Abstract
Anticoagulation has been shown to reduce ischemic stroke in atrial fibrillation (AF). However, concerns remain regarding their safety and efficacy in those ≥70 years of age who comprise most AF patients. Of the 4060 patients (mean age, 65 years; range, 49–80 years) in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial, 2248 (55% of 4060) were 70–80 years of age, 1901 of whom were receiving warfarin. Propensity score for warfarin use, estimated for each of the 2248 patients, were used to match 227 of the 347 no-warfarin patients (in 1:1, 1:2 or 1:3 sets) with 616 warfarin patients, who were balanced on 45 baseline characteristics. All-cause mortality occurred in 18% and 33% of matched patients receiving and not receiving warfarin, respectively, during up to six (mean, 3.4) years of follow-up (hazard ratio {HR} when warfarin use was compared with its non-use, 0.58; 95% confidence interval {CI}, 0.43–0.77; p<0.001). All-cause hospitalization occurred in 64% and 67% of matched patients receiving and not receiving warfarin, respectively (HR associated with warfarin use, 0.93; 95% CI, 0.77–1.12; p=0.423). Ischemic stroke occurred in 4% and 8% of matched patients receiving and not receiving warfarin, respectively (HR associated with warfarin use, 0.57; 95% CI, 0.31–1.04; p=0.068). Major bleeding occurred in 7% and 10% of matched patients receiving and not receiving warfarin, respectively (HR associated with warfarin use, 0.73; 95% CI, 0.44–1.22; p=0.229). In conclusion, warfarin use was associated with reduced mortality in septuagenarian AF patients but had no association with hospitalization or major bleeding.
Keywords: atrial fibrillation, warfarin, mortality, propensity score, older adults
Anticoagulation has been shown to reduce the risk of ischemic stroke among older adults with atrial fibrillation (AF).1 Although most high risk patients with AF are over 70 years of age,2 the safety and efficacy of warfarin in these patients remain unclear.3 Additionally, there is little data on the effect of long-term anticoagulation on mortality in these patients. Therefore, we conducted a propensity-matched study of the association of warfarin and outcomes in older adults with AF.
Methods
We analyzed a public-use copy of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) data obtained from the National Heart, Lung, and Blood Institute. The design and the primary results of AFFIRM have been previously published.4, 5 Briefly, AFFIRM was a multicenter randomized clinical trial for rate versus rhythm control treatment strategies for AF conducted in 213 centers in the United States and Canada. Patients with recurrent AF without contraindication to anticoagulant therapy (as determined by their physician) and with high risk for stroke were recruited. Because age was considered a risk factor for stroke in AF, those ≥65 years of age could be enrolled regardless of other risk factors. However, to be eligible for enrollment, those <65 years were required to have at least one other risk factor for stroke, which included prior stroke or transient ischemic attacks, hypertension, heart failure, diabetes mellitus, increased left atrial enlargement, and left ventricular systolic dysfunction. AFFIRM participants had a mean age of 65 years (range, 49 to 80 years) and 76% (3091/4060) of patients were ≥65 years of age.
The current analysis was restricted to 2248 (55% of 4,060) patients who were 70–80 years of age. We chose a cut-off of 70 years because of the high prevalence of AF in this age group.6 Of the 2,248 patients, 1,901 (85%) were receiving warfarin, with goal International normalized ratio (INR) between 2.0 and 3.0. Patients were followed up for up to 6 years (with mean follow-up time of 3.4 years) with interval follow-up visits every 4 months. All outcomes were blindly adjudicated by the AFFIRM events committee. The primary outcome for the current analysis was all-cause mortality. Secondary outcomes included all-cause hospitalization, ischemic stroke, and major bleeding defined as bleeding requiring transfusion and/or surgery and/or permanent cessation of warfarin.
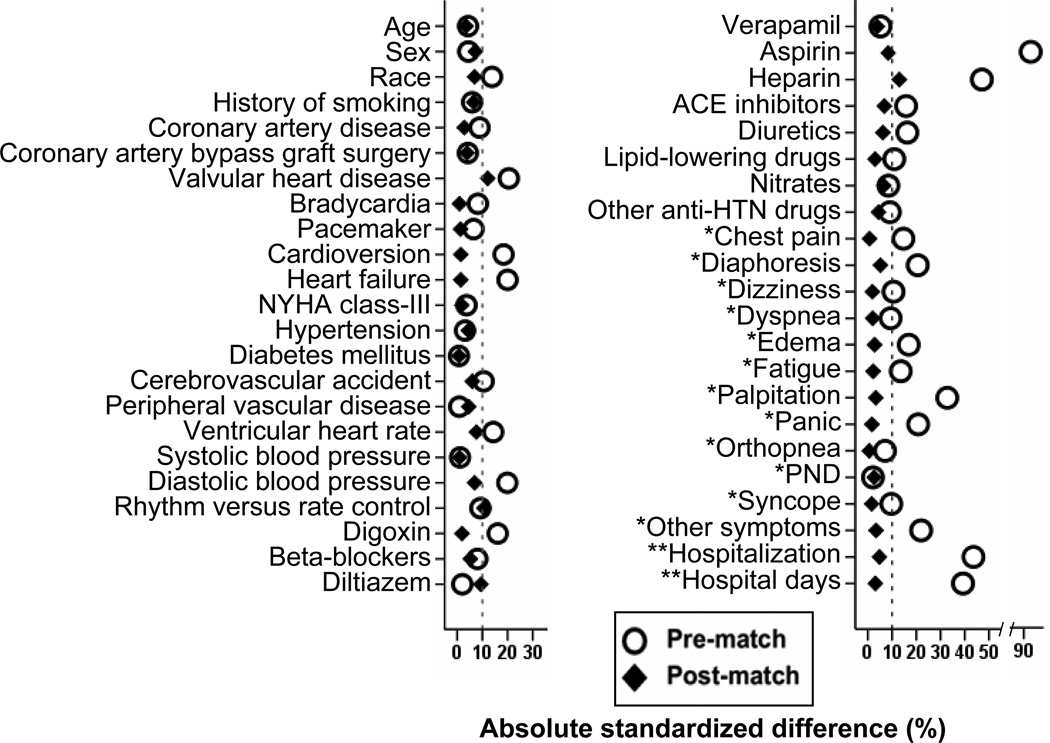
Considering the significant imbalances in baseline characteristics between the two groups (Table 1), we used propensity scores to assemble a matched cohort.7, 8 Propensity scores for warfarin use were estimated for each of the 2,248 patients using a non-parsimonious multivariable logistic regression model.9–11 We were able to match 227 of the 347 patients not receiving warfarin with 616 patients receiving warfarin using a greedy algorithm to match warfarin patients to sets of 1, 2 or 3 patients not receiving warfarin with similar propensity scores.12–16 The matched cohort of 843 patients was well-balanced between warfarin recipients and non-recipients on the 45 baseline characteristics used in the propensity score model. Absolute standardized differences were estimated to evaluate the pre-match imbalance and post-match balance, and are presented in a Love plot (Figure 1).17–19 Absolute standardized differences directly quantify biases in the means (or proportions) of covariates across the groups, and are expressed as percentages of the pooled standard deviations. An absolute standardized difference of 0% indicates no residual bias and differences <10% are considered inconsequential.
Table 1.
Baseline characteristics of atrial fibrillation (AF) patients ≥70 years by warfarin use, before and after propensity matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| n (%) or mean (±SD) | No warfarin (n=347) |
Warfarin (n=1901) |
P value |
No warfarin (n=227) |
Warfarin (n=616) |
P value |
| Age (years) | 76 (±3) | 75 (±3) | 0.458 | 76 (±3) | 76 (±3) | 0.655 |
| Female | 164 (47%) | 857 (45%) | 0.453 | 98 (43%) | 288 (47%) | 0.355 |
| African American | 37 (11%) | 129 (7%) | 0.011 | 20 (9%) | 67 (11%) | 0.382 |
| Current smoker | 28 (8%) | 124 (7%) | 0.291 | 19 (8%) | 41 (7%) | 0.391 |
| Systolic blood pressure (mm Hg) | 136 (±20) | 136 (±19) | 0.847 | 136 (±19) | 136 (±19) | 0.909 |
| Diastolic blood pressure (mm Hg) | 73 (±10) | 75 (±10) | 0.001 | 74 (±10) | 74 (±10) | 0.379 |
| Ventricular rate, bpm | 71 (±13) | 73 (±14) | 0.016 | 71 (±13) | 72 (±14) | 0.332 |
| Maximum ventricular rate during AF, bpm | 122 (±30) | 104 (±31) | <0.001 | 120 (±30) | 109 (±33) | <0.001 |
| Duration of AF ≥2 days | 118 (34%) | 1446 (76%) | <0.001 | 78 (34%) | 392 (64%) | <0.001 |
| CHADS2 score | 1.8 (±1.1) | 2.0 (±1.2) | 0.020 | 1.8 (±1.1) | 1.9 (±1.2) | 0.343 |
| CHA2DS2VASc score | 3.8 (±1.5) | 3.9 (±1.4) | 0.232 | 3.7 (±1.5) | 3.8 (±1.4) | 0.332 |
| Hospitalization due to AF | 227 (65%) | 841 (44%) | <0.001 | 142 (63%) | 371 (60%) | 0.539 |
| Hospitalizations duration (days) | 4 (±4) | 2 (±3) | <0.001 | 4 (±4) | 3 (±4) | 0.686 |
| Critical care duration (days) | 0.4 (±1.2) | 0.2 (±0.8) | <0.001 | 0.4 (±1.2) | 0.3 (±1.0) | 0.053 |
| Non-critical care duration (days) | 3.3 (±4.0) | 2.1 (±3.2) | <0.001 | 3.1 (±3.8) | 3.1 (±3.8) | 0.956 |
| Past medical history | ||||||
| Coronary artery disease | 151 (44%) | 744 (39%) | 0.125 | 96 (42%) | 252 (41%) | 0.718 |
| Acute myocardial infarction | 64 (18%) | 359 (19%) | 0.847 | 40 (18%) | 122 (20%) | 0.475 |
| Vulvular heart disease | 32 (9%) | 304 (16%) | 0.001 | 14 (6%) | 58 (9%) | 0.134 |
| Stroke or transient ischemic attack | 40 (12%) | 287 (15%) | 0.083 | 24 (11%) | 77 (13%) | 0.445 |
| Heart failure | 58 (17%) | 471 (25%) | 0.001 | 43 (19%) | 120 (20%) | 0.861 |
| Bradycardia | 38 (11%) | 162 (9%) | 0.144 | 22 (10%) | 58 (9%) | 0.903 |
| Diabetes mellitus | 59 (17%) | 328 (17%) | 0.909 | 40 (18%) | 107 (17%) | 0.932 |
| Hypertension | 234 (67%) | 1309 (69%) | 0.599 | 143 (63%) | 401 (65%) | 0.572 |
| Peripheral arterial disease | 27 (8%) | 152 (8%) | 0.892 | 21 (9%) | 49 (8%) | 0.545 |
| Pacemaker | 34 (10%) | 151 (8%) | 0.248 | 23 (10%) | 60 (10%) | 0.865 |
| Cardioversion | 117 (34%) | 810 (43%) | 0.002 | 80 (35%) | 213 (35%) | 0.857 |
| Randomization to rhythm treatment | 161 (46%) | 969 (51%) | 0.117 | 117 (52%) | 285 (46%) | 0.174 |
| Anti-arrhythmic drug failures | 49 (14%) | 309 (16%) | 0.318 | 34 (15%) | 92 (15%) | 0.988 |
| Symptoms during AF | ||||||
| Chest pain | 97 (28%) | 411 (22%) | 0.009 | 60 (26%) | 161 (26%) | 0.931 |
| Diaphoresis | 84 (24%) | 304 (16%) | <0.001 | 46 (20%) | 138 (22%) | 0.505 |
| Dizziness | 135 (39%) | 643 (34%) | 0.067 | 88 (39%) | 233 (38%) | 0.803 |
| Dyspnea | 172 (50%) | 1031 (54%) | 0.109 | 118 (52%) | 314 (51%) | 0.795 |
| Edema | 54 (16%) | 421 (22%) | 0.006 | 43 (19%) | 110 (18%) | 0.717 |
| Fatigue | 176 (51%) | 1093 (58%) | 0.019 | 120 (53%) | 319 (52%) | 0.781 |
| Palpitation | 209 (60%) | 838 (44%) | <0.001 | 129 (57%) | 360 (58%) | 0.674 |
| Panic | 53 (15%) | 163 (9%) | <0.001 | 26 (12%) | 74 (12%) | 0.824 |
| Syncope | 21 (6%) | 75 (4%) | 0.074 | 13 (6%) | 33 (5%) | 0.834 |
| Flutter | 71 (21%) | 841 (44%) | <0.001 | 47 (21%) | 250 (41%) | <0.001 |
| Other symptoms | 56 (16%) | 169 (9%) | <0.001 | 28 (12%) | 83 (14%) | 0.664 |
| Medications | ||||||
| Digoxin | 162 (47%) | 1040 (55%) | 0.006 | 116 (51%) | 309 (50%) | 0.809 |
| Beta-blockers | 132 (38%) | 798 (42%) | 0.171 | 89 (39%) | 226 (37%) | 0.503 |
| Diltiazem | 108 (31%) | 574 (30%) | 0.729 | 77 (34%) | 182 (30%) | 0.222 |
| Verapamil | 30 (9%) | 193 (10%) | 0.388 | 24 (11%) | 58 (9%) | 0.615 |
| Aspirin | 213 (61%) | 380 (20%) | <0.001 | 112 (49%) | 278 (45%) | 0.277 |
| Heparin | 118 (34%) | 273 (14%) | <0.001 | 75 (33%) | 167 (27%) | 0.091 |
| ACE inhibitors | 106 (31%) | 723 (38%) | 0.008 | 68 (30%) | 204 (33%) | 0.384 |
| Diuretics | 134 (39%) | 887 (47%) | 0.006 | 98 (43%) | 247 (40%) | 0.421 |
| Lipid lowering agents | 57 (16%) | 393 (21%) | 0.069 | 34 (15%) | 99 (16%) | 0.699 |
CHADS2 scoring system for risk of stroke in atrial fibrillation is based on the presence of each of the following conditions (with points assigned to each of them are indicated in the parenthesis): Congestive heart failure (1), Hypertension (1), Age >75 years (1), Diabetes mellitus (1), history of Stroke (2).
CHA2DS2VASc scoring system for risk of stroke in atrial fibrillation is based on the presence of each of the following conditions (with points assigned to each of them are indicated in the parenthesis): Congestive heart failure or left ventricular dysfunction (1), Hypertension (1), Age >75 years (2), Diabetes mellitus (1), a history of Stroke (2), Vascular disease (1), Age 65–74 years (1), Sex category (1 for Female)
Figure 1.
Absolute standardized differences of 45 baseline characteristics between patients receiving and not receiving warfarin, before and after propensity score matching (*Symptoms experienced during atrial fibrillation in the last six months; **Hospitalization for qualifying episodes of atrial fibrillation; ACE = angiotensin-converting enzyme; HTN = hypertension; NYHA = New York Heart Association; PND = paroxysmal nocturnal dyspnea)
For descriptive analyses, we used Pearson's chi-square and Wilcoxon rank-sum tests for the pre-match comparisons, and paired sample t-tests for post-match comparisons of baseline characteristics of patients with and without warfarin use, as appropriate. We used Kaplan-Meier plots and Cox regression analyses to determine associations between warfarin use and outcomes during follow-up. We conducted formal sensitivity analyses to quantify the degree of hidden bias that would need to be present to invalidate our conclusions based on a significant association between use of warfarin and all-cause mortality among matched patients.20–23 Subgroup analyses were conducted to determine the homogeneity of association between use of warfarin and all-cause mortality. Finally, to assess the generalizability of the findings of the current study based on trial-eligible AFFIRM participants 70–80 years with AF to community-dwelling AF patients in that age group, we compared the baseline characteristics and outcomes of participants included in our study with AF patients 70–80 years in the Cardiovascular Health Study (CHS). All statistical tests were two-tailed with a p-value <0.05 considered significant and all data analyses were performed using SPSS for Windows (Rel. 18; Chicago, IL).
Results
Patients (n=843) had a mean (SD) age of 76 (3) years, 45% were women, and 7% were non-white. Before matching, patients receiving warfarin were more likely to have heart failure and valvular heart disease, have higher CHADS2 scores but similar CHA2DS2VASc scores. These and other baseline imbalances were balanced after matching (Table 1 and Figure 1).
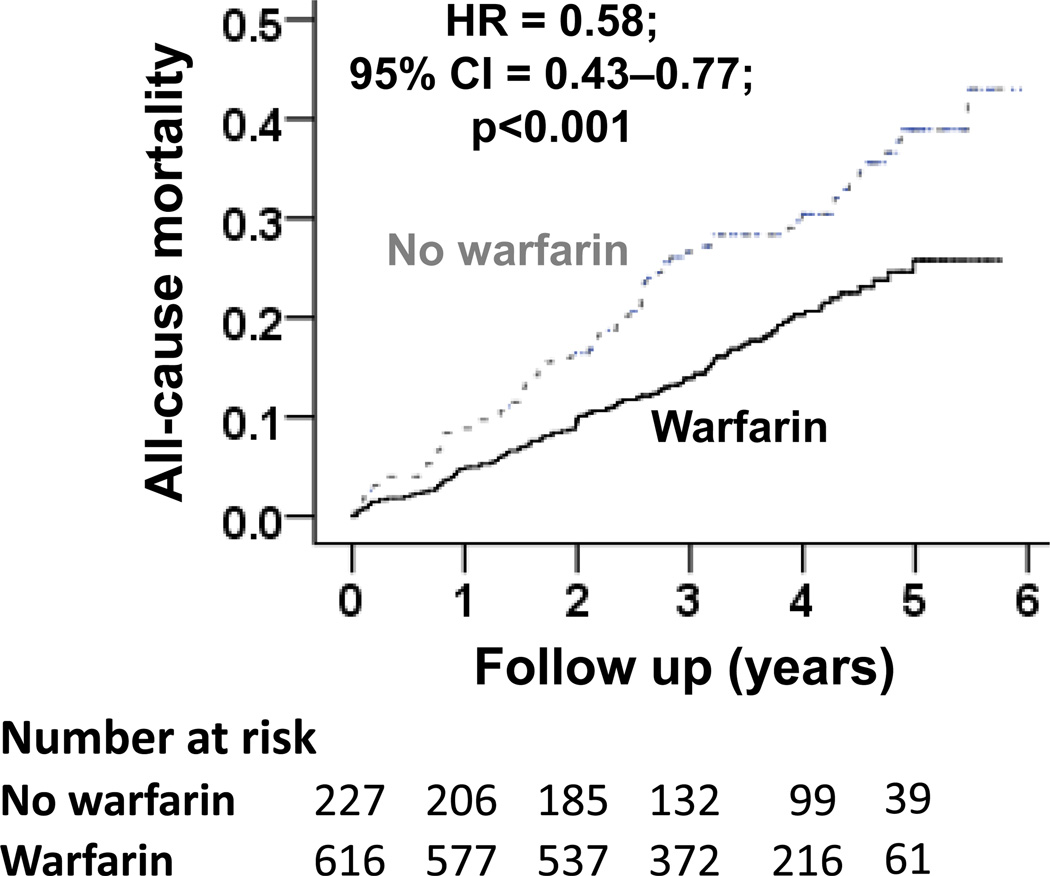
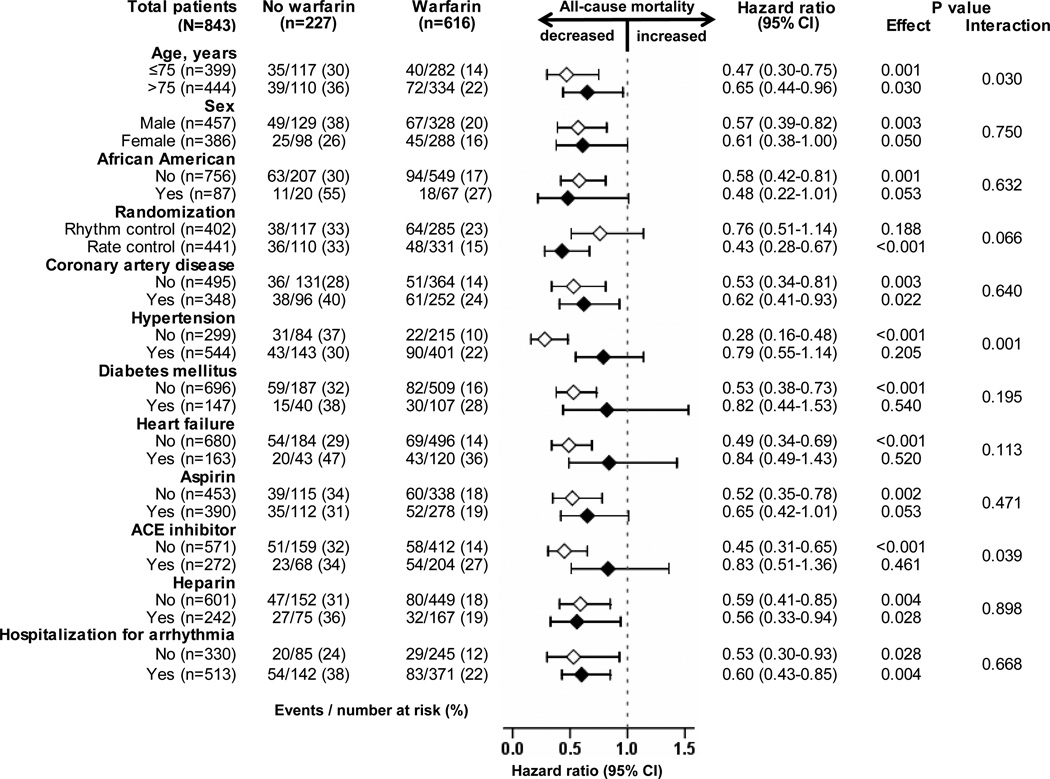
All-cause mortality occurred in 18% and 33% of matched warfarin and no-warfarin patients, respectively during 6 years of follow-up (hazard ratio {HR} when use of warfarin was compared with its non-use, 0.58; 95% confidence interval {CI}, 0.43–0.77; p<0.001; Table 2 and Figure 2). A hidden covariate that is a near-perfect predictor of mortality would need to increase the odds of warfarin use by 48% to explain away this association. The association of warfarin use with mortality in various subgroups of patients are displayed in Figure 3. The associations of warfarin use with various cause-specific mortalities are displayed in Tables 3 and 4.
Table 2.
Association of warfarin use with all-cause mortality
| Events (%) | Absolute risk difference* |
Hazard ratio (95% confidence interval) |
P value |
||
|---|---|---|---|---|---|
| No warfarin | Warfarin | ||||
| All-cause mortality | |||||
| Before matching (N=2248) | n=347 | n=1901 | |||
| Unadjusted | 97 (28%) | 365 (19%) | − 9% | 0.71 (0.57–0.89) | 0.003 |
| Multivariable-adjusteda | --- | --- | 0.70 (0.54–0.91) | 0.007 | |
| Propensity-adjustedb | --- | --- | 0.67 (0.52–0.87) | 0.003 | |
| After matching (N=843) | n=227 | n=616 | |||
| Propensity-matched | 74 (33%) | 112 (18%) | − 14% | 0.58 (0.43–0.77) | <0.001 |
Absolute risk difference was calculated by subtracting the percentage of events in the warfarin group from that of the no-warfarin group (before values were rounded)
Adjusted for all 45 baseline characteristics
Adjusted for propensity score.
Figure 2.
Kaplan-Meier plots for (a) all-cause mortality, and (b) all-cause hospitalization by warfarin use (HR=hazard ratio; CI=confidence interval)
Figure 3.
Association of warfarin use with all-cause mortality in subgroups of propensity-matched atrial fibrillation patients 70 years of age (CI=confidence interval)
Table 3.
Associations of warfarin use with other outcomes among 843 propensity-matched atrial fibrillation patients 70 years of age or older
| Events (%) | Absolute risk difference* |
Hazard ratio (95% confidence interval) |
P value |
||
|---|---|---|---|---|---|
| No warfarin (n=227) |
Warfarin (n=616) |
||||
| Cardiovascular mortality | 27 (12%) | 56 (9%) | − 3% | 0.80 (0.51–1.27) | 0.346 |
| Due to cardiac causes | 20 (9%) | 43 (7%) | − 2% | 0.84 (0.49–1.43) | 0.516 |
| Arrhythmic | 12 (5%) | 24 (4%) | − 1% | 0.76 (0.38–1.52) | 0.432 |
| Non-arrhythmic | 8 (4%) | 19 (3%) | − 1% | 0.96 (0.42–2.20) | 0.931 |
| Due to vascular causes | 7 (3%) | 13 (2%) | − 1% | 0.70 (0.28–1.75) | 0.442 |
| Non-cardiovascular mortality | 40 (18%) | 48 (8%) | − 10% | 0.45 (0.30–0.70) | <0.001 |
| Cancer | 14 (6%) | 24 (4%) | − 2% | 0.61 (0.31–1.19) | 0.609 |
| Pulmonary | 8 (4%) | 10 (2%) | − 2% | 0.43 (0.18–1.07) | 0.068 |
| Others | 17 (8%) | 15 (2%) | − 6% | 0.33 (0.17–0.67) | 0.002 |
| All-cause hospitalization | 152 (67%) | 394 (64%) | − 3% | 0.93 (0.77–1.12) | 0.423 |
| Due to cardiovascular causes | 103 (44%) | 252 (41%) | − 3% | 0.90 (0.72–1.14) | 0.386 |
| Due to non-cardiovascular causes | 104 (46%) | 258 (42%) | − 4% | 0.90 (0.71–1.13) | 0.355 |
| Ischemic stroke | 17 (8%) | 26 (4%) | − 4% | 0.57 (0.31–1.04) | 0.068 |
| Major bleeding** | 22 (10%) | 44 (7%) | −3% | 0.73 (0.44–1.22) | 0.229 |
Absolute risk difference was calculated by subtracting the percentage of events in the warfarin group from that of the no-warfarin group (before values were rounded)
Major bleeding was defined as bleeding requiring transfusion and/or surgery and/or permanent cessation of warfarin
Table 4.
Adjusted associations of warfarin use with other outcomes among 2248 atrial fibrillation patients 70 years of age or older
| Unadjusted events (%) | Absolute risk difference** |
Hazard ratio* (95% confidence interval) |
P value |
||
|---|---|---|---|---|---|
| No warfarin (n=347) |
Warfarin (n=1901) |
||||
| Cardiovascular mortality | 36 (10%) | 182 (10%) | − 0% | 0.92 (0.66–1.39) | 0.700 |
| Due to cardiac causes | 28 (8%) | 143 (8%) | − 0% | 0.92 (0.58–1.45) | 0.708 |
| Arrhythmic | 15 (4%) | 82 (4%) | − 0% | 0.96 (0.51–1.78) | 0.888 |
| Non-arrhythmic | 13 (4%) | 61 (3%) | − 1% | 0.87 (0.44–1.72) | 0.684 |
| Due to vascular causes | 8 (2%) | 39 (2%) | − 0% | 0.95 (0.40–2.25) | 0.904 |
| Non-cardiovascular mortality | 52 (15%) | 154 (8%) | − 7% | 0.51 (0.35–0.73) | <0.001 |
| Cancer | 20 (6%) | 64 (3%) | − 3% | 0.56 (0.31–1.01) | 0.053 |
| Pulmonary | 13 (4%) | 40 (2%) | − 2% | 0.54 (0.26–1.12) | 0.096 |
| Others | 19 (6%) | 50 (3%) | − 3% | 0.43 (0.23–0.80) | 0.008 |
| All-cause hospitalization | 227 (65%) | 1218 (64%) | − 1% | 0.96 (0.82–1.12) | 0.600 |
| Due to cardiovascular causes | 154 (44%) | 779 (41%) | − 3% | 1.12 (0.93–1.36) | 0.235 |
| Due to non-cardiovascular causes | 152 (44%) | 780 (41%) | − 3% | 1.04 (0.85–1.27) | 0.701 |
| Ischemic stroke | 21 (6%) | 77 (4%) | − 2% | 0.79 (0.45–1.38) | 0.400 |
| Major bleeding*** | 35 (10%) | 140 (7%) | − 3% | 0.76 (0.50–1.15) | 0.195 |
Adjusted for propensity score for warfarin use
Absolute risk difference was calculated by subtracting the percentage of events in the warfarin group from that of the no-warfarin group (before values were rounded)
Major bleeding was defined as bleeding requiring transfusion and/or surgery and/or permanent cessation of warfarin
All-cause hospitalization occurred in 64% and 67% of matched warfarin and no-warfarin patients, respectively (HR associated with warfarin use, 0.93; 95% CI, 0.77–1.12; p=0.423; Table 3). Ischemic stroke occurred in 4% and 8% of matched patients receiving and not receiving warfarin, respectively (HR associated with warfarin use, 0.57; 95% CI, 0.31–1.04; p=0.068; Table 3). Major bleeding occurred in 7% and 10% of matched patients receiving and not receiving warfarin, respectively (HR associated with warfarin use, 0.73; 95% CI, 0.44–1.22; p=0.229; Table 3). Pre-match associations of warfarin use with other outcomes are displayed in Table 4. Baseline characteristics of AF patients 70–80 years enrolled in the AFFIRM trial and community-dwelling AF patients 70–80 years in CHS are displayed in Table 5.
Table 5.
Characteristics of atrial fibrillation (AF) patients 70–80 years in AFFIRM and CHS
| n (%) or mean (±SD) | CHS (n=102) |
AFFIRM (n=2248) |
P value |
|---|---|---|---|
| Age, years | 74.6 (±3.5) | 75.4 (±3.4) | 0.019 |
| Female | 46 (45%) | 1021 (45%) | 0.949 |
| African American | 10 (10%) | 166 (7%) | 0.364 |
| Current smoker | 9 (9%) | 152 (7%) | 0.343 |
| Systolic blood pressure, mm Hg | 137 (±21) | 136 (±19) | 0.438 |
| Diastolic blood pressure, mm Hg | 72 (±12) | 75 (±10) | 0.003 |
| Ventricular rate, bpm | 71 (±13) | 73 (±14) | 0.144 |
| CHADS2 score | 1.8 (±1.2) | 2.0 (±1.2) | 0.351 |
| CHA2DS2VASc score | 3.6 (±1.5) | 3.8 (±1.4) | 0.176 |
| Past medical history | |||
| Coronary artery disease | 23 (23%) | 895 (40%) | <0.001 |
| Acute myocardial infarction | 12 (12%) | 423 (19%) | 0.073 |
| Hypertension | 62 (61%) | 1543 (69%) | 0.095 |
| Diabetes mellitus | 28 (28%) | 387 (17%) | 0.008 |
| Heart failure | 24 (24%) | 529 (24%) | 1.000 |
| Stroke or transient ischemic attack | 19 (19%) | 327 (15%) | 0.255 |
| Medications | |||
| Warfarin | 50 (49%) | 1901 (85%) | <0.001 |
| Heparin | 10 (10%) | 391 (17%) | 0.046 |
| Aspirin | 13 (13%) | 593 (26%) | 0.002 |
| Digoxin | 79 (78%) | 1202 (54%) | <0.001 |
| Beta-blockers | 16 (16%) | 930 (41%) | <0.001 |
| ACE inhibitors | 8 (8%) | 829 (37%) | <0.001 |
| Diuretics | 45 (44%) | 1021 (45%) | 0.796 |
| Lipid lowering agents | 1 (1%) | 450 (20%) | <0.001 |
| One year mortality | |||
| Unadjusted events | 4 (4%) | 117 (5%) | 0.566 |
| Unadjusted hazard ratio (95% CI) | Reference (1) | 1.36 (0.52–3.69) | 0.543 |
| Age-sex-race adjusted hazard ratio (95% CI) | Reference (1) | 1.29 (0.48–3.50) | 0.616 |
| Six-year mortality | |||
| Unadjusted events | 29 (28%) | 462 (21%) | 0.056 |
| Unadjusted hazard ratio (95% CI) | Reference (1) | 1.24 (0.83–1.83) | 0.292 |
| Age-sex-race adjusted hazard ratio (95% CI) | Reference (1) | 1.15 (0.77–1.70) | 0.494 |
Overall, the 2248 pre-match patients had a mean CHADS2 and CHA2DS2VASc scores of 1.96 (range, 0 to 6) and 3.85 (range, 1 to 9). Unadjusted HR for all-cause mortality associated with every unit increase in CHADS2 score was 1.45 (95% CI, 1.36–1.56; p<0.001), which remained unchanged despite multivariable adjustment for all covariates except those used to estimate CHADS2 score (adjusted HR, 1.32; 95% CI, 1.22–1.43; p<0.001). Similarly, unadjusted HR all-cause mortality associated with every unit increase in CHA2DS2VASc score was 1.38 (95% CI, 1.30–1.46; p<0.001), which remained unchanged despite multivariable adjustment for all covariates except those used to estimate CHA2DS2VASc score (adjusted HR, 1.26; 95% CI, 1.17–1.35; p<0.001).
Unadjusted HR for incident ischemic stroke associated with every unit increase in CHADS2 score was 1.26 (95% CI, 1.08–1.47; p=0.004), which remained essentially unchanged after multivariable adjustment for all covariates except those used to estimate CHADS2 score (adjusted HR, 1.21; 95% CI, 1.01–1.44; p=0.040). Similarly, unadjusted HR incident ischemic stroke associated with every unit increase in CHA2DS2VASc score was 1.30 (95% CI, 1.14–1.48; p<0.001), and this estimate did not change after multivariable adjustment for all covariates except those used to estimate CHA2DS2VASc score (adjusted HR, 1.30; 95% CI, 1.11–1.52; p=0.001). Similar associations were observed in the matched cohort.
Discussion
Findings from the current study demonstrate that septuagenarian AF patients had high rates of all-cause mortality and that the use of warfarin was associated with a significant reduction in mortality in these patients. These findings are consistent with those based on AFFIRM participants of all age groups.1 Despite high rates of all-cause and cardiovascular hospitalizations, warfarin use had no association with these events. Warfarin use was associated with a near-significant reduction in incident ischemic stroke but had no association with incident major bleeding. These findings are important, as the incidence of AF increases with age yet warfarin may be underused in this population due to concern for adverse effects and outcomes. This is particularly significant as the incidence of AF is projected to increase with the aging of the population.
The increased mortality without associated increased hospitalization in those not receiving warfarin suggests that these patients had a higher incidence of sudden death that may have precluded hospitalization. However, warfarin use was not associated with a reduction in cardiac death including those due to arrhythmias. Further, warfarin use was also not associated with vascular death including those due to stroke. The observation that warfarin-associated mortality reduction was largely due to reduction in non-cardiovascular mortality is intriguing. However, warfarin has been shown to be associated with reduction in the risk of various cancers including pulmonary neoplasm, and pulmonary embolism and associated deaths.24–26 Potential explanations for the lack of a significant association of warfarin with major bleeding include selection bias, close monitoring during the trial, lack of power due to small number of events and/or chance. However, the CHADS2 and CHA2DS2VASc scores of trial-eligible older AF patients in AFFIRM were generally similar to those of community-dwelling older AF patients in CHS.
Our results are consistent with the findings from the Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF) in which randomization to warfarin was associated with a significant reduction in mortality over 2 years among 420 AF patients (mean age, 68 years), which was also primarily driven by reduction in non-cardiac mortality.27 However, in that study, there was also a significant reduction in ischemic stroke. In contrast, patients in our study were older and were receiving contemporary medications such as lipid lowering agents and ACE-inhibitors, which may in part explain the small number of stroke events in AFFIRM.4
Current guidelines focus on stroke prevention as the main benefit of warfarin therapy using stroke risk stratification tools such as the CHADS2 score,28 which recommends warfarin for patients who have a prior history of stroke or have 2 of the following: heart failure, age ≥75, hypertension, or diabetes. However, findings from our subgroup analyses suggest that warfarin-associated mortality reduction may be greater in age 70–75 years and in those without hypertension. Although warfarin use was not associated with major bleeding in septuagenarian AF patients in our study, warfarin should be used with caution in older adults.29 In the National Consortium of Anticoagulation Clinics study, although the overall risk of bleeding did not increase with age, among AF patients receiving warfarin, the risk of life-threatening or fatal bleeding was significantly higher among those ≥80 years versus <50 years of age.29 However, in that study, overall bleeding rates for patients 70–79 years (37%; 157/432) was similar to those ≥80 years of age (30%; 28/93). Corresponding rates for serious (0.9% versus 1.1% among those ≥80 years) and life-threatening (0.1% versus 0.4% among those ≥80 years) bleeding were also comparable.
There were several limitations to our study. Despite balance on a large and diverse set of baseline characteristics, bias due to imbalances on unmeasured baseline characteristics remains possible, as in any observational study. Our sensitivity analysis suggests, however, that the association of warfarin use with mortality reduction observed here was sensitive only to fairly strong confounding from unmeasured variables. Patients in the warfarin group may have discontinued their use during follow-up and vice-versa. The resultant regression dilution may have attenuated the true association between warfarin and mortality in our study.30 AF patients in the current study were enrolled in clinical trial and excluded those >80 years of age, which may limit generalizability. However, these patients were similar in key baseline characteristics and outcomes to a cohort of community-dwelling AF patients. In conclusion, in a propensity-matched balanced cohort of septuagenarian AF patients, the use of warfarin was associated with reduced mortality but had no association with hospitalization or major bleeding.
Acknowledgments
Funding: Dr. Ahmed is supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG, Investigators A. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 2.Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999;84:131R–138R. doi: 10.1016/s0002-9149(99)00713-4. [DOI] [PubMed] [Google Scholar]
- 3.Morley J, Marinchak R, Rials SJ, Kowey P. Atrial fibrillation, anticoagulation, and stroke. Am J Cardiol. 1996;77:38A–44A. doi: 10.1016/s0002-9149(97)89116-3. [DOI] [PubMed] [Google Scholar]
- 4.The Planning and Steering Committees of the AFFIRM Study for the NHLBI AFFIRM Investigators. Atrial Fibrillation Follow-up investigation of Rhythm Management - the AFFIRM study design. Am J Cardiol. 1997;79:1198–1202. [PubMed] [Google Scholar]
- 5.The AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 7.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 8.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 9.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed MI, White M, Ekundayo OJ, Love TE, Aban I, Liu B, Aronow WS, Ahmed A. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, Ahmed A. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009;53:458–465. doi: 10.1161/HYPERTENSIONAHA.108.119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banach M, Bhatia V, Feller MA, Mujib M, Desai RV, Ahmed MI, Guichard JL, Aban I, Love TE, Aronow WS, White M, Deedwania P, Fonarow G, Ahmed A. Relation of baseline systolic blood pressure and long-term outcomes in ambulatory patients with chronic mild to moderate heart failure. Am J Cardiol. 2011;107:1208–1214. doi: 10.1016/j.amjcard.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujib M, Rahman AA, Desai RV, Ahmed MI, Feller MA, Aban I, Love TE, White M, Deedwania P, Aronow WS, Fonarow G, Ahmed A. Warfarin use and outcomes in patients with advanced chronic systolic heart failure without atrial fibrillation, prior thromboembolic events, or prosthetic valves. Am J Cardiol. 2011;107:552–557. doi: 10.1016/j.amjcard.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai RV, Ahmed MI, Fonarow GC, Filippatos GS, White M, Aban IB, Aronow WS, Ahmed A. Effect of serum insulin on the association between hyperuricemia and incident heart failure. Am J Cardiol. 2010;106:1134–1138. doi: 10.1016/j.amjcard.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai RV, Banach M, Ahmed MI, Mujib M, Aban I, Love TE, White M, Fonarow G, Deedwania P, Aronow WS, Ahmed A. Impact of baseline systolic blood pressure on long-term outcomes in patients with advanced chronic systolic heart failure (insights from the BEST trial) Am J Cardiol. 2010;106:221–227. doi: 10.1016/j.amjcard.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed A, Pitt B. A history of systemic hypertension and incident heart failure hospitalization in patients with acute myocardial infarction and left ventricular systolic dysfunction. Am J Cardiol. 2009;103:1374–1380. doi: 10.1016/j.amjcard.2009.01.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer P, Ekundayo OJ, Adamopoulos C, Mujib M, Aban I, White M, Aronow WS, Ahmed A. A propensity-matched study of elevated jugular venous pressure and outcomes in chronic heart failure. Am J Cardiol. 2009;103:839–844. doi: 10.1016/j.amjcard.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aronow WS, Ahmed MI, Ekundayo OJ, Allman RM, Ahmed A. A propensity-matched study of the association of peripheral arterial disease with cardiovascular outcomes in community-dwelling older adults. Am J Cardiol. 2009;103:130–135. doi: 10.1016/j.amjcard.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekundayo OJ, Muchimba M, Aban IB, Ritchie C, Campbell RC, Ahmed A. Multimorbidity due to diabetes mellitus and chronic kidney disease and outcomes in chronic heart failure. Am J Cardiol. 2009;103:88–92. doi: 10.1016/j.amjcard.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer P, White M, Mujib M, Nozza A, Love TE, Aban I, Young JB, Wehrmacher WH, Ahmed A. Digoxin and reduction of heart failure hospitalization in chronic systolic and diastolic heart failure. Am J Cardiol. 2008;102:1681–1686. doi: 10.1016/j.amjcard.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippatos GS, Adamopoulos C, Sui X, Love TE, Pullicino PM, Lubsen J, Bakris G, Anker SD, Howard G, Kremastinos DT, Ahmed A. A propensity-matched study of hypertension and increased stroke-related hospitalization in chronic heart failure. Am J Cardiol. 2008;101:1772–1776. doi: 10.1016/j.amjcard.2008.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giamouzis G, Sui X, Love TE, Butler J, Young JB, Ahmed A. A propensity-matched study of the association of cardiothoracic ratio with morbidity and mortality in chronic heart failure. Am J Cardiol. 2008;101:343–347. doi: 10.1016/j.amjcard.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris GK, Mitchell JR. Warfarin sodium in prevention of deep venous thrombosis and pulmonary embolism in patients with fractured neck of femur. Lancet. 1976;2:869–872. doi: 10.1016/s0140-6736(76)90536-5. [DOI] [PubMed] [Google Scholar]
- 25.Schulman S, Lindmarker P. Incidence of cancer after prophylaxis with warfarin against recurrent venous thromboembolism. Duration of Anticoagulation Trial. N Engl J Med. 2000;342:1953–1958. doi: 10.1056/NEJM200006293422604. [DOI] [PubMed] [Google Scholar]
- 26.Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ, Jr, Forcier RJ, Edwards R, Headley E, Kim SH, O'Donnell JR, O'Dell R, Tornyos K, Kwaan HC. Effect of warfarin on survival in small cell carcinoma of the lung. Veterans Administration Study No. 75. JAMA. 1981;245:831–835. [PubMed] [Google Scholar]
- 27.The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505–1511. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 28.Gage B, Waterman A, Shannon W, Boechler M, Rich M, Radford M. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 29.Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996;124:970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]