Abstract
Background
Coronary heart disease (CHD) risk increases with age; yet lipid-lowering therapies are significantly under-utilized in patients > 65 years. The objective was to evaluate the safety and efficacy of lipid-lowering therapies in older patients treated with atorvastatin 10 mg + ezetimibe 10 mg (EZ/Atorva) vs. increasing the atorvastatin dose to 40 mg.
Methods
Patients ≥ 65 years with atherosclerotic vascular disease (LDL-C ≥ 1.81 mmol/L) or at high risk for coronary heart disease (LDL-C ≥ 2.59 mmol/L) were randomized to EZ/Atorva for 12 wk vs. uptitration to atorvastatin 20 mg for 6 wk followed by atorvastatin 40 mg for 6 wk. The percent change in LDL-C and other lipid parameters and percent patients achieving prespecified LDL-C levels were assessed after 12 wk.
Results
EZ/Atorva produced greater reductions in most lipid parameters vs. uptitration of atorvastatin in patients ≥ 75 years (n = 228), generally consistent with patients 65–74 years (n = 812). More patients achieved LDL-C targets with combination therapy vs. monotherapy in both age groups at 6 wk and in patients ≥ 75 years at 12 wk. At 12 wk, more patients ≥ 75 years achieved LDL-C targets with monotherapy vs. combination therapy. EZ/Atorva produced more favorable improvements in most lipids vs. doubling or quadrupling the atorvastatin dose in patients ≥ 75 years, generally consistent with the findings in patients 65–74 years.
Conclusions
Our results extended previous findings demonstrating that ezetimibe added to a statin provided a generally well-tolerated therapeutic option for improving the lipid profile in patients 65 to 74 years and ≥ 75 years of age.
Keywords: cholesterol absorption inhibitor, LDL-C, hypercholesterolemia, statin
1. Introduction
Coronary heart disease (CHD) risk increases with age.[1],[2] Despite this, lipid-lowering therapies are significantly under-utilized in patients > 65 years old.[3],[4] Aside from the potential for increased side effects or drug interactions due to concomitant medication use, there are limitations and a perceived lack of current evidence that statins provide benefits in this population.[5] Clinical trials designed to assess older patients are somewhat limited and data are especially limited in patients 75 years and older. Most support for statin use is derived from post hoc analyses showing that statins improve lipids and outcomes in patients > 65 years.[6]–[8] Studies designed to assess the efficacy of statins specifically in older patients, such as the PROSPER and SAGE studies, show favorable lipid results with intensive statin use in patients up to 85 years of age and support the use of statin treatment for the improvement of cardiovascular outcomes in older patients.[9],[10] There are also limited data on combination therapy in older patients.
This analysis assessed the lipid-altering efficacy and tolerability of ezetimibe added to atorvastatin 10 mg vs. uptitration of atorvastatin to 20 mg and 40 mg in subjects grouped by age (65–74 years and ≥ 75 years) with hypercholesterolemia at high risk for CHD. After 6 wk of treatment with ezetimibe plus atorvastatin 10 mg vs. uptitration to atorvastatin 20 mg followed by uptitration to atorvastatin 40 mg for an additional 6 wk of treatment, percent change from baseline in lipids, lipoproteins, and high-sensitivity C-reactive protein (hs-CRP) and the percentage of patients achieving prespecified low-density lipoprotein cholesterol (LDL-C) levels were evaluated. Tolerability was also assessed.
2. Methods
2.1. Study design
This was a subgroup analysis of a previously published, 12-week multicenter, randomized, double-blind, parallel-arm study conducted between February 2007 and October 2008 at 115 sites: 75 sites in the USA, 18 sites in Russia, 8 sites in Ukraine, 5 sites in Canada, 5 sites in Romania, and 4 sites in Poland.[11] The protocol and amendments were reviewed and approved by institutional review boards, and patients provided written informed consent prior to any study procedures being performed.
2.2. Patients
Patients included males and females ≥ 65 years with atherosclerotic vascular disease (AVD) or at high risk for CHD who had not reached LDL-C level < 1.81 mmol/L or < 2.59 mmol/L, respectively, on atorvastatin 10 mg/d. Patients were potentially eligible if they were taking atorvastatin 10 mg or 20 mg, a stable daily dose of a statin of equal or lesser potency than atorvastatin 20 mg with good compliance (≥ 80% of daily doses for 6 wk prior to visit 1), or who were naïve to lipid-lowering therapy. In addition, patients who were switched to atorvastatin 10 mg during run-in and had not reached LDL-C level < 1.81 mmol/L or < 2.59 mmol/L depending on risk status were eligible. All patients were instructed to follow a cholesterol-lowering diet throughout the study.
Patients were eligible for entry if they met the following criteria: established CHD and other AVD and LDL-C ≥ 1.81 mmol/L and ≤ 4.14 mmol/L; patients without AVD who had diabetes mellitus (type 1 or 2), or multiple risk factors and a 10-year risk for CHD > 20% (as determined by the Framingham calculation)[1],[2] and LDL-C ≥ 2.59 mmol/L and ≤ 4.92 mmol/L; triglycerides ≤ 33.96 mmol/L; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 1.5 × the upper limit of normal (ULN) with no active liver disease; creatine kinase (CK) ≤ 2 × ULN, thyroid stimulating hormone (TSH) ≥ 0.3 mcIU/mL or ≤ 5.0 mcIU/mL; and hemoglobin A1C (HbA1c) < 8.5%.
Patients were excluded if they had uncontrolled hypertension (systolic blood pressure > 160 mmHg or diastolic > 100 mmHg) or impaired renal function (creatinine ≥ 2.0 mg/d or a history of nephrotic range proteinuria), were taking lipid-lowering agents (except atorvastatin 10 or 20 mg; simvastatin 10, 20 or 40 mg; pravastatin 10, 20, or 40 mg; fluvastatin 20, 40 or 80 mg; ezetimibe 10 mg; lovastatin 10, 20 or 40 mg; or rosuvastatin 5 mg) within 6 wk or fibrates within 8 wk of screening, or were taking prescription and/or over-the-counter-drugs with potential drug interactions with statins within 6 wk of study start. Patients on maintenance therapy with psyllium or other over-the-counter lipid-lowering therapies for at least 6 wk prior to study entry were allowed into the study if they agreed to maintain the same treatment regimen throughout the study.
2.3. Treatments
During the run-in period, all patients received single-blind atorvastatin 10 mg. Patients already taking atorvastatin 10 mg received the same dose of atorvastatin for 4 wk. Switch and naïve patients received atorvastatin 10 mg for 5 wk. After the run-in period, patients were randomized to 1 of the following treatments for 6 wk: (1) ezetimibe 10 mg and atorvastatin 10 mg or (2) atorvastatin 20 mg. After the first 6 wk, patients in the ezetimibe 10 mg plus atorvastatin 10 mg group continued on the same treatment for an additional 6 wk. Patients in the atorvastatin 20 mg group were titrated to atorvastatin 40 mg for an additional 6 wk.
2.4. Efficacy endpoints
In patients grouped by age (65–74 years and ≥ 75 years), prespecified endpoints included percent change from baseline in LDL-C and the proportion of patients achieving the following LDL-C treatment targets after 6 wk and after 12 wk of treatment: (1) LDL-C < 1.81 mmol/L (all patients regardless of risk strata), (2) LDL-C < 2.59 mmol/L for high-risk patients without AVD and < 1.81 mmol/L for high-risk patients with AVD. To extend these results, post hoc analyses assessed mean change from baseline in high-density lipoprotein cholesterol (HDL-C), non-HDL-C, total cholesterol, triglycerides, apolipoprotein (Apo) B, Apo A-I, total Cholesterol/HDL-C, Apo B/Apo A-I, and non-HDL-C/HDL-C and hs-CRP in patients grouped by age after 6 wk of treatment.
All lipid determinations used were obtained through central laboratories (PPD, Highland Heights, KY, USA; and PPD at Zaventem, Belgium). Plasma cholesterol and triglyceride levels were determined using enzymatic methods. For patients with triglycerides ≤ 400 mg/dL, LDL-C measurements were calculated by the Friedewald equation.[12] For patients whose triglycerides reached > 400 mg/dL during the study, LDL-C measurement was obtained directly using the beta quantification method.[13]
2.5. Safety/Tolerability Endpoints
The safety and tolerability profiles were assessed by treatment in patients grouped by age (65–74 years and ≥ 75 years). Adverse events were summarized by system organ class and specific adverse experience term. Prespecified safety parameters included ALT and/or AST consecutive elevations ≥ 3 × ULN, CK elevations ≥ 10 × ULN, CK elevations ≥ 10 × ULN with muscle symptoms (within ±7 days of lab result), CK elevations ≥ 10 × ULN with muscle symptoms (within ± 7 days of lab result) that are considered by the investigators to be related to study drug; Hy's Law Condition based on FDA guidance on drug-induced liver injury,[14] hepatitis-related adverse events, gallbladder-related adverse events, gastrointestinal-related adverse events, and allergic reaction or rash adverse events.
2.6. Statistics
Efficacy endpoints (with the exception of triglycerides and hs-CRP) were evaluated using analysis of covariance (ANCOVA) with terms for treatment, baseline covariate (i.e., the baseline of the dependent variable being modeled), AVD status, age subgroup, and treatment by age subgroup interaction. The analysis of triglycerides was based on the nonparametric method. Normal scores were calculated using the Tukey method. The between-treatment group difference in medians was estimated based on the Hodges-Lehmann location shift, and the distribution-free 95% confidence interval for the between-treatment difference was based on Wilcoxon's rank sum test statistic. The least squares mean percent change from baseline in hs-CRP was calculated as follows: [Back-transformed value of the log(week 6 or 12/baseline)-1] x 100% where log(week 6 or 12/baseline) is the log of the ratio of the week 6 or 12 value to the baseline value estimated from the ANCOVA model with terms for treatment, baseline covariate (i.e., the baseline of the dependent variable being modeled), AVD status, age subgroup, and treatment by age subgroup interaction. All randomized patients who took at least 1 dose of study medication were included in the safety analyses. For change from baseline in vital signs or laboratory measurements, patients were required to have a baseline measurement and at least 1 on-treatment measurement to be included in these analyses.
3. Results
The flow of participants through the study was previously published.[11] Of the 2276 patients screened, 1053 were randomized, 526 were assigned to the atorvastatin 10 mg + ezetimibe group, and 527 were assigned to the atorvastatin 20/40 mg group. Twenty-three patients from the atorvastatin 10 mg + ezetimibe group and 20 patients from the atorvastatin 20/40 mg group discontinued the study. There were 812 patients aged 65–74 years and 218 patients aged ≥ 75 years enrolled in the study.
Baseline demographics and National Cholesterol Education Program (NCEP)-specified risk factors for each treatment group and by age are summarized in Table 1. Of the 1053 patients randomized, the majority were white (1008, 96%) and female (563, 53%). The mean age was about 69 years in the < 75 year age group and about 78 years in the ≥ 75 years age group. About 1/3 of the population in each treatment group and age group had BMI ≥ 30 kg/m2. Baseline characteristics and risk factors were similar between treatment groups and age groups. Baseline lipid values between treatment groups were similar in the 65–74 years age group (Table 2). In the older patients (≥ 75 years), LDL-C, Apo B and total cholesterol values were higher in the atorvastatin 10 mg plus ezetimibe group vs. the atorvastatin treatment group. In both age groups, baseline values for the other lipid parameters were similar between treatment groups.
Table 1. Baseline Demographics and Risk Factors.
| A10 + E10 |
A20/40 |
|||
| Age 65-74 n = 410 | Age ≥ 75 n = 116 | Age 65-74 n = 418 | Age ≥ 75 n = 109 | |
| Characteristic | ||||
| Female, n (%) | 216 (52.7) | 61 (52.6) | 222 (53.1) | 64 (58.7) |
| Mean age, yr (SD) | 69.2 (2.6) | 78.4 (2.9) | 69.3 (2.5) | 78.6 (3.5) |
| Race, n (%) | ||||
| White | 394 (96.1) | 109 (94.0) | 401 (95.9) | 104 (95.4) |
| Black | 14 (3.4) | 7 (6.0) | 14 (3.3) | 3 (2.8) |
| Other | 2 (0.5) | 0 | 3 (0.7) | 2 (1.8) |
| Body mass index ≥ 30 kg/m2 | 130 (31.7) | 35 (30.2) | 135 (32.3) | 30 (27.5) |
| Risk factors, n (%) | ||||
| Coronary heart disease | 327 (79.8) | 91 (78.4) | 332 (79.4) | 91 (83.5) |
| Other forms of atherosclerosis* | 110 (26.8) | 38 (32.8) | 111 (26.6) | 30 (27.5) |
| Diabetes mellitus | 82 (20.0) | 28 (24.1) | 91 (21.8) | 22 (20.2) |
| Metabolic syndrome† | 208 (50.7) | 60 (51.7) | 222 (53.1) | 53 (48.6) |
| Visit 2 low-density lipoprotein cholesterol strata | ||||
| ≥1.81 & <2.59 mmol/L | 189 (46.1) | 55 (47.4) | 186 (44.5) | 58 (53.2) |
| ≥2.59 & <3.37 mmol/L | 161 (39.3) | 47 (40.5) | 167 (40.0) | 42 (38.5) |
| ≥3.37 mmol/L | 60 (14.6) | 14 (12.1) | 65 (15.6) | 9 (8.3) |
| AVD | 357 (87.1) | 99 (85.3) | 364 (87.1) | 93 (85.3) |
A: atorvastatin; E: ezetimibe 10 mg; AVD: atherosclerotic vascular disease. *Other forms of atherosclerosis are peripheral arterial disease, abdominal aortic aneurysm, symptomatic carotid artery disease, transient ischemic attack, and stroke. †Defined as at least 3 of the 5 following characteristics: Waist circumference ≥ 102 cm (males) or ≥88 cm (females); Triglycerides ≥ 1.7 mmol/dL; HDL-C < 1.04 mmol/L (males) or < 1.30 mmol/L (females); Blood pressure ≥ 130/85 mmHg or on antihypertensive medication or diagnosis of hypertension based on medical history; Fasting glucose ≥ 2.59 mmol/L or on drug therapy for elevated glucose.
Table 2. Baseline Values and Change from Baseline in Lipids, Lipid Ratios, and hs-CRP.
| Week 6 |
||||||||||
| Age 65-74 | Age ≥ 75 | |||||||||
| Parameter | A10 + E | A20 | Treatment difference* | A10 + E | A20 | Treatment difference* | ||||
| LDL-C (mmol/L) | n = 404 | n = 408 | n = 111 | n = 107 | ||||||
| Baseline (SD) | 2.63 (0.56) | 2.66 (0.61) | 2.78 (1.12) | 2.50 (0.48) | ||||||
| LS mean % change | −26.1 | −12.5 | −13.6 | −28.4 | −14.0 | −14.5 | ||||
| (95% CI) | (−28.3, −24.0) | (−14.6, −10.4) | (−16.0, −11.2) | (−31.9, −25.0) | (−17.5, −10.5) | (−19.1, −9.8) | ||||
| Apo B (g/L) | n = 397 | n = 407 | n = 110 | n = 107 | ||||||
| Baseline (SD) | 1.03 (.23) | 1.02 (.22) | 1.06 (.22) | 1.00 (.18) | ||||||
| LS mean % change | −16.2 | −7.6 | −8.6 | −19.0 | −8.0 | −11.0 | ||||
| (95% CI) | (−18.0, −14.4) | (−9.4, −5.8) | (−10.7, −6.6) | (−21.9, −16.0) | (−10.9, −5.0) | (−15.0, −7.0) | ||||
| Total C (mmol/L) | n = 404 | n = 409 | n = 111 | n = 107 | ||||||
| Baseline (SD) | 4.70 (0.71) | 4.74 (0.69) | 4.89 (1.16) | 4.55 (0.61) | ||||||
| LS mean % change | −15.5 | −7.7 | −7.8 | −17.4 | −9.0 | −8.4 | ||||
| (95% CI) | (−16.9, −14.1) | (−9.1, −6.3) | (−9.4, −6.2) | (−19.7, −15.1) | (−11.3, −6.7) | (−11.5, −5.3) | ||||
| non-HDL-C (mmol/L) | n = 404 | n = 408 | n = 111 | n = 107 | ||||||
| Baseline (SD) | 3.28 (0.66) | 3.32 (0.67) | 3.46 (1.17) | 3.16 (0.56) | ||||||
| LS mean % change | −22.9 | −11.0 | −11.9 | −25.6 | −12.1 | −13.5 | ||||
| (95% CI) | (−24.8, −20.9) | (−12.9, −9.1) | (−14.0, −9.7) | (−28.7, −22.4) | (−15.3, −8.9) | (−17.7, −9.3) | ||||
| Triglycerides (mmol/L) | n = 404 | n = 409 | n = 111 | n = 107 | ||||||
| Baseline (Robust SD)† | 1.25 (0.61) | 1.30 (0.67) | 1.36 (0.56) | 1.32 (0.67) | ||||||
| Median % change | −12.7 | −5.5 | −5.7 | −14.1 | −7.9 | −7.6 | ||||
| (95% CI) | (−15.5, −10.0) | (−8.1, −2.8) | (−8.9, −2.4) | (−18.3, −9.9) | (−12.7, −3.0) | (−13.3, −2.0) | ||||
| Total C/HDL-C ratio | n = 404 | n = 408 | n = 111 | n = 107 | ||||||
| Baseline (SD) | 3.5 (0.8) | 3.5 (0.8) | 3.6 (1.3) | 3.4 (0.7) | ||||||
| LS mean % change | −16.2 | −7.9 | −8.4 | −19.0 | −7.8 | −11.3 | ||||
| (95% CI) | (−17.9, −14.6) | (−9.5, −6.2) | (−10.3, −6.5) | (−21.8, −16.3) | (−10.5, −5.0) | (−14.9, −7.6) | ||||
| HDL-C (mmol/L) | n = 404 | n = 408 | n = 111 | n = 107 | ||||||
| Baseline (SD) | 1.42 (0.35) | 1.42 (0.33) | 1.43 (0.37) | 1.39 (0.29) | ||||||
| LS mean % change | 2.2 | 1.0 | 1.3 | 3.6 | −0.6 | 4.2 | ||||
| (95% CI) | (0.7, 3.8) | (−0.6, 2.5) | (−0.5, 3.1) | (1.0, 6.2) | (−3.2, 2.1) | (0.7, 7.6) | ||||
| Apo A-I (g/L) | n = 397 | n = 407 | n = 110 | n = 107 | ||||||
| Baseline (SD) | 1.65 (0.29) | 1.65 (0.27) | 1.65 (0.30) | 1.64 (0.26) | ||||||
| LS mean % change | −1.2 | −1.6 | 0.4 | −0.5 | −1.9 | 1.4 | ||||
| (95% CI) | (−2.7, 0.3) | (−3.1, −0.2) | (−1.3, 2.1) | (−2.9, 1.9) | (−4.3, 0.5) | (−1.8, 4.7) | ||||
| hs-CRP (mg/L) | n = 388 | n = 396 | n = 109 | n = 104 | ||||||
| Baseline‡ | 1.9 | 1.9 | 2.1 | 1.9 | ||||||
| LS mean % change | −9.4 | −3.7 | 5.7 | 3.0 | −5.7 | −8.7 | ||||
| (95% CI) | (−18.1, 0.2) | (−12.8, 6.4) | (−5.1, 16.6) | (−12.6, 21.5) | (−20.3, 11.6) | (−30.7, 13.3) | ||||
| Week 12 |
||||||||||
| Age 65-74 | Age ≥ 75 | |||||||||
| Parameter | A10 + E | A20/40 | Treatment difference§ | A10 + E | A20/40 | Treatmentdifference§ | ||||
| LDL-C (mmol/L) | n = 405 | n = 403 | n = 111 | n = 106 | ||||||
| Baseline (SD) | 2.63 (0.56) | 2.67 (0.54) | 2.78 (1.12) | 2.51 (0.47) | ||||||
| LS mean % change | −23.0 | −17.3 | −5.7 | −20.6 | −20.2 | −0.4 | ||||
| (95% CI) | (−25.7, −20.2) | (−20.0, −14.5) | (−8.8, −2.6) | (−25.1, −16.0) | (−24.8, −15.7) | (−6.5, 5.7) | ||||
| Apo B (g/L) | n = 400 | n = 400 | n = 111 | n = 106 | ||||||
| Baseline (SD) | 1.03 (0.23) | 1.03 (0.22) | 1.07 (0.22) | 1.00 (0.18) | ||||||
| LS mean % change | −13.7 | −10.4 | −3.3 | −14.8 | −12.0 | −2.8 | ||||
| (95% CI) | (−16.0, −11.4) | (−12.7, −8.2) | (−5.9, −0.7) | (−18.5, −11.1) | (−15.8, −8.3) | (−7.7, 2.2) | ||||
| Total C (mmol/L) | n = 405 | n = 403 | n = 111 | n = 106 | ||||||
| Baseline (SD) | 4.70 (.0.71) | 4.75 (0.68) | 4.89 (1.16) | 4.55 (0.61) | ||||||
| LS mean % change | −13.5 | −11.3 | −2.3 | −13.6 | −12.7 | −0.8 | ||||
| (95% CI) | (−15.3, −11.8) | (−13.0, −9.5) | (−4.3, −0.3) | (−16.5, −10.7) | (−15.7, −9.8) | (−4.7, 3.1) | ||||
| non-HDL-C (mmol/L) | n = 405 | n = 403 | n = 111 | n = 106 | ||||||
| Baseline (SD) | 3.28 (0.66) | 3.33 (0.67) | 3.46 (1.17) | 3.16 (0.56) | ||||||
| LS mean % change | −20.1 | −15.5 | −4.6 | −19.7 | −17.0 | −2.7 | ||||
| (95% CI) | (−22.6, −17.6) | (−18.0, −12.9) | (−7.5, −1.8) | (−23.8, −15.5) | (−21.1, −12.8) | (−8.3, 2.8) | ||||
| Triglycerides (mmol/L) | n = 405 | n = 403 | n = 111 | n = 106 | ||||||
| Baseline (Robust SD)† | 1.25 (0.61) | 1.31 (0.67) | 1.36 (0.56) | 1.32 (0.67) | ||||||
| Median % change | −11.0 | −10.4 | 0.4 | −14.0 | −4.4 | −11.0 | ||||
| (95% CI) | (−14.2, −7.8) | (−13.5, −7.4) | (−3.1, 3.9) | (−18.8, −9.1) | (−9.6, 0.8) | (−17.5, −4.8) | ||||
| Total C/HDL-C ratio | n = 405 | n = 403 | n = 111 | n = 106 | ||||||
| Baseline (SD) | 3.5 (0.8) | 3.5 (0.8) | 3.6 (1.3) | 3.4 (0.7) | ||||||
| LS mean % change | −14.5 | −9.6 | −4.9 | −14.2 | −10.8 | −3.5 | ||||
| (95% CI) | (−16.6, −12.3) | (−11.7, −7.4) | (−7.3, −2.5) | (−17.7, −10.8) | (−14.3, −7.3) | (−8.1, 1.2) | ||||
| HDL-C (mmol/L) | n = 405 | n = 403 | n = 111 | n = 106 | ||||||
| Baseline (SD) | 1.42 (0.35) | 1.41 (0.33) | 1.43 (0.37) | 1.39 (0.29) | ||||||
| LS mean % change | 2.4 | −0.6 | 3.0 | 2.4 | −1.4 | 3.8 | ||||
| (95% CI) | (0.8, 3.9) | (−2.2, 1.0) | (1.1, 4.8) | (−0.2, 5.0) | (−4.1, 1.3) | (0.2, 7.3) | ||||
| Apo A-I (g/L) | n = 400 | n = 400 | n = 111 | n = 106 | ||||||
| Baseline (SD) | 1.64 (0.29) | 1.64 (0.27) | 1.65 (0.30) | 1.63 (0.26) | ||||||
| LS mean % change | 0.8 | −1.8 | 2.6 | 0.2 | −2.6 | 2.8 | ||||
| (95% CI) | (−0.7, 2.3) | (−3.3, −0.3) | (0.9, 4.4) | (−2.2, 2.7) | (−5.1, −0.1) | (−0.5, 6.2) | ||||
| hs-CRP (mg/L) | n = 396 | n = 394 | n = 110 | n = 106 | ||||||
| Baseline‡ | 1.9 | 1.9 | 2.1 | 1.9 | ||||||
| LS mean % change | −19.7 | −13.4 | 6.3 | −23.2 | −17.8 | 5.4 | ||||
| (95% CI) | (−27.3, −11.3) | (−21.6, −4.3) | (−3.3, 15.9) | (−34.7, −9.6) | (−30.4, −3.0) | (−12.1, 22.9) | ||||
A: atorvastatin; E: ezetimibe 10 mg; LS: least squares; n: number of patients in full analysis set population; LDL-C:low-density lipoprotein cholesterol; Apo B: apolipoprotein B; HDL-C: high-density lipoprotein cholesterol; hs-CRP: high-sensitivity C-reactive protein. *Between treatment difference at week 6 (A10+EZ minus A20). †Robust SD is interquartile range (IQR)/1.075 where IQR = 3rd quartile minus 1st quartile. ‡Baseline is geometric mean back-transformed from log of the value. §Between treatment difference after additional 6 wk of treatment and uptitration to A 40 mg (A10+EZ minus A20/40).
The within and between-treatment estimates of mean percent change from baseline in LDL-C are summarized in Table 2. In both age groups, the addition of ezetimibe to ongoing atorvastatin 10 mg therapy resulted in a greater reduction from baseline in LDL-C levels compared with uptitration to atorvastatin 20 mg after 6 wk of treatment (65–74 years: −26.1% vs. −12.5% and ≥ 75 years: −28.4% vs. −14.0%; Figure 1) and compared with uptitration to atorvastatin 40 mg for an additional 6 wk of treatment in the 65–74 years (−23.0% vs. −17.3%). In the ≥ 75 years group, the percent change in LDL-C was similar between treatment groups (−20.6% vs. −20.2%; Figure 1). In both age groups, there was a greater reduction in LDL-C in the ezetimibe add-on to atorvastatin 10 mg groups vs. uptitration to atorvastatin 20 mg groups. The magnitude of difference was generally similar for both age groups at week 6 (Table 2). Compared with the first 6 wk reduction (i.e., treatment with atorvastatin to 20 mg for 6 wk) after uptitration to atorvastatin 40 mg for an additional 6 wk, the magnitude of between-treatment differences in lipid and lipoprotein levels were less (Table 2).
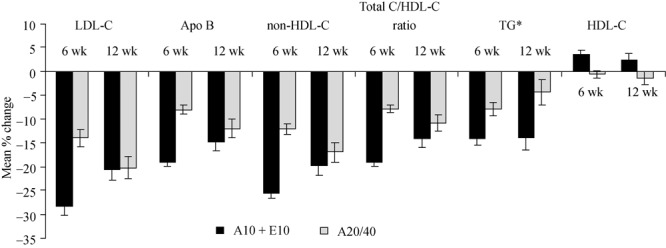
Figure 1. Change in lipid parameters. Change from baseline in lipid parameters in patients ≥ 75 years after 6 wk and 12 wk of treatment with atorvastatin 10 mg + ezetimibe (A10 + E10) vs. uptitration to atorvastatin 20 mg for 6 wk followed by uptitration to atorvastatin 40 mg for an additional 6 weeks (A20/40). *presented as median values. A: atorvastatin; E: ezetimibe; Apo: apolipoprotein; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein; TG: triglycerides.
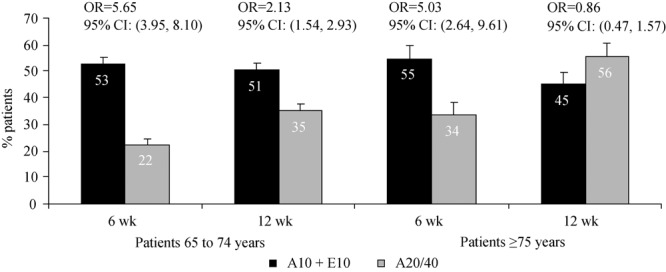
The percentage of patients reaching prespecified LDL-C targets at week 6 and 12 is illustrated in Figure 2. At week 6, the proportion of patients achieving LDL-C < 2.59 mmol/L (without AVD) or < 1.81 mmol/L (with AVD) was greater in the atorvastatin 10 mg + ezetimibe treatment group vs. the atorvastatin 20 mg treatment group in both age groups (Figure 2). After uptitration to atorvastatin 40 mg for an additional 6 wk (Figure 2), the proportion of patients achieving LDL-C < 2.59 mmol/L (without AVD) or < 1.81 mmol/L (with AVD) was greater in the atorvastatin 10 mg plus ezetimibe treatment group vs. the atorvastatin 20/40 mg treatment group in patients 65–74 years, but was numerically greater in the atorvastatin 20/40 mg treatment group vs. the atorvastatin 10 mg plus ezetimibe treatment group in patients ≥ 75 years.
Figure 2. Achievement of prespecified LDL-C levels. Proportion of patients achieving prespecified LDL-C levels after 6 wk of treatment with atorvastatin 10 mg + ezetimibe vs. atorvastatin 20 mg and after an additional 6 wk of treatment with atorvastatin 10 mg + ezetimibe vs. uptitration to atorvastatin to 40 mg (12 wk). OR: Odds ratio, 95% CI: 95% confidence interval.
In both age groups, greater changes in favor of atorvastatin 10 mg plus ezetimibe occurred in the majority of lipids and lipoproteins compared with uptitration to atorvastatin 20 mg with similar changes between treatments in HDL-C, Apo A-I and hs-CRP after 6 wk (Table 2). After uptitration to atorvastatin 40 mg for an additional 6 wk, greater changes in favor of atorvastatin 10 mg plus ezetimibe occurred in the majority of lipids with similar changes between treatments in triglycerides, Apo A-I, and hs-CRP in patients 65–74 years (Table 2). In patients aged ≥ 75 years, numerically greater changes were observed in favor of atorvastatin 10 mg plus ezetimibe vs. uptitration to atorvastatin 40 mg in most lipid and lipoprotein levels and hs-CRP (Table 2 and Figure 1). There was a greater reduction in triglycerides in patients treated with atorvastatin 10 mg plus ezetimibe for 12 wk vs. patients whose dose was uptitrated to atorvastatin 20/40 mg over the course of the study (Table 2 and Figure 1).
The magnitude of difference between treatments was generally similar for both age groups at week 6. Compared with the first 6 wk reduction (i.e., treatment with atorvastatin to 20 mg for 6 wk) after uptitration to atorvastatin 40 mg for an additional 6 wk, the magnitudes of between-treatment differences were less overall, although generally similar for both age groups except for LDL-C.
The overall safety and tolerability profiles for the 2 treatment regimens in this population of patients 65 years and older were previously reported.[11] Briefly, the overall incidence of adverse events of interest was similar between treatment groups. The analysis by age group indicated that the overall incidence of reported adverse events was generally similar in both treatment groups regardless of age during the course of the 12-week study (Table 3). In patients 65–74 years, the incidence of discontinuations due to adverse events was 1.5% in patients treated with atorvastatin 10 mg + ezetimibe compared with 0.7% in the atorvastatin 20 mg treatment group. In patients ≥75 years the incidence of discontinuations due to adverse events was 5.2% in patients treated with atorvastatin 10 mg + ezetimibe compared with 1.8% in the atorvastatin 20 mg treatment group. The highest incidence of prespecified adverse events was gastrointestinal-related, with a numerically higher incidence occurring in both age subgroups in the atorvastatin 10 mg + ezetimibe group vs. the atorvastatin 20/40 mg group. However, no specific pattern of gastrointestinal-related adverse events or clinically meaningful difference was seen between treatment groups. One death occurred in the atorvastatin 10 mg + ezetimibe treatment group in patients 65–74 years and was not attributed to study drug. In patients 64–75 years, there was 1 case of increased ALT (consecutive) and 1 case of increased AST (consecutive) > 3 × ULN in the atorvastatin plus ezetimibe treatment group and 1 case of increased ALT (consecutive) > 3X ULN in the atorvastatin 20 mg treatment group. There were no cases of increased ALT, AST, or CK reported in patients 75 years or older during the study.
Table 3. Adverse Events Summary for 12 wk by age subgroup (All patients as treated population).
| Atorva 10 mg + EZ, n (%) | A20/40, n (%) | |
| Age 65-74 years | n = 410 | n = 416 |
| With one or more AE | 108 (26) | 125 (30) |
| Drug related† | 22 (5) | 19 (5) |
| Serious | 12 (3) | 14 (3) |
| Serious drug related† | 1 (<1) | 0 |
| Discontinuations‡ due to AEs | 7 (2) | 6 (1) |
| Drug related† | 4 (1) | 2 (1) |
| Serious | 2 (1) | 3 (1) |
| Serious drug related† | 0 | 0 |
| Death | 2 (1) | 1 (<1) |
| Pre-specified AEs, m/n (%)§ | ||
| ALT ≥3X ULN, consecutive | 1/405 (0.2) | 3/412 (0.7) |
| AST ≥3X ULN, consecutive | 2/405 (0.5) | 5/412 (1.2) |
| ALT and/or AST ≥ 3X ULN, consecutive | 2/405 (0.5) | 5/412 (1.2) |
| CK ≥10X ULN | 0/405 (0.0) | 1/412 (0.2) |
| CK ≥10X ULN with muscle symptoms | 0/405 (0.0) | 0/412 (0.0) |
| Gastrointestinal-related | 24/410 (5.9) | 14/416 (3.4) |
| Gallbladder-related | 0/410 (0.0) | 1/416 (0.2) |
| Allergic reaction or rash | 3/410 (0.7) | 4/416 (1.0) |
| Hepatitis-related | 0/410 (0.0) | 0/416 (0.0) |
| Age ≥ 75 years | n = 116 | n = 109 |
| With one or more AE | 35 (30) | 34 (31) |
| Drug related† | 8 (7) | 7 (6) |
| Serious | 3 (3) | 0 |
| Serious drug related† | 0 | 0 |
| Discontinuations‡ due to AEs | 7 (6) | 2 (2) |
| Drug related† | 2 (2) | 1 (1) |
| Serious | 2 (2) | 0 |
| Serious drug related† | 0 | 0 |
| Death | 0 | 0 |
| Pre-specified AEs, m/n (%)§ | ||
| ALT ≥3X ULN, consecutive | 0/115 (0.0) | 0/108 (0.0) |
| AST ≥3X ULN, consecutive | 0/115 (0.0) | 0/108 (0.0) |
| ALT and/or AST ≥ 3X ULN, consecutive | 0/115 (0.0) | 0/108 (0.0) |
| CK ≥10X ULN | 0/115 (0.0) | 0/108 (0.0) |
| Gastrointestinal-related | 7/116 (6.0) | 4/109 (3.7) |
| Gallbladder-related | 1/116 (0.9) | 0/109 (0.0) |
| Allergic reaction or rash | 2/116 (1.7) | 0/109 (0.0) |
| Hepatitis-related | 0/116 (0.0) | 0/109 (0.0) |
ALT: alanine aminotransferase; CK: creatine kinase; AST: aspartate aminotransferase; ULN: upper limit of normal. †Determined by the investigator to be related to the drug. ‡Study medication withdrawn. §%=m/n × 100 = (number of patients within the AE category/number of treated patients) × 100.
4. Discussion
The present analysis showed that in patients aged 65–74 years and aged >75 years, treatment with ezetimibe added to atorvastatin 10 mg resulted in greater favorable changes in most lipids compared with the recommended starting and next higher doses of atorvastatin. In addition, more patients achieved prespecified LDL-C levels with ezetimibe added to atorvastatin 10 mg compared with uptitration to commonly used doses of atorvastatin in patients 65–74 years old.
Overall, no differences in responses between older and younger patients have been identified in previous studies with ezetimibe.[15],[16] A pooled analysis of patients grouped by age, including < 65 years, 65–74 years, and ≥ 75 years, showed that when added to statin (pooled), ezetimibe consistently lowered LDL-C by an additional 17% vs. statin monotherapy (pooled) in all age groups assessed.[17] In a separate pooled analysis of elderly patients (grouped by < 65 years vs. > 65 years and < 75 years vs. > 75 years) similar results were demonstrated: treatment with ezetimibe added to a statin resulted in greater reductions in LDL-C, ranging from −13% to −16% difference between treatments, and greater proportions of patients achieved NCEP adult treatment panel (ATP) II LDL-C targets vs. statin monotherapy regardless of age group.[18] In a sub-analysis of patients 65 years and older in the EASE study, the ezetimibe plus statin group demonstrated significantly reduced LDL-C compared with the placebo-statin group, with mean differences of −22% to −25% in both age groups (P < 0.001).[19] In all 3 analyses, adverse events were similar across age groups and treatment groups.[17]–[19]
The results of the current study along with the previously published reports support the efficacy of ezetimibe added to a statin for LDL-C lowering and achievement of NCEP ATP III recommended LDL-C targets in patients 65 years and older. In patients > 75 years, ezetimibe add-on resulted in numerically greater changes in most lipids than doubling and quadrupling the atorvastatin dose, although the magnitude of differences between the treatment groups diminished after uptitration to atorvastatin 40 mg. These results demonstrate improved LDL-C reduction with the addition of ezetimibe even when using the lowest dose of atorvastatin (10 mg) in patients older than 65 years, consistent with previous reports indicating that addition of ezetimibe to the recommended starting and next higher dose of atorvastatin is more effective than doubling the dose of atorvastatin regardless of age.[20],[21] In contrast, the proportion of patients achieving prespecified targets was greater in the atorvastatin 20/40 mg treatment group vs. the atorvastatin 10 mg plus ezetimibe treatment group in patients ≥ 75 years. In this age group, the differences in baseline LDL-C levels (2.78 mmol/L in the atorvastatin plus ezetimibe group vs. 2.50 mmo/L in the atorvastatin 20/40 mg group) may account, for this discrepancy between percent reduction in LDL-C and percent achieving the prespecified targets. Previous analyses have reported that higher baseline LDL-C is a significant negative predictor of LDL-C goal attainment, and the results of this analysis are consistent with that effect.[22],[23] Although no between-age groups comparisons were made in these analyses, the younger and older patients experienced similar lipid-altering effects at each time point with ezetimibe added to atorvastatin. Likewise, atorvastatin-treated patients experienced similar lipid-lowering effects at each time point regardless of age, albeit in most cases these changes were not as great as those treated with ezetimibe plus atorvastatin. Larger studies in this patient population that are powered to incorporate inferential statistics are warranted.
An attenuation of LDL-C lowering treatment effect was noted at the 12-week time point in patients treated with atorvastatin 10 mg + ezetimibe in both age subgroups, but particularly in the oldest patients. This finding is in contrast to most ezetimibe plus statin studies, where no attenuation over a 12-week (or longer) period is generally observed.[24]–[26] It could be a chance finding (the 95% confidence intervals just overlap) or possibly due to a waning of compliance as the study progressed (since the atorvastatin monotherapy group was uptitrated, no comparison can be made as to whether the same effect might be observed over the course of 12 wk in that group). It would be of interest to evaluate whether attenuation of treatment effect on ezetimibe plus statin in patients ≥ 75 is observed in other studies.
Safety and tolerability are important considerations in choosing the right treatment, especially in elderly patients. There have been concerns about the tolerability of statins in the elderly. There are differences in metabolism, and a potentially greater risk of myopathy may exist in older patients.[27]–[31] No difference in safety and effectiveness, and no reported clinical experience in response has been observed previously between older and younger patients with ezetimibe therapy.[15],[17],[18] Overall, comparable safety and tolerability profiles have been observed between treatments throughout 12 wk of study. In this study, there was a generally similar incidence of adverse events between treatments and by age group. No differences in clinically relevant laboratory abnormalities have been reported between patients younger than 65 and ≥ 65 being treated with atorvastatin.[31] No inferential statistics were done to show whether differences in this study were significant between treatments. However, in the primary analysis, P-values showed no statistical difference between treatment groups. Taken together, these results provide support for the tolerability of intensive lipid-altering therapy in patients ≥ 65 years, although the results must be interpreted with caution due to the short-term nature of the study.
This study was not of sufficient duration, nor was it powered to detect very rare adverse events. The benefits of the addition of ezetimibe to atorvastatin with regard to clinical outcomes have yet to be determined in any patient population.
In conclusion, this is the first study to date that specifically assesses the efficacy in lowering LDL-C and tolerability of ezetimibe added to a low dose of atorvastatin compared with higher doses of atorvastatin in patients ≥ 65 years of age at high risk for CHD. The results of this analysis provided additional support and extended previous findings that have demonstrated that ezetimibe added to a statin provides a generally well-tolerated therapeutic option for improving the lipid profile in patients 65 to 74 years and ≥ 75 years of age. Although not powered to detect initial, very rare adverse events, these results suggest that both treatments have generally similar safety and tolerability profiles in both age groups assessed.
Acknowledgments
The authors wish to thank Martha Vollmer, MA, of Merck for editorial assistance. This study was supported by Merck, Sharp & Dohme, One Merck Drive, Whitehouse Station, NJ 08889, USA.
References
- 1.Expert panel on detection evaluation and treatment of high blood cholesterol in adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.JBS 2: Joint British Societies' guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl. 5):v1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA. 2004;291:1864–1870. doi: 10.1001/jama.291.15.1864. [DOI] [PubMed] [Google Scholar]
- 4.Williams D, Bennett K, Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. Br J Clin Pharmacol. 2003;55:604–608. doi: 10.1046/j.1365-2125.2003.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaw A. The care gap: underuse of statin therapy in the elderly. Int J Clin Pract. 2004;58:777–785. doi: 10.1111/j.1368-5031.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: Results from the LIPID trial. Ann Intern Med. 2001;134:931–940. doi: 10.7326/0003-4819-134-10-200105150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med. 1998;129:681–689. doi: 10.7326/0003-4819-129-9-199811010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen TA, Pyörälä K, Olsson AG, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S) Circulation. 1997;96:4211–4218. doi: 10.1161/01.cir.96.12.4211. [DOI] [PubMed] [Google Scholar]
- 9.Deedwania P, Stone PH, Bairey Merz CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the Study Assessing Goals in the Elderly (SAGE) Circulation. 2007;115:700–707. doi: 10.1161/CIRCULATIONAHA.106.654756. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 11.Zieve F, Wenger NK, Ben-Yehuda O, et al. Safety and Efficacy of Ezetimibe Added to Atorvastatin Versus Up Titration of Atorvastatin to 40 mg in Patients > or =65 Years of Age (from the ZETia in the ELDerly [ZETELD] Study) Am J Cardiol. 2010;105:656–663. doi: 10.1016/j.amjcard.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Lipid and lipoprotein analysis, manual for laboratory operations. Volume 1. DHEW Publication; Washington: 1974. [Google Scholar]
- 14.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) Guidance for industry: Drug-induced liver injury: premarketing clinical evaluation. 2009.
- 15. Merck/Schering-Plough Pharmaceuticals. Zetia (ezetimibe) [package insert]. 2008; USA.
- 16. Merck/Schering-Plough Pharmaceuticals. Vytorin (ezetimibe/simvastatin) [package insert]. 2009; USA.
- 17.Robinson JG, Davidson M, Shah A, et al. Efficacy and safety of ezetimibe and ezetimibe plus statin therapy in patients aged under 65, 65–74 and 75 years and older. Aging Health. 2007;3:691–705. [Google Scholar]
- 18.Lipka L, Sager P, Strony J, et al. Efficacy and safety of coadministration of ezetimibe and statins in elderly patients with primary hypercholesterolaemia. Drugs Aging. 2004;21:1025–1032. doi: 10.2165/00002512-200421150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Pearson T, Denke M, McBride P, et al. Effectiveness of the addition of ezetimibe to ongoing statin therapy in modifying lipid profiles and attaining low-density lipoprotein cholesterol goals in older and elderly patients: subanalyses of data from a randomized, double-blind, placebo-controlled trial. Am J Geriatr Pharmacother. 2005;3:218–228. [PubMed] [Google Scholar]
- 20.Conard SE, Bays HE, Leiter LA, et al. Efficacy and safety of ezetimibe added on to atorvastatin (20 mg) versus uptitration of atorvastatin (to 40 mg) in hypercholesterolemic patients at moderately high risk for coronary heart disease. Am J Cardiol. 2008;102:1489–1494. doi: 10.1016/j.amjcard.2008.09.075. [DOI] [PubMed] [Google Scholar]
- 21.Leiter LA, Bays H, Conard S, et al. Efficacy and safety of ezetimibe added on to atorvastatin (40 mg) compared with uptitration of atorvastatin (to 80 mg) in hypercholesterolemic patients at high risk of coronary heart disease. Am J Cardiol. 2008;102:1495–1501. doi: 10.1016/j.amjcard.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 22.Lee KK, Lee VW, Chan WK, et al. Cholesterol goal attainment in patients with coronary heart disease and elevated coronary risk: results of the Hong Kong hospital audit study. Value Health. 2008;11(Suppl 1):S91–S98. doi: 10.1111/j.1524-4733.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 23.Schultz JS, O'Donnell JC, McDonough KL, et al. Determinants of compliance with statin therapy and low-density lipoprotein cholesterol goal attainment in a managed care population. Am J Manag Care. 2005;11:306–312. [PubMed] [Google Scholar]
- 24.Reckless JP, Henry P, Pomykaj T, et al. Lipid-altering efficacy of ezetimibe/simvastatin 10/40 mg compared with doubling the statin dose in patients admitted to the hospital for a recent coronary event: the INFORCE study. Int J Clin Pract. 2008;62:539–554. doi: 10.1111/j.1742-1241.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 25.Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 26.Davidson MH, Ballantyne CM, Kerzner B, et al. Efficacy and safety of ezetimibe coadministered with statins: randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemia. Int J Clin Pract. 2004;58:746–755. doi: 10.1111/j.1368-5031.2004.00289.x. [DOI] [PubMed] [Google Scholar]
- 27. Novartis. Lescol (fluvastatin sodium) capsules [package insert]. 2006; USA.
- 28. Bristol-Myers Squibb. Pravachol (pravastatin sodium) tablets [package insert]. 2007; USA.
- 29. Merck & Co I. Mevacor tablets (lovastatin) [package insert]. 2002; USA.
- 30. AstraZeneca. Crestor (rosuvastatin calcium) tablets [package insert]. 2007; USA.
- 31. Parke-Davis. Lipitor (atorvastatin calcium) tablets [package insert]. 2007; USA.


