indicates the AFFF manufacturer Fire Service Plus, Inc.
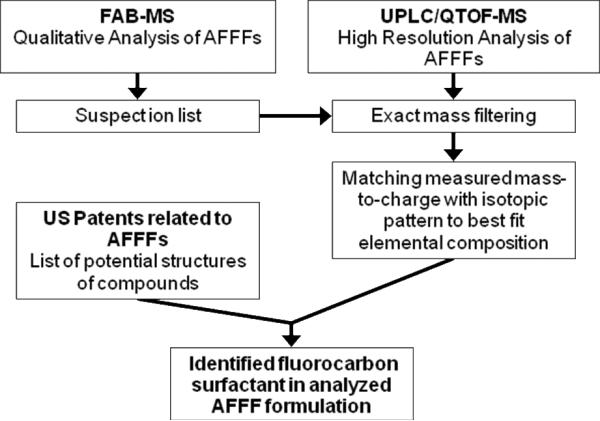
Both MSDS and patents pertaining to the AFFFs used by the military list that these mixtures contain fluorinated surfactants, although the exact elemental composition of these compounds are proprietary. The single exception is the presence of perfluoroalkyl sulfonate salts, as indicated in MSDS for 3M AFFFs.18 For this reason, analytical tools are needed to determine (e.g. reverse engineer) the composition of AFFFs sold to the military. Fast atom bombardment mass spectrometry (FAB-MS) with unit mass resolution is an established qualitative technique that requires minimal sample preparation and that favorably ionizes hydrocarbon and fluorocarbon surfactants in commercial and environmental mixtures.8, 19–21 As opposed to most LC-MS/MS methods, FAB-MS does not require prior knowledge of analytes of interest in order to analyze the samples (e.g. mass ranges, acidity/basicity, mixture composition and concentration). In contrast, high resolution mass spectrometry (HRMS) with chromatographic separation allows for the accurate determination of ion masses, which can be used to determine specific elemental compositions.22 However, the major obstacle is that full scan HRMS provides a large quantity of data that must be reduced in order to identify compounds of interest.23–25 For this reason, multiple samples of AFFF formulations spanning a range of manufacturing years were first screened by FAB-MS to identify target analytes for further analysis by HRMS in order to determine the final elemental compositions of the fluorochemicals (Figure 2). Finally, the information on chemical structure was compared to structures given in patents.
Figure 2.
Workflow scheme for the elucidation of fluorochemical surfactants in AFFF formulations.
Experimental
Materials
All solvents used for sample preparation and analysis by FAB-MS were HPLC-Grade quality or better from Sigma Aldrich (St. Louis, MO). Laboratory water at Oregon State University was deionized and cleaned with a Millipore Synergy UV Water System (Bedford, MA) that included a LC-Pak C18 polisher. For FAB-MS analysis, MS-grade 3-nitrobenzyl alcohol (3-NBA) was purchased from Sigma Aldrich.
UPLC/QTOF-MS analysis was performed at the Waters Corporation Facility in Pleasanton, CA. Solvents used for mobile phases and sample dilutions included Fisher Optima LCMS grade methanol from Fisher Scientific (Fair Lawn, NJ) and Millipore MilliQ laboratory water (Bedford, MA). Ammonium acetate buffer was made using laboratory deionized water and high purity ammonium acetate (Sigma Aldrich).
Sample Collection
Sample containers (60 mL HDPE Nalgene bottles) purchased from VWR International (Radnor, PA) were shipped to 21 different US Navy and Air Force military bases within the United States. Sampling instructions also were sent that included sample handling and recording of pertinent AFFF formulation information. Sampling instructions specifically stated to sample AFFF from their original product container in order to avoid mixtures of products. Additional AFFF samples were sent by Bradley Williams of the US Naval Research Laboratory. In total, 74 QPL-listed AFFF samples were received with manufacturing dates ranging from 1984 to 2011. AFFF product names have changed over time; therefore products were categorized by their manufacturer rather than product name and were reported as such (“3M AFFF”, “Chemguard AFFF”, “National Foam AFFF” etc.). After receipt, AFFF samples were stored in the dark at room temperature until analysis.
Fast Atom Bombardment Mass Spectrometry
FAB-MS analyses were performed with a JEOL MS-ROUTE JMS-600H magnetic sector mass spectrometer that was equipped with a FAB interface (JEOL, Ltd., Peabody, MA). Prior to analysis, the instrument was calibrated using a polyethylene glycol mixture (with average molecular weight of 300 g/mol) over the m/z 100 – 1000 and the ionization energy was set to 5 keV, while xenon gas was used as the ionization gas.
Each AFFF sample was diluted at least 10:1 with HPLC-grade methanol and an aliquot was mixed with 3-NBA on the FAB probe. Samples were scanned over an m/z range from 100 – 1000 in both positive and negative ionization mode. A minimum of 7 scans were performed for each sample and the mass spectra were calculated as an average of the 7 scans. Blank samples, consisting of only 3-NBA, were also analyzed to provide background mass spectra and to verify no compound carryover and/or contamination between AFFF samples. A number of AFFF samples from each AFFF manufacturer were analyzed in order to cover the entire range of available lot numbers and manufacturing dates.
Multiple parameters were used to identify target masses for subsequent screening by high resolution mass spectrometry. Ions in a series characterized by spacings of +/− m/z 50, which corresponds to −CF2- units, were selected because they are indicative of fluorochemicals produced by electrochemical fluorination. Ions with spacings of m/z 100 correspond to −C2F4 units were selected because they can be characteristic of fluorochemicals produced by telomerization or electrofluorination (Figure S1, S2).11, 20 In addition, other masses that were identified in the FAB-MS spectra of multiple lots of the same AFFF were also added to the list of target masses.
Ultra Performance Liquid Chromatography / Quadrupole-Time of Flight Mass Spectrometry
For analysis by UPLC/QTOF-MS, all AFFF formulations were prepared in HPLC-grade methanol and diluted to ~12 ppb concentrations of fluorochemical surfactants as estimated from information provided by the available MSDS. Blank samples (consisting of 50% 0.5 mM ammonium acetate in water and 50% methanol) were injected regularly throughout the sequence to verify that there was neither system contamination nor analyte carryover.
Separations were performed on a Waters Acquity H-Class UPLC (Waters Corp., Milford, MA); the chromatographic conditions are reported in the Supporting Information (SI). The chromatographic conditions selected provided the minimum resolution required to separate the suspect ions of interest. A Waters Xevo G2 Quadrupole-Time of Flight (QTOF) mass spectrometer with electrospray ionization (ESI) was operated as the high resolution mass spectrometer. Voltages for the cone and capillary were 30 V and 1.50 kV, respectively. Additional parameters included a source temperature of 130 °C, a desolvation temperature of 350 °C, a cone gas flow of 25 L/hr, and a desolvation gas flow of 1000 L/hr. MS scan time was 0.1 s with an MS scan range of 150 – 1000 m/z. Every 15 s, the system was recalibrated using leucine-enkelphalin as the lockmass and the resolution was set to be 20,000 (unitless, defined as the peak width at half-maximum). All samples were analyzed in both positive and negative ionization modes.
UPLC/QTOF chromatograms for each of the AFFF formulations were first screened for only compounds that had mass defects from −0.100 to +0.150, which is typical of fluorochemicals. Mass defects are the difference between the actual/theoretical ion mass from the nominal ion mass. For example: PFOS has an actual ion m/z 499.9375 and a nominal ion m/z 500.0000, for a mass defect of m/z −0.0625. The low-to-negative mass defects of fluorochemicals are due to the cumulative negative mass defect of multiple fluorine atoms (m/z − 0.0016), and can be compared to the positive mass defect created by multiple hydrogen atoms (m/z +0.0079).
Chromatograms were extracted for each target mass. High accuracy masses (to the ten-thousandth of a mass-to-charge unit) were calculated as an average over the entire peak width, which has been reported to give the most reproducible results (Figure S3, S4).26 Possible elemental compositions of the high-accuracy masses were calculated along with the error, which is reported as the deviation of the detected mass from the calculated elemental composition's mass (in parts-per-million [ppm]). In addition, the elemental composition of the +1 and +2 isotopes were used to rank the likely parent elemental compositions. The elemental composition constraints include an error limit of +/− 5 ppm and elemental limits of carbon: 0–50; hydrogen: 0–50; oxygen: 0–7; nitrogen: 0–7; sulfur: 0–7; and fluorine: 0–25.
Patent Information and Structure Confirmation
US Patents related to AFFF formulations contain limited information on the functional groups and possible perfluoroalkyl chain lengths of fluorochemical components. A database was compiled, which contained the masses and elemental formulas for all potential AFFF fluorochemicals identified in patents. The high accuracy masses detected by the UPLC/QTOF analysis and their calculated elemental composition were then matched to those in the structural database derived from patents to confirm the final structures of the identified fluorochemical compounds.
Results and Discussion
Electrochemical fluorination-Based AFFF
3M AFFF
From the sampling program, 19 samples of 3M AFFF were received from US Air Force and Navy bases within the United States. The samples had a range of manufacturing dates from 1988 to 2001. Although 3M AFFFs were placed on the QPL in 1976, attempts to locate samples older than 1988 were unsuccessful. Six representative 3M AFFF samples were qualitatively analyzed by FAB-MS.
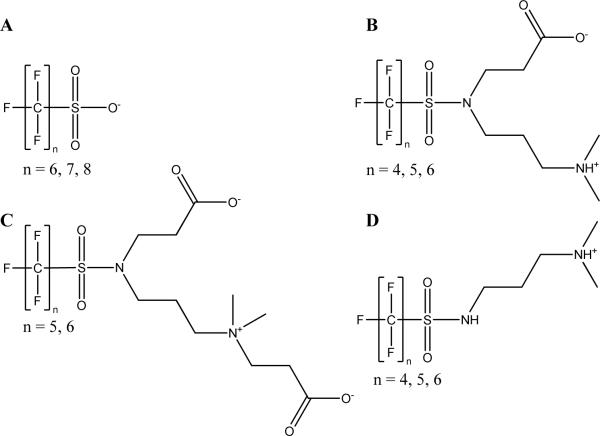
The FAB-MS spectra of 3M AFFF obtained in negative ionization mode contained spacings of m/z 50, which is characteristic of compounds synthesized from electrochemical fluorination.20 In the 3M AFFF, C6–C8 perfluoroalkyl sulfonates (Figure 3A) were identified components in all the 3M AFFF tested (Table S1), and is consistent with the frequent detection of perfluoroalkyl sulfonates found in AFFF-impacted groundwater.4, 5, 7, 8, 27 Contrary to these findings, however, no perfluoroalkyl carboxylates were detected in any AFFF product, with dates that ranged from 1988 to 2001. However, PFCAs are reported as primary components in early 3M AFFFs.11 A limitation of the FAB-MS/QTOF-MS method is that it can only captures the major components and that minor (approximately < 0.1%) fluorochemical compounds may go undetected, therefore if PFCAs were an impurity and/or minor component of the analyzed AFFF products they could not be detected with the current method. Current research using LC/MSMS to determine trace components in AFFF has determined PFCAs are present in some 3M AFFF (unpublished work). While chemical degradation could occur during long term storage of any AFFF product, it was beyond the scope of the study to determine the stability of fluorochemicals in commercial AFFF mixtures during long-term (e.g., decades) storage.
Figure 3.
Electrofluorination-based fluorinated surfactants identified in AFFF. The perfluoroalkyl chain lengths identified in AFFF are shown as number of n fluorocarbons. The ionic species shown are estimated at an environmentally relevant pH.
In addition, 3M AFFF were comprised of zwitterionic C4–C6 perfluoroalkyl sulfonamides containing carboxylic acid and tertiary amine functionalities (Figure 3B), which are consistent with patent information28 and Material Safety Data Sheets (MSDS) that list “amphoteric fluoroalkylamide derivatives”.29 The identification of these compounds was made in positive ionization mode, an uncommon method of mass spectrometric ionization for fluorochemical detection. Of the six 3M AFFF analyzed, the zwitterionic compounds were found only in AFFFs manufactured in 1993, 1998 and 2001 but not in those dating 1988 or 1989. The 3M AFFFs were recertified in 1992 but the addition of zwitterionic fluorochemicals to 3M AFFFs is not well documented.30, 31 AFFF formulation recertification would occur if there were changes to military specifications or if the AFFF formulation itself was significantly changed (i.e. a change in chemical components). An additional set of ions of lower abundance were observed in positive ionization FAB-MS that corresponded to the zwitterionic sulfonamide class but with masses that were +/− m/z 72 different (Table S1) from the chemical class shown in Figure 3B. The addition of m/z 72 indicate C5–C6 perfluoroalkyl sulfonamide compounds with an additional propanoic acid branch (Figure 3C) and the loss of m/z 72 indicates the absence of the propanoic acid branch (Figure 3D). These derivatives are impurities from the synthesis as indicated in the AFFF patent.28 No C8-based homologues of the zwitterionic class (Figure 3B) or the corresponding impurities (Figure 3C–D) were identified.
Telomerization-based AFFF
National Foam AFFF
Nineteen samples were collected from military bases with manufacturing dates ranging from 2003 to 2008. Although National Foam has AFFFs on the QPL since 1976 (Figure 1), no samples from 1976 to 2003 were acquired. Six representative samples were analyzed by FAB-MS.
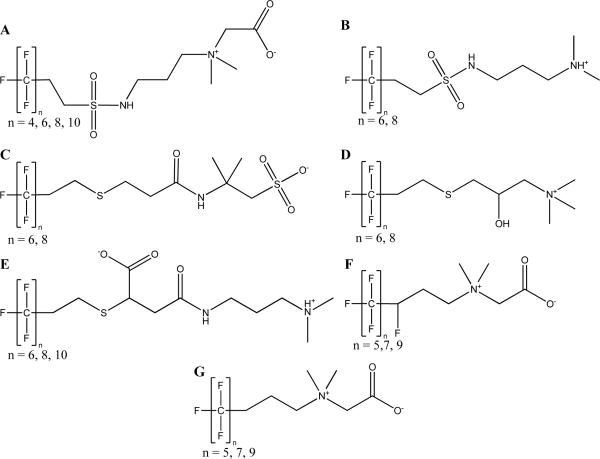
The primary fluorochemicals of National Foam AFFF were detected by m/z 100 spacings in both positive and negative mode FAB-MS, which correspond to −C2F4– units that are characteristic of telomer-based fluorochemicals. The targeted ions were then identified by QTOF-MS as the 4:2, 6:2, 8:2 and 10:2 fluorotelomer sulfonamide with dimethyl quaternary amine and carboxylic acid functional groups (Figure 4A; Table S1).32 Less abundant ions were identified with m/z −58 differences from the 4:2 and 6:2 fluorotelomer ions, which are related to the same structure but without the terminal acetic acid functionality (Figure 4B). In the related patent, Norman et al. suggest that these compounds could result as a byproduct in the synthesis of the major betaine compound.32
Figure 4.
Telomerization-based fluorinated surfactants identified in AFFF. The perfluoroalkyl chain lengths identified in AFFF are shown as number of n fluorocarbons. The ionic species shown are estimated at an environmentally relevant pH.
Ansul AFFF
Ansul AFFF, along with 3M and National Foam, was placed on the AFFF QPL in 1976 (Figure 1). Fifteen samples of Ansul AFFF were collected from the sampling program, with manufacturing dates that ranged from 1984 to 2010 (Figure 1), of these eight representative samples were analyzed by FAB-MS.
Negative ionization mode FAB-MS analyses for Ansul AFFF revealed two abundant ions with characteristic fluorotelomer mass spacings of +/− m/z 100 (Table S1). The primary components identified in the Ansul AFFF were the 6:2 and 8:2 fluorotelomer thioether amido sulfonates at m/z 586 and 686, respectively (Figure 4C). This structure is supported by multiple patents33–35 and a limited number of other reports on AFFF composition.8, 20 An ion of lower abundance was identified at m/z 602, corresponding to a mass difference of m/z 15.9940 from the 6:2 thioether amido sulfonate and is proposed to be the addition of an oxygen atom (structure not shown). The identity of this fluorochemical class could not be definitively determined from the mass spectral data nor from the patents and may be a synthetic impurity. The 6:2 fluorotelomer sulfonate was also reported as being detected by LC/MS/MS in Ansul AFFF,8 but with the current method no fluorotelomer sulfonates (FTS) were detected. The lack of identification of FTS in AFFF formulations is most likely due to the aforementioned high detection limits, and current work developing a quantitative LC-MS/MS method will determine these trace components.
Angus AFFF
Only one sample of Angus AFFF was received and analyzed. Because there was no recertification from the time that the product met Mil-Spec in 1994 to present (Figure 1),30, 31 and there were no formulation changes that necessitate recertification, the single sample may well represent the entirety of Angus AFFFs regardless of the year of manufacture.
In the Angus AFFF formulation, the 6:2 fluorotelomer thioether amido sulfonate (Figure 4C) and corresponding oxygenated impurity (structure not shown) were detected. In addition, two masses at m/z 496 and 596 were identified through positive ionization FAB-MS analysis. By QTOF-MS analysis, the structure was determined to be a 6:2 and 8:2 fluorotelomer thioether with hydroxyl and trimethyl quaternary amine functionalities (Figure 4D; Table S1).33
Chemguard AFFF
From the sampling program, 11 samples were received from US military bases and the manufacturing dates ranged from 2006 to 2010. While this is a narrow range of dates there was no AFFF sample recertification and therefore there have been no official formulation changes.30, 31 Therefore these samples are likely to be representative of the QPL-listed AFFF product. Five representative samples were analyzed by FAB-MS.
Within the samples analyzed by FAB-MS, there were distinct differences between Chemguard products with manufacturing dates from 2006–2007 and 2008–2010. The FAB-MS spectra of the later manufacturing years had no patterns characteristic of fluorochemicals detected through positive and negative ionization FAB-MS, but there was a single strong peak detected at m/z 586, which was previously identified as the 6:2 fluorotelomer thioether amido sulfonate (Figure 4C) and verified by QTOF-MS. The other homologues of the fluorotelomer thioether amido sulfonate (4:2, 8:2, 10:2) may be present at concentrations below the above-specified detection limit. In the earlier manufacturing years, fluorochemical patterning was identified for m/z 602, 702, and 802, which was identified by QTOF-MS to be the sodium-adducted compounds of compounds with m/z 581, 681, 781. These compounds were identified as 6:2, 8:2, 10:2 fluorotelomer thioether amido amino carboxylic acid (Figure 4E; Table S1).36
Buckeye AFFF
Buckeye AFFF was initially certified for military use in 2004, making it the second most recent product to be added to the QPL (Figure 1).30, 31 Only one sample of QPL-listed Buckeye AFFF was received from a military base and an additional sample was supplied by the US Naval Research Laboratory, both of these samples were analyzed by FAB-MS.
No characteristic mass spacings of fluorochemicals were identified by analysis under negative ionization FAB-MS. Two different series of fluorotelomer-based homologues (m/z 100 spacing) were detected in positive ionization mode at m/z 432, 532, and 632 and m/z 414, 514, and 614 (Table S1). Based on AFFF patent information,33 the fluorochemicals were identified as fluorotelomer betaines with quaternary amine and carboxylic acid functionalities (Figure 4F and 4G). The difference between the two series of homologues is 18 mass units, which is identified as the substation of a hydrogen atom with a fluorine atom near the fluorotelomer chain. Both compounds have perfluoroalkyl chains with lengths of 5, 7, and 9. The compounds with the additional fluorine atom near the fluorotelomer chains are referred to as x:y:z fluorotelomer betaine (Figure 4F), indicating that the compound has x fully fluorinated carbons, y singly fluorinated carbons, and z non-fluorinated carbons prior to the first functional group (quaternary amine) (Table S1). These compounds do not follow the typical telomerization pattern of even fluorocarbon chain lengths.11 In addition, the structure of the x:y:z fluorotelomer betaine does not follow the typical telomerization paradigm of a fully fluorinated carbon chain (with the singly fluorinated carbon linkage). The synthesis of this unique structure results from the use of an unsaturated fluoroalkyl amine.37, 38
Fire Service Plus AFFF
No Fire Service Plus AFFF samples were received from the sampling program, which was expected as the AFFF joined the military QPL in 2011. However, two Fire Service Plus samples (from the same manufacturing batch) were received from the Naval Research Laboratory and analyzed.
Positive ionization mode FAB-MS analysis of Fire Service Plus AFFF showed fluorotelomer characteristic spacings (m/z 100) at the same masses as the National Foam AFFF. This was verified as the fluorotelomer sulfonamide betaine class with perfluoroalkyl chain lengths of 4, 6, 8 and 10 (Figure 4A). In addition, the 4:2 and 6:2 fluorotelomer sulfonamide amine impurities were also identified in the formulation (Figure 4B).
As the newest addition to the AFFF QPL for US military use, it is very unlikely that there has been any environmental exposure of this AFFF due to uncontrolled or controlled releases of the material.
Environmental Implications and Research Needs
This is one of the first studies to report the identities of per- and polyfluorinated surfactants contained in military-use AFFF. While the specific compounds are now known, the environmental behavior and toxicity of the individual fluorinated surfactants (and as mixtures) are still unknown.
Previous studies have examined the presence of PFOS and the other perfluoroalkyl sulfonic acids in environmental samples due to AFFF-use and have detected relatively high concentrations of these compounds in groundwater.5, 7, 8 While Schultz et al. reported the identity of the fluorotelomer thioamido sulfonate in AFFF formulations, no data on its environmental occurrence was obtained.8 Oakes et al. also included the 6:2 and 8:2 fluorotelomer compound in their analytical method although no values for environmental presence were reported.39 The scope of the current study was to qualitatively identify the fluorochemical components in AFFF, which are listed in various MSDS to range in concentrations of 0.5 – 25% (by weight) in the product concentrate. On-going research is underway to develop LC-MS/MS methods with the capability for quantifying trace levels all of the newly identified fluorochemicals in groundwater, sediment, and soil. Such methodology can be applied to future studies on the fate of the newly-identified fluorochemicals in natural and engineered systems and to evaluate their occurrence and effects in biota.
Of the 10 fluorinated surfactant classes reported in this study, 8 were determined to have cationic or zwitterionic functionalities at environmental conditions (Figure 3B–D, 4A, B, D–G). The nature of these fluorinated surfactants in the environment has not been investigated in the peer-reviewed literature. Cationic (non-fluorinated) surfactants have different environmental transport characteristics than anionic surfactants. For instance Lee et al. reported that the studied cationic surfactants would cation-exchange onto the negatively charged surfaces of sediments and therefore retard the transport of the compounds through the environmental system.40 In addition, the adsorbed cationic surfactants could act as a carbon loading surface that further retained other hydrocarbon compounds at the source of contamination.40 Cationic and zwitterionic fluorinated surfactants may also behave in a similar manner, suggesting that groundwater sampling may not be sufficient in the detection of these compounds in the environment. Furthermore, the cationic fluorocarbon surfactants may act as a sink to retain fluorochemicals or other priority pollutants and create long-term source zones of high fluorocarbon contamination.
Most of the studies also found detectable levels of perfluoroalkyl carboxylic acids (PFCAs) in AFFF-impacted groundwater,5–8, 27, 39, 41 but none of the analyzed AFFF contained PFCAs as a major component. As previously alluded, PFCAs may have been major components of 3M AFFF prior to 1988 or are minor (e.g., < 0.1%) components of current AFFF at trace levels. In addition, the presence of PFCAs may be due to the degradation of other fluorochemicals. Wang et al. reported the degradation of fluorotelomers to the corresponding carboxylates through aerobic biotransformation in activated sludge.42 Work by Houtz and Sedlak has shown, through the advanced oxidation process, that more functionalized fluorocarbon surfactants can be degraded down to the more oxidation-resistant fluorinated carbon backbone, resulting in the production of corresponding perfluoroalkyl carboxylates.43 This has important implications toward the application of in situ chemical oxidation (ISCO) remediation processes that may be used to clean up contaminated sites that may also contain these AFFF-based fluorochemicals. These examples suggest that not only do the AFFF compounds present their own environmental and toxiocological concerns, they also could be potential sources of perfluoroalkyl carboxylates through environmental and anthropogenic transformation.
Future research studying the fate of the fluorochemicals during biodegradation and upon exposure to chemical remediation approaches (e.g., ISCO) is needed. The data from these experiments will have important ramifications toward the site closure of fluorochemical-contaminated military bases. The targeted approach based on FAB-MS described in this study may be useful in the identification of transformation products of the fluorochemicals identified in this study if they continue to exhibit surface-active properties. However, FAB-MS analysis has poor sensitivity (approximately mg/L levels) compared to that of LC-MS/MS (ng/L), which is necessary detect trace levels of intermediates. Therefore, LC-MS/MS combined with QTOF analyses may be more suitable for environmental transformation and /or bioaccumulation studies.
In addition to understanding the environmental behavior of these fluorochemicals, it is important to understand the implications of remedial strategies applied in the field. For example, `pump and treat' remediation may not be able to access the positively-charged fluorochemicals that could cation-exchange to the sediments. In addition, advanced oxidation could potentially result in the increase of `dead end products' (such as the perfluorinated carboxylates), some of which are compounds of concern. Development of new approaches to fluorochemical remediation may be important to fully account for the various classes identified in this research.
As previously noted, 3M ceased production of their PFOS-based AFFF in 2002, while the rest of the AFFF manufacturers agreed to the voluntary regulations of the EPA PFOA/PFOS Stewardship Program, which calls for the complete phase-out of C8-based products from materials. As reported in this study, while most AFFF formulations did contain C8 and above fluorinated surfactants, the major homologue (identified as the most intense signal via FAB-MS) in the telomerization-based AFFF were of perfluoroalkyl chain lengths less than 8, although fluorochemical homologues of chain length 8 or greater were identified at lesser intensities. The method described in this research could be applied to future AFFF formulations, after the 2015 deadline, to verify the removal of C8-based fluorochemicals from these products.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Mike Wakefield and Greg Witkop of the Waters Corporation for their assistance with UPLC/QTOF-MS and data analysis. We would like to acknowledge Bradley Williams of the U.S. Naval Research Laboratory, Donald Warner of the U.S. Air Force, and all of the participating U.S. Navy and Air Force bases for the collection and shipment of the AFFF materials. In addition, we thank the Fire Fighting Foam Coalition, especially Executive Director Tom Cortina, for their technical assistance and historical knowledge on the use of AFFF.
This study was supported by Oregon State University's Department of Chemistry N.L Tartar Fellowship and the Strategic Environmental Research and Defense Program (SERDP) grant number ER-2128. This publication was made possible, in part, by the Mass Spectrometry Facilities and Services Core of the Environmental Health Sciences Center, Oregon State University, grant number P30 ES00210, National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
Supporting Information Available This information is free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Tuve RL, J.Jablonski E. Method of extinguishing liquid hydrocarbon fires. U.S. Patent 3,258,423. 1966 Jun 28;
- 2.Schaefer T, Dlugogorski B, Kennedy E. Sealability Properties of Fluorine-Free Fire-Fighting Foams (FfreeF) Fire Technol. 2008;44(3):297–309. [Google Scholar]
- 3.Long-Chain Perfluorinated Chemicals (PFCs) Action Plan. US Environmental Protection Agency; Dec 30, 2009. http://www.epa.gov/opptintr/existingchemicals/pubs/pfcs_action_plan1230_09.pdf. [Google Scholar]
- 4.de Solla SR, De Silva AO, Letcher RJ. Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada. Environ. Int. 2012;39(1):19–26. doi: 10.1016/j.envint.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Moody CA, Field JA. Determination of Perfluorocarboxylates in Groundwater Impacted by Fire-Fighting Activity. Environ. Sci. Technol. 1999;33(16):2800–2806. [Google Scholar]
- 6.Moody CA, Hebert GN, Strauss SH, Field JA. Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. J. Environ. Monitor. 2003;5(2):341–345. doi: 10.1039/b212497a. [DOI] [PubMed] [Google Scholar]
- 7.Moody CA, Martin JW, Kwan WC, Muir DCG, Mabury SA. Monitoring Perfluorinated Surfactants in Biota and Surface Water Samples Following an Accidental Release of Fire-Fighting Foam into Etobicoke Creek. Environ. Sci. Technol. 2001;36(4):545–551. doi: 10.1021/es011001+. [DOI] [PubMed] [Google Scholar]
- 8.Schultz MM, Barofsky DF, Field JA. Quantitative Determination of Fluorotelomer Sulfonates in Groundwater by LC MS/MS. Environ. Sci. Technol. 2004;38(6):1828–1835. doi: 10.1021/es035031j. [DOI] [PubMed] [Google Scholar]
- 9.Darwin RL. Estimated Quantities of Aqueous Film Forming Foam (AFFF) in the United States. Baltimore, MD: Aug, 2004. 2004. [Google Scholar]
- 10.Military Specifications MIL-F-24385F: Fire Extinguishing Agents, Aqueous Film-forming Foam (AFFF) Liquid Concentrate, for Fresh and Seawater. US Naval Research Laboratory; 1994. [Google Scholar]
- 11.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2005;40(1):32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- 12.Paul AG, Jones KC, Sweetman AJ. A First Global Production, Emission, And Environmental Inventory For Perfluorooctane Sulfonate. Environ. Sci. Technol. 2008;43(2):386–392. doi: 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
- 13.Kissa E. Fluorinated Surfactants and Repellents. 2nd ed Marcel Dekker, Inc; New York, NY: 2001. [Google Scholar]
- 14.Chemical & Material Emerging Risk Alert: Aqueous Film Forming Foam (AFFF) Department of Defense, Chemical and Risk Management Directorate; Jul, 2011. [Google Scholar]
- 15.Environment Canada [(accessed Feb. 23, 2012)];Perfluorooctane Sulfonate (PFOS), Its Salts and Its Precursors. http://www.ec.gc.ca/toxiques-toxics/default.asp?lang=En&n=ECD5A576-1.
- 16.Directive 2006/122/ECOF the European Parliament and of the Council. European Union; Dec 27, 2006. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:372:0032:0034:EN:PDF. [Google Scholar]
- 17.US Environmental Protection Agency [(accessed Feb. 23, 2012)];2010/2015 PFOA Stewardship Program. http://www.epa.gov/oppt/pfoa/pubs/stewardship/index.html.
- 18.MSDS No. BJRHL. Minnesota Mining and Manufacturing Company; St. Paul, MN: Feb 22, 1991. FC-203CE. [Google Scholar]
- 19.Barber M, Bordoli RS, Elliott GJ, Sedgwick RD, Tyler AN. Fast atom bombardment mass spectrometry (FABMS). A study of surface coverage effects in FABMS. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases. 1983;79(5):1249–1255. [Google Scholar]
- 20.Field JA, Schultz M, Barofsky D. Identifying Hydrocarbon and Fluorocarbon Surfactants in Specialty Chemical Formulations of Environmental Interest by Fast Atom Bombardment/Mass Spectrometry. Chimia. 2003;57(9):556–560. [Google Scholar]
- 21.Ventura F, Caixach J, Figueras A, Espalder J, Fraisse D, Rivera J. Identification of surfactants in water by fab mass spectrometry. Wat. Res. 1989;23(9):1191–1203. [Google Scholar]
- 22.Gross JH. Mass Spectrometry. Springer; New York: 2004. [Google Scholar]
- 23.Kellmann M, Muenster H, Zomer P, Mol H. Full Scan MS in Comprehensive Qualitative and Quantitative Residue Analysis in Food and Feed Matrices: How Much Resolving Power is Required? J. Am. Soc. Mass Spectr. 2009;20(8):1464–1476. [Google Scholar]
- 24.Kosjek T, Žigon D, Kralj B, Heath E. The use of quadrupole-time-of-flight mass spectrometer for the elucidation of diclofenac biotransformation products in wastewater. J. Chrom. A. 2008;1215(1–2):57–63. doi: 10.1016/j.chroma.2008.10.111. [DOI] [PubMed] [Google Scholar]
- 25.Krauss M, Singer H, Hollender J. LC-high resolution MS in environmental analysis: from target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010;397(3):943–951. doi: 10.1007/s00216-010-3608-9. [DOI] [PubMed] [Google Scholar]
- 26.Köfeler HC, Gross ML. Correction of accurate mass measurement for target compound verification by quadrupole time-of-flight mass spectrometry. J. Am. Soc. Mass Spectr. 2005;16(3):406–408. doi: 10.1016/j.jasms.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Screening of polyfluorinated organic compounds at four fire training facilities in Norway. Norwegian Pollution Control Authority; Dec 2, 2008. [Google Scholar]
- 28.Alm RR, Stern RM. Aqueous film-forming foamable solution useful as fire extinguishing concentrate. U.S. Patent 5,085,786. 1992 Feb 4;
- 29.MSDS No. CKQCB. 3M Company; St. Paul, MN: Dec 17, 1999. FC-203CF Lightwater (TM) AFFF 3% [Google Scholar]
- 30.MIL-F-24385 QPL/QPD History for Type 6 AFFF. US Naval Sea Systems Command; Jul 1, 2011. http://www.dcfpnavymil.org/Systems/AFFF/QPL%2024385%20HISTORY%20-%20TYPE%206.pdf. [Google Scholar]
- 31.MIL-F-24385 QPL/QPD History for Type 3 AFFF. US Naval Sea Systems Command; Jul 1, 2011. http://www.dcfpnavymil.org/Systems/AFFF/QPL%2024385%20HISTORY%20-%20TYPE%206.pdf. [Google Scholar]
- 32.Norman EC, Regina AC. Alcohol resistant aqueous film forming firefighting foam. U.S. Patent 5,207,932. 1993 May 4;
- 33.Clark KP, Kleiner EK. Synergistic surfactant compositions and fire fighting concentrates thereof. U.S. Patent 5,616,273. 1997 Apr 1;
- 34.Dear REA, Kleiner EK. Fluorinated sulfonic acids and derivatives thereof. U.S. Patent 4,014,926. 1977 Mar 29;
- 35.Falk RA. Aqueous wetting and film forming compositions. U.S. Patent 4,090,967. 1978 May 23;
- 36.Mueller KF. Perfluoroalkyl Substituted Anhydrides and Polyacids, and Derivatives Thereof. U.S. Patent 4,153,590. 1979 May 8;
- 37.Prossel G, Knaup W, Wehowsky F. Saturated fluoroalkylamines and their derivatives, and mixtures thereof. U.S. Patent 5,648,527. 1997 Jul 15;
- 38.Hauptschein M, Fainberg AH, Hager RB. Unsaturated Fluoroalkyl Amines and Process for the Preparation Thereof. U.S. Patent 3,535,381. 1970 Oct 20;
- 39.Oakes KD, Benskin JP, Martin JW, Ings JS, Heinrichs JY, Dixon DG, Servos MR. Biomonitoring of perfluorochemicals and toxicity to the downstream fish community of Etobicoke Creek following deployment of aqueous film-forming foam. Aquat. Toxicol. 2010;98(2):120–129. doi: 10.1016/j.aquatox.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Lee JF, Crum JR, Boyd SA. Enhanced retention of organic contaminants by soils exchanged with organic cations. Environ. Sci. Technol. 1989;23(11):1365–1372. [Google Scholar]
- 41.Awad E, Zhang X, Bhavsar SP, Petro S, Crozier PW, Reiner EJ, Fletcher R, Tittlemier SA, Braekevelt E. Long-Term Environmental Fate of Perfluorinated Compounds after Accidental Release at Toronto Airport. Environ. Sci. Technol. 2011;45(19):8081–8089. doi: 10.1021/es2001985. [DOI] [PubMed] [Google Scholar]
- 42.Wang N, Liu J, Buck RC, Korzeniowski SH, Wolstenholme BW, Folsom PW, Sulecki LM. 6:2 Fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. Chemosphere. 2011;82(6):853–858. doi: 10.1016/j.chemosphere.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Houtz E, Sedlak D. Quantification of Perfluorinated Acid Precursors in Urban Runoff. Presented at American Chemical Society National Meeting; Anaheim, CA. March 27, 2011; Paper ENVR 22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.