Abstract
Oxomanganese(V) species have been implicated in a variety of biological and synthetic processes, including as a key reactive center within the oxygen-evolving complex in photosynthesis. Nearly all mononuclear MnV–oxo complexes have tetragonal symmetry, producing low-spin species. A new MnV–oxo complex that is high-spin is now reported, which was prepared from a well-characterized oxomanganese(III) complex having trigonal symmetry. Titration experiments with [FeCp2]+ were monitored with optical and electron paramagnetic resonance (EPR) spectroscopies and support a high-spin oxomanganese(V) complex formulation. The parallel-mode EPR spectrum has a distinctive S = 1 signal at g = 4.01 with a six-line hyperfine pattern having Az = 113 MHz. The presence of an oxo ligand was supported by resonance Raman spectroscopy, which revealed O-isotope sensitive peaks at 737 cm−1 and 754 cm−1 assigned as a Fermi doublet centered at 746 cm−1(Δ18O = 31 cm−1). Kβ Mn X-ray emission spectra showed Kβ' and Kβ1,3 bands at 6475.92 and 6490.50 eV, which are characteristic of a high-spin MnV center.
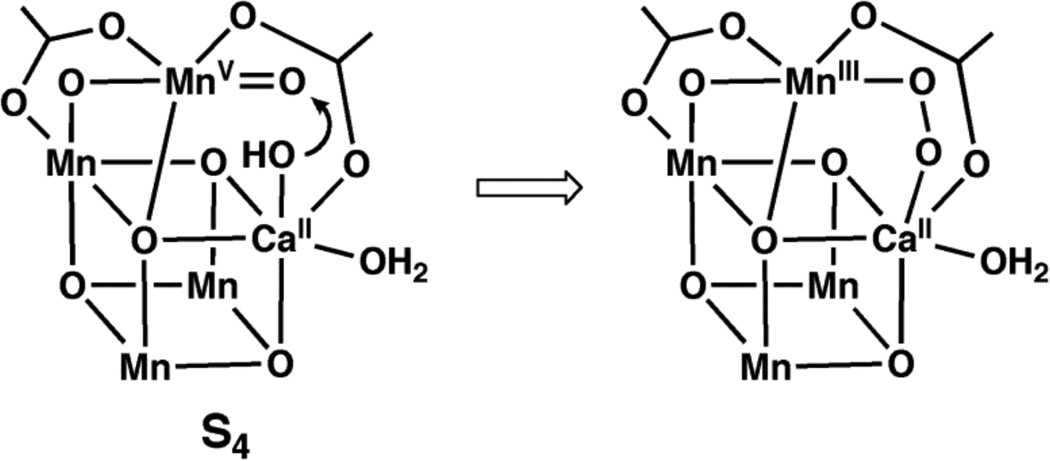
Manganese-oxo complexes have prominent roles in several chemical and biological processes.1 In biology, high valent Mn–oxo species may be consequential in the oxidation of water to dioxygen within the photosynthetic membrane protein (Photosystem II, PSII).2 Catalysis occurs at the oxygen-evolving complex (OEC) in PSII, which contains a Mn4Ca cluster surrounded by a network of H-bonds. The structure of the cluster can be described as a Mn3CaO4 distorted cube with an oxo-bridged external manganese center. 3 The mechanism of water oxidation is still debated, yet one common proposal invokes the generation of high valent Mn species prior to the formation of the O-O bond.4 In this premise, photo-oxidation would produce a high-energy transient state (S4) containing MnV–oxo and CaII–OH centers that initially couple to form a peroxo intermediate and, ultimately, dioxygen (Figure 1).
Figure 1.
Hypothetical representation of the S4 state in the OEC and a possible route to the formation of the O–O bond.
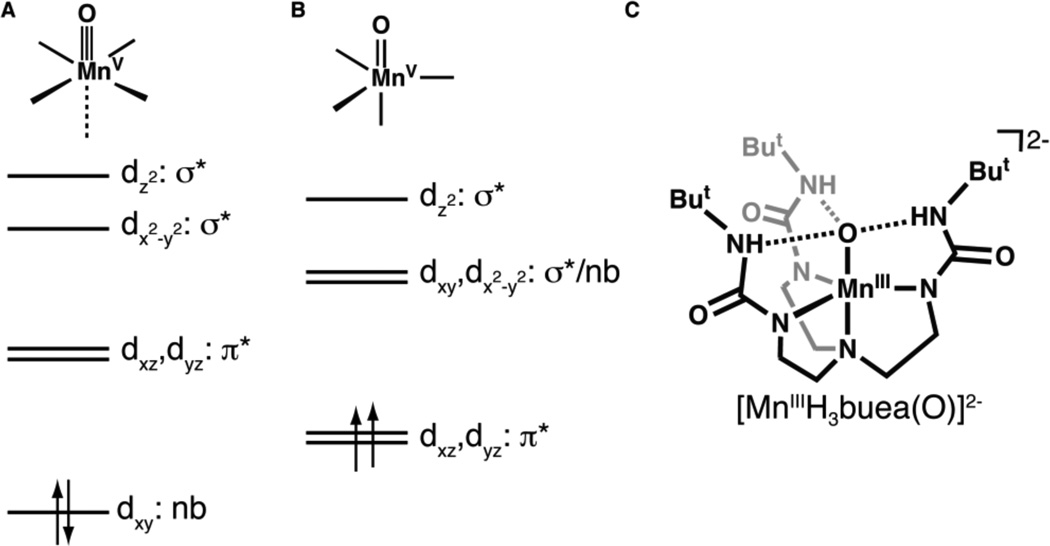
The properties of a MnV–oxo center that can lead to O–O bond formation are unknown, leading to efforts to prepare synthetic systems that probe this process. Some successes have been reported, including the work of Nam and Åkermark, 5 who showed that dioxygen can be produced from Mn(V)–O species and hydroxide ions. In addition to water oxidation, MnV–oxo complexes have been invoked as oxiddants in O-atom transfer reactions and C–H bond function-alization. 6 Most of these complexes contain ancillary porphyrin, 7 corrole,8 corrolazine,9 or salen ligands,10 producing species that are low-spin with S = 0 spin ground states. This observation follows from the established theory that predicts that all tetragonal d2 metal-oxo complexes will be diamagnetic, resulting in a triple bond between the metal center and the terminal oxo ligand (Figure 2A).11 Results from the few characterized tetragonal MnV–oxo species support this prediction, insofar as the observed spectroscopic and structural properties indicate the existence of an S = 0 Mn≡O unit.12
Figure 2.
Qualitative orbital splitting diagrams for oxometal complexes with (A) tetragonal11a and (B) trigonal15 symmetries, and (C) the structure of the oxomanganese(III) precursor.
The lone MnV–oxo site in the OEC could also be high spin (S = 1). Less is known about the chemistry of metal–oxo complexes in this spin state: we are aware of only one report concerning a high-spin MnV–oxo complex, a MnV(O)porphyrin species for which only a room temperature magnetic moment was used to evaluate its magnetic properties.13 In addition, an isoelectronic MnV –imido complex has been prepared,14 the properties of which suggested that it is paramagnetic. One approach toward preparing high-spin metal-oxo species is to enforce local trigonal symmetry within the complex. Mayer and Thorn15 were the first to suggest that metal–oxo complexes with trigonal symmetry possess two low-lying degenerate orbitals that will produce S = 1 spin ground states for a d2 metal-oxo complex (Figure 2B).16 This theoretical prediction suggests that the metal–oxo interactions in such complexes are weaker compared to those with tetragonal symmetry, making the former a more reactive species.
This report describes the preparation and spectroscopic properties of a high-spin MnV–oxo complex, in which the oxo ligand arises from H2O. The complex is supported by the ligand [H3buea]3−, which promotes C3 molecular symmetry and influences the secondary coordination sphere through an intramolecular H-bonding network around the MnV–oxo unit. Our findings demonstrate the feasibility of high-spin MnV–oxo complexes and provide a spectroscopic framework for probing their role in chemical processes.
We have previously synthesized [MnIIIH3buea(O)]2−, the only example of a monomeric MnIII–oxo complex (Figure 2C).17 Unlike most monomeric metal-oxo complexes, there are three intramolecular H-bonds between the oxo ligand and [H3buea]3− which aids in the isolation of the complex. [MnIIIH3buea(O)]2− has unusually low oxidation potentials because of the highly anionic ligand field surrounding the manganese center, making it a convenient synthon to prepare higher valent Mn–oxo species. We showed that the one-electron oxidation of [MnIIIH3buea(O)]2− to its MnIV–oxo derivative at room temperature in DMSO can be accomplished at −1.0 V vs [FeCp2]+/0 (−0.34 V vs NHE).18 A second oxidative process was observed at −0.076 V vs [FeCp2]+/0 (+0.59 V vs NHE), which we assigned to the MnV/IV–oxo redox couple. However, this process was only reversible under the relatively fast scan velocity of 50 V/s, which hindered further characterization of this oxidized species at room temperature. We have found that measuring the redox potential at −60°C in 1:1 THF/DMF produced a quasi-reversible one-electron redox process at −0.08 V vs [FeCp2]+/0 at significantly slower scan velocities of 0.100 V/s (Figure S1), suggesting the possibility for detecting this putative MnV–oxo species at lower temperatures.
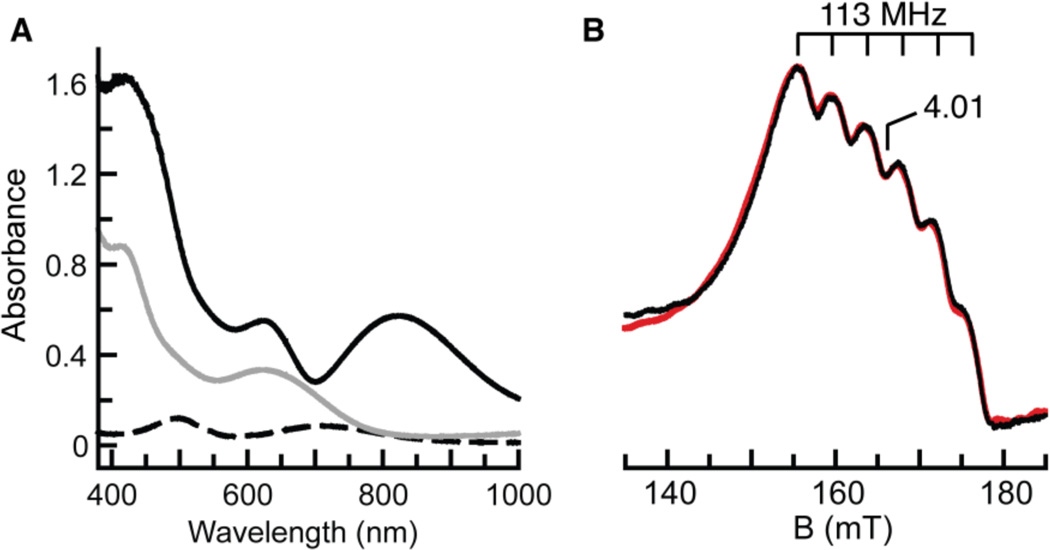
The sequential oxidation of [MnIIIH3buea(O)]2− was followed spectrophotometrically at −80°C in a DMF/THF solution (Scheme 1, Figure 3A). The optical properties of [MnIIIH3buea(O)]2− at λmax = 710 and 500 nm disappear upon the addition of 1 equiv of [FeCp2]+ to produce a spectrum with bands at λmax = 640 and 420 nm. These features are identical to those obtained for [MnIVH3buea(O)]− when measured at room temperature in DMSO.18a Treating the solution with another equivalent of [FeCp2]+ caused a new spectrum to appear, containing peaks at λmax, nm (εM)= 820 (3600), 620 (3400) and 430 (10,000), which persists for hours at −80°C. We assign these features to the MnV–oxo complex, [MnVH3buea(O)].
Scheme 1.
Conditions: (a) 1.0 equiv [FeCp2]+, 1:1 DMF/THF, −80°C; (b) 1–1.5 equiv [FeCp2]+, 1:1 DMF/THF, −80°C
Figure 3.
(A) Electronic absorbance spectra of [MnI–IIH3buea(O)]2− (- - -), [MnIVH3buea(O)]− (—), and [MnVH3buea(O)] (—) collected at −80°C in 1:1 THF/DMF. (B) ‖-mode EPR spectrum of [MnVH3buea(O)] (black) and simulated spectrum (red). See Figure 4 for experimental conditions.
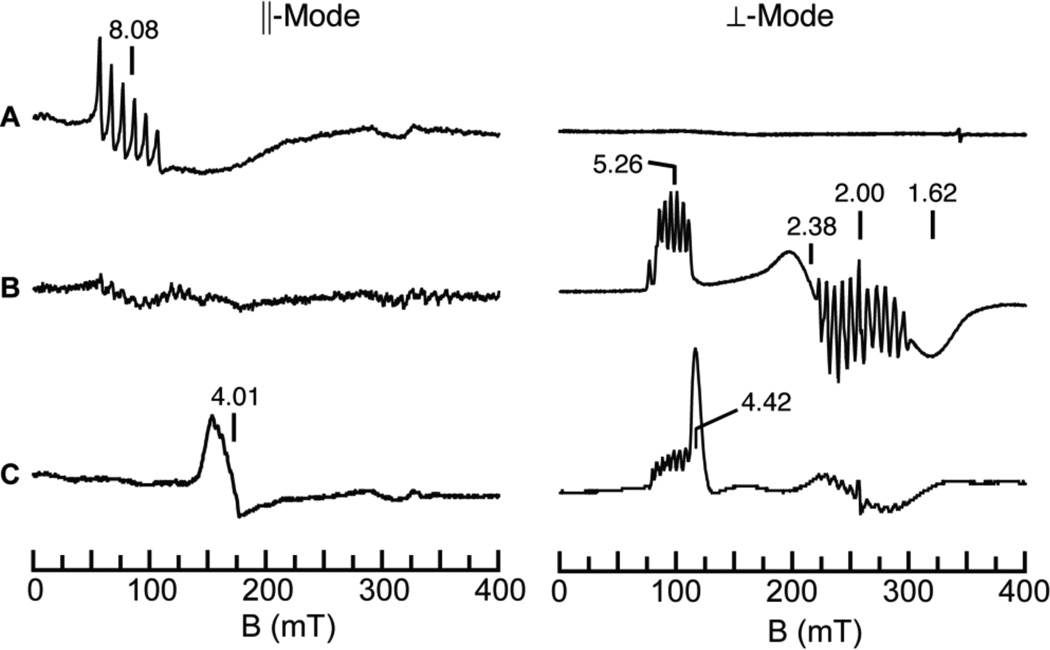
The oxidation of [MnIIIH3buea(O)]2− by [FeCp2]+ was also studied using parallel (‖) and perpendicular (⊥) mode EPR spectroscopy. We found that [MnIIIH3buea(O)]2− has no signals in ⊥-mode but shows a 6-line hyperfine pattern (Az = 280 MHz, 10 mT) in ‖-mode at g = 8.08 (Figure 4A), which is indicative of an S = 2 spin state.19 One-electron oxidation of [MnIIIH3buea(O)]2− was evident by the loss of the g = 8.08 signal in the ‖-mode EPR spectrum and the appearance of features at g = 5.26, 2.38 and 1.62 in the ⊥-mode spectrum (Figure 4B). These signals are associated with [MnIVH3buea(O)]− as reported previously.18a The relative signal intensities of [MnIIIH3buea(O)]2− and the simulations of the MnIV-oxo showed that 80% of the manganese in the sample was converted to [MnIVH3buea(O)]−. The 11-line hyperfine pattern centered at g = 2 is proposed to originate from a minority mixed-valent species (< 1% in manganese).
Figure 4.
‖- (left) and ⊥-mode (right) EPR spectra of a (A) [MnIIIH3buea(O)]2−); and after addition of approximately (B) one and (C) three equiv of [FeCp2]+. The peak at g = 4.42 in the ⊥-mode spectrum in C is from excess [FeCp2]+. Experimental conditions, temperature, 10 K; microwave frequency, 9.29 (‖) or 9.62 (⊥) GHz.
Further oxidation of the sample produced a new signal in ‖-mode centered at g = 4.01 (Figure 4C),20 which displayed a 6-line hyperfine splitting of 113 MHz (4 mT) (Figure 3B). The position of the signal is indicative of a transition from the |1±> doublet of an S = 1 spin manifold,20,21 and, taken together with the hyperfine splitting, is consistent with a monomeric MnV species that is assigned to [MnVH3buea(O)]. Simulations of this signal indicated that 60% of the initial MnIII–oxo species was converted to [MnVH3buea(O)].
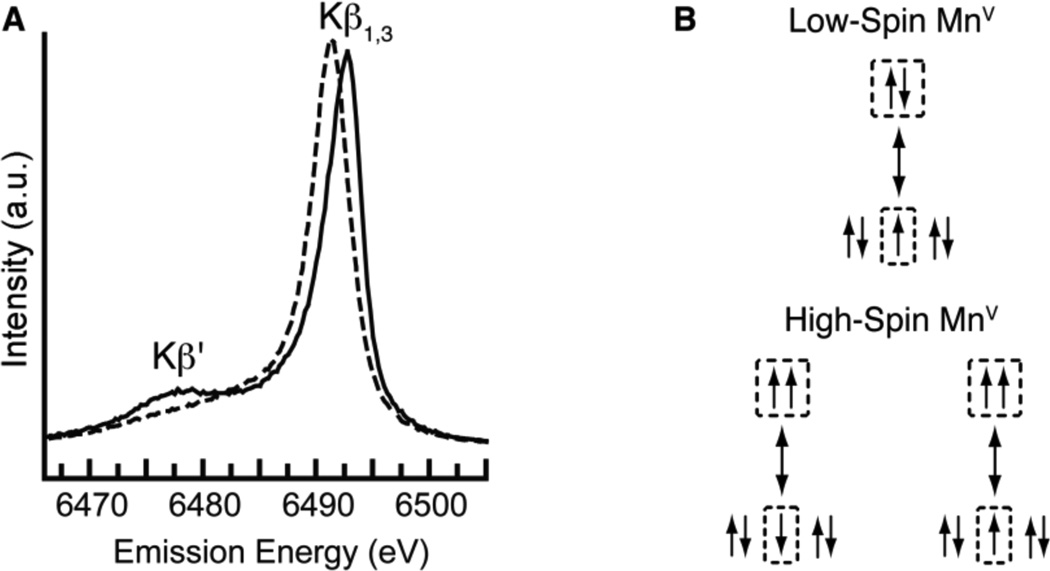
Additional support for the S = 1 spin state for [MnVH3buea(O)] came from Kβ manganese X-ray emission spectroscopy (XES). Figure 5 shows the Mn Kβ emission spectrum of [MnVH3buea(O)] and of the low-spin oxomanganese(V) complex [MnVDMB(O)]−,12b recorded at 10 K. The low-spin oxomanganese(V) shows a single peak (Kβ1,3) at 6489.06 eV, while the spectrum of [MnVH3buea(O)] is split into two peaks, Kβ1,3 at 6490.50 eV and its satellite peak (Kβ') at ~6476 eV. The Kβ1,3 peak of [MnVH3buea(O)] is shifted to higher energy by 1.44 eV compared to that of [MnVDMB(O)]−.
Figure 5.
(A) XES spectra of [MnVH3buea(O)] (—) and the low-spin oxomanganese(V) complex [MnVDMB(O)] (- - -) collected at 10K with an excitation energy of 10.0 keV and (B) schematic diagrams of the 3d-3p exchange interactions in low- and high-spin MnV systems.
Kβ1,3 XES detects the X-ray emission from the relaxation of a 3p electron to a 1s hole, which is created by excitation of an 1s electron. The energy splitting in Kβ spectra is caused by the exchange interaction between 3p and 3d orbitals. 22 Two final states (Kβ1,3 and Kβ') exist because of constructive (Kβ1,3) or destructive (Kβ') spin-exchange interactions between the unpaired electrons in the 3p and 3d orbitals (Figure 5B). The Kβ spectrum is thus sensitive to the effective number of unpaired 3d electrons and has been used to determine the Mn oxidation states in synthetic complexes and the OEC.23 Splitting of Kβ1,3 peak has been observed previously between low- and high-spin FeIII species: 24 a shift of ~ 0.7 eV for the Kβ1,3 peak position was found between the low- and high-spin species. For the low-spin complex, the Kβ' peak moved towards and merged into the low energy side of the Kβ1,3 line because of a decrease in valence spin. Analogous trends were observed in the Kβ XES spectra of [MnVH3buea(O)] and [MnVDMB(O)]−, findings that are consistent with [MnVH3buea(O)] having an S = 1 spin ground state.
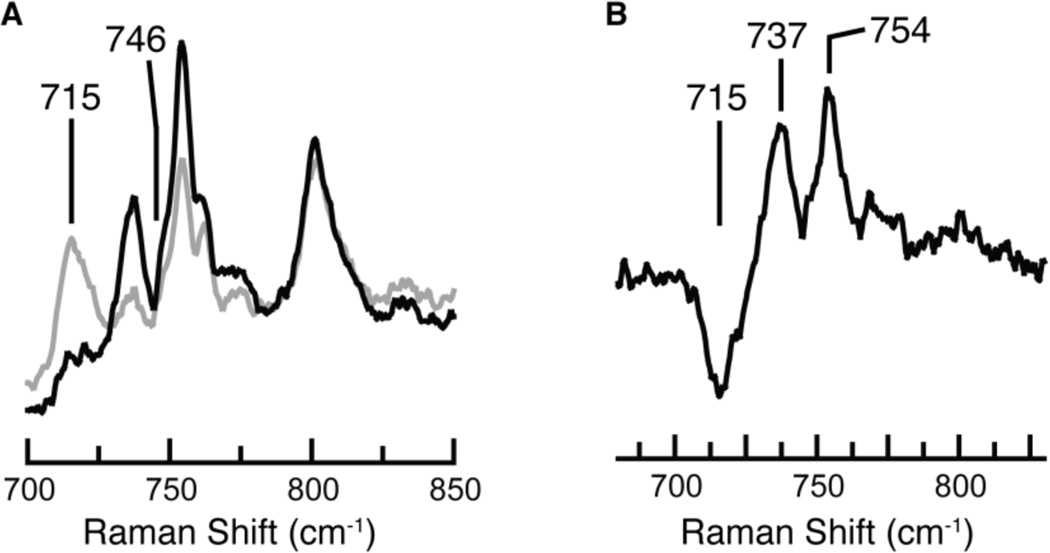
In order to confirm the presence of the oxo ligand in [MnVH3buea(O)], resonance Raman (rR) spectroscopy was performed (λex = 647.1 nm, 77 K). The rR spectrum of [MnVH3buea(16O)] contained oxygen-sensitive features at 737 cm−1 and 754 cm−1 that converted to a single feature at 715 cm−1 when the sample was prepared with H2 18O (Figure 6). On the basis of this behavior, and the fact that none of the peaks were observed in rR spectra of the MnIII or MnIV precursors,25 we postulate that the two peaks in the 16O sample comprise a Fermi doublet, a relatively common phenomenon in metal-oxygen compounds.26 In support of assigning these features to a MnV-O vibration, the observed difference of 31 cm−1 between the average position of the two peaks in the 16O sample (746 cm−1) and the 715 cm−1 peak in the 18O sample agrees with the value expected based on a harmonic Mn—O oscillator (ν(Mn–16O)/ν(Mn–18O) = 1.043; calc'd = 1.046). The MnV–O vibration occurs at a slightly higher energy than that found by FTIR spectroscopy for [MnIVH3buea(O)] (ν(Mn–O) = 737 cm−1),18a which is consistent with the electron being removed from orbitals not involved in Mn–O bonding (Figure 2B). The Mn–O vibration observed for [MnVH3buea(O)] is similar to the ν(Mn–O) of 759 cm−1 reported for a 6-coordinate MnV–oxo porphyrin complex; however, it is at significantly lower energy than those reported for nonporphyrinic low-spin oxomanganese(V) complexes. For example, the low-spin [MnVDMB(O)]− complex has an Mn–O vibration of 973 cm−1.12b This difference in vibrational energies reflects the disparate Mn–O bond orders predicted for high- and low-spin oxomanganese(V) complexes (Figure 2) and the likelihood that the Mn–O bond in [MnVH3buea(O)] forms intramolecular H-bonds with the [H3buea]3− ligand.
Figure 6.
Resonance Raman spectra of (A) [MnVH3buea(16O)] (black) and [MnVH3buea(18O)] (gray) collected at 77 K with λex = 647.1 nm and (B) their difference spectrum (16O – 18O).
With the characterization of [MnVH3buea(O)] we have now prepared oxomanganese complexes in three different oxidation levels, which have similar primary and secondary coordination spheres. The chemistry within this series of complexes is currently under investigation but preliminary results are consistent with our spectroscopic findings that all are monomeric oxomanganese species. For instance, treating the newly discovered [MnVH3buea(O)] complex with 1 equiv of [MnIIIH3buea(O)]2− produced 2 equiv of [MnIVH3buea(O)]– as determined by EPR and optical spectroscopies (Figure S2). This reaction is consistent with the comproportionation reaction described by eq 1
| (1) |
Our work has further shown that changes in spin state within the series can be monitored with EPR spectroscopy, illustrating the utility of using ‖-mode methods to probe integer-spin states. In addition, we have obtained the first high-spin manganese(V) EPR and XES spectra that can now be used as references in the study of other high valent manganese systems, such as those that may be present in the OEC of PSII. These results also establish the viability of high-spin oxomanganese(V) species as possible reactive intermediates in a variety of chemical processes.
Supplementary Material
ACKNOWLEDGMENT
We thank Drs. J. Kern, R. A. Mori, and T.-C. Weng for their help during the XES data collection and Professor T. J. Collins for providing [NEt4][MnVDMB(O)].
Funding Sources
The authors thank the NIH (GM50781 to ASB; GM77387 to MPH; GM47365 to WBT; and GM55302 to VKY)) and the Office of Science, Basic Energy Sciences (BES), Division of Chemical Sciences, Geosciences and Biosciences, Department of Energy under Contract No. DE-AC02-05CH11231 (VKY and JY) for financial support. Portions of this research were carried out at Stanford Synchrotron Radiation Lightsource (SSRL) operated by DOE, OBES.
ABBREVIATIONS
- [H3buea]3−
tris[(N'-tert-butylureaylato)-N-ethylene]aminato
DMB is a tetra-amido marcocylic ligand
Footnotes
ASSOCIATED CONTENT
Experimental details for all chemical reactions and measurements, and figures for comproportionation reactions and electrochemical experiments. This material is available free of charge via the Internet at (http://pubs.acs.org/page/jacsat/submission/authors.html).
REFERENCES
- 1.(a) Groves JT, Han YZ. In: Cytochrome P-450. Structure, Mechanism and Biochemistry. Ortiz de Montellano RR, editor. New York: Plenum Press; 1995. pp. 3–48. [Google Scholar]; (b) Holm RH. Chem. Rev. 1987;87:1401–1449. [Google Scholar]; (c) Gardner KA, Kuehnert LL, Mayer JM. Inorg. Chem. 1997;36:2069–2078. doi: 10.1021/ic961297y. [DOI] [PubMed] [Google Scholar]
- 2.Meyer TJ, Nuynh MHV, Thorp HH. Angew. Chem. Int. Ed. 2007;46:5284–5304. doi: 10.1002/anie.200600917. [DOI] [PubMed] [Google Scholar]
- 3.Umena Y, Kawakami K, Shen J-R, Kamiya N. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- 4.(a) McEvoy JP, Brudvig GW. Chem. Rev. 2006;106:4455–4483. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]; (b) Cady CW, Crabtree RH, Brudvig GW. Coord. Chem. Rev. 2008;252:444–455. doi: 10.1016/j.ccr.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Kim SH, Park H, Seo MS, Kubo M, Ogura T, Klajn J, Gryko DT, Valentine JS, Nam W. J. Am. Chem. Soc. 2010;132:14030–14032. doi: 10.1021/ja1066465. [DOI] [PubMed] [Google Scholar]; (b) Gao Y, Akermark T, Liu J, Sun L, Akermark B. J. Am. Chem. Soc. 2009;131:8726–8727. doi: 10.1021/ja901139r. [DOI] [PubMed] [Google Scholar]
- 6.Groves JT, Lee J, Marla SS. J. Am. Chem. Soc. 1997;119:6269–6273. [Google Scholar]
- 7.(a) Jin N, Groves JT. J. Am. Chem. Soc. 1999;121:2923–2924. [Google Scholar]; (b) Song WJ, Seo MS, George SD, Ohta T, Song R, Kang M-J, Tosha T, Kitagawa T, Solomon EI, Nam W. J. Am. Chem. Soc. 2007;129:1268–1277. doi: 10.1021/ja066460v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross Z, Golubkov G, Simkhovich L. Angew. Chem. Int. Ed. 2000;39:4045–4047. doi: 10.1002/1521-3773(20001117)39:22<4045::aid-anie4045>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Prokop KA, de Visser SP, Goldberg DP. Angew. Chem. Int. Ed. 2010;49:5091–5095. doi: 10.1002/anie.201001172. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen EN, Zhang W, Muci AR, Ecker JR, Deng L. J. Am. Chem. Soc. 1991;113:7063–7064. [Google Scholar]
- 11.(a) Ballhausen CJ, Gray HB. Inorg. Chem. 1962;1:111–122. [Google Scholar]; (b) Mayer JM. Comm. Inorg. Chem. 1988;8:125–135. [Google Scholar]; (c) Winkler JR, Gray HB. Struct. Bond. 2011 ASAP. [Google Scholar]
- 12.(a) Collins TJ, Powell RD, Slebonick C, Uffelman ES. J. Am. Chem. Soc. 1990;112:899–901. [Google Scholar]; (b) Workman JM, Powell RD, Procyk AD, Collin TJ, Bosian DF. Inorg. Chem. 1992;31:1548–1550. [Google Scholar]; (c) Lansky DE, Mandimutsira B, Ramdhanie B, Clausen M, Penner-Hahn J, Zvyagin SA, Telser J, Krzystek J, Zhan R, Ou Z, Kadish KM, Zakharov L, Rheingold AL, Goldberg DP. Inorg. Chem. 2005;44:4485–4498. doi: 10.1021/ic0503636. [DOI] [PubMed] [Google Scholar]
- 13.Groves JT, Kruper WJ, Haushalter RC. J. Am. Chem. Soc. 1980;102:6375–6377. [Google Scholar]
- 14.Zdilla MJ, Dexheimer JL, Abu-Omar MM. J. Am. Chem. Soc. 2007;129:11505–11511. doi: 10.1021/ja073027s. [DOI] [PubMed] [Google Scholar]
- 15.Mayer JM, Thorn DL, Tulip TH. J. Am. Chem. Soc. 1985;107:7454–7462. [Google Scholar]
- 16.For examples of high-spin oxochromiun(IV) complexes see: Hess A, Hörz MR, Liable-Sands LM, Lindner DC, Rheingold AL, Theopold KH. Angew. Chem. Int. Ed. 1999;38:166–168. Qin K, Incarvito CD, Rheingold AL, Theopold KH. J. Am. Chem. Soc. 2002;124:14008–14009. doi: 10.1021/ja028382r.
- 17.Borovik AS. Acc. Chem. Res. 2005;38:54–61. doi: 10.1021/ar030160q. [DOI] [PubMed] [Google Scholar]
- 18.(a) Parsell TH, Behan RK, Hendrich MP, Green MT, Borovik AS. J. Am. Chem. Soc. 2006;128:8728–8729. doi: 10.1021/ja062332v. [DOI] [PubMed] [Google Scholar]; (b) Parsell TH, Yang M-Y, Borovik AS. J. Am. Chem. Soc. 2009;131:2762–2763. doi: 10.1021/ja8100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrich MP, Debunner P. Biophys. J. 1989;56:489–506. doi: 10.1016/S0006-3495(89)82696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A total of 3 equiv of [FeCp2]+was used to ensure complete oxidation at −80°C (see Figure 4C).
- 21.Details of this spectrum will be discussed in a forthcoming report.
- 22.(a) Tsutsumi K, Nakamori H, Ichikawa K. Phys. Rev. B: Condens. Matter. 1976;13:929–933. [Google Scholar]; (b) Thole BT, Cowan RD, Sawatzky GA, Fink J, Fuggle JC. Phys. Rev. B. 1985;31:6856–6858. doi: 10.1103/physrevb.31.6856. [DOI] [PubMed] [Google Scholar]
- 23.(a) Messinger J, Robblee JH, Bergmann U, Fernandez C, Glatzel P, Visser H, Cinco RM, McFarlane KL, Bellacchio E, Pizarro SA, Cramer SP, Sauer K, Klein MP, Yachandra VK. J. Am. Chem. Soc. 2001;123:7804–7820. doi: 10.1021/ja004307+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Glatzel P, Bergmann U. Coord. Chem. Rev. 2005;249:65–95. [Google Scholar]
- 24.Wang X, Randall CR, Peng G, Cramer SP. Chem. Phys. Lett. 1995;243:469–473. [Google Scholar]
- 25.O-sensitive peaks were not observed in rRaman spectra for independently prepared samples of [MnIIIH3buea(O)]𢈒 and [MnIVH3buea(O)]− that were collected under the same experimental conditions. Spectra were also collected for [MnIVH3buea(O)]− with λex = 457.9 and 488.0 nm and no O-isotope sensitive peaks were observed, indicating that the observed peaks in the rR spectra of the MnV species originated exclusively from [MnVH3buea(O)].
- 26.(a) Holland PL, Cramer CJ, Wilkinson EC, Mahapatra S, Rodgers KR, Itoh S, Taki M, Fukuzumi S, Que L, Jr, Tolman WB. J. Am. Chem. Soc. 2000;122:792–802. [Google Scholar]; (b) Wilkinson EC, Dong Y, Zang Y, Fujii H, Fraczkiewicz R, Fraczkiewicz G, Czernuszewicz RS, Que L., Jr J. Am. Chem. Soc. 1998;120:955–962. [Google Scholar]; (c) Namuswe F, Hayashi T, Jiang Y, Kasper GD, Narducci Sarjeant AA, Moënne-Loccoz P, Goldberg DP. J. Am. Chem. Soc. 2010;132:157–167. doi: 10.1021/ja904818z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.