Abstract
Background
Heart failure (HF) is an increasing problem for the aging population, specifically among women. The etiology of HF influences both the selection and outcome of the treatment. There are variations between genders in morbidity and mortality in different studies, possibly reflecting etiology. The objective of this study was to examine the strength of evidence available for gender differences in the etiology of chronic heart failure.
Methods
Computer-assisted searches from 1980–2009 for gender differences in the etiology of heart failure were performed (Medline, EMBASE and PubMed). From 2347 abstracts reviewed based on inclusion criteria, 35 original articles were chosen for review. Data extraction was based on observational studies (prospective/retrospective cohort or cross sectional) with a mean follow up of 3 months. There was no interrater variability between the 2 reviewers on data-extraction.
Results
Ventricular systolic dysfunction being more associated with male sex, but female sex was more reported to be associated with preserved left ventricular function. Ischemic etiology and associated coronary heart disease were strongly correlated with male sex. The risk for HF was dramatically more elevated for women with systolic hypertension but the association for diabetes mellitus as the etiology of HF was somewhat equal between males and females.
Conclusions
One of the limitations in reaching conclusions about gender differences in cardiovascular disease is that many major clinical trials do not include a gender analysis nor they are powered to do so as women are under-represented in most of the HF studies. The need remains for a well designed prospective study of sufficient numbers of male and female patients with and without heart failure and analyzing etiology and risk factors based on the sex differences.
Keywords: heart failure, gender differences, etiology
1. Introduction
Heart failure (HF) is an increasing problem for the aging population, specifically among older women. Individuals with HF experience debilitating symptoms and functional limitations. Based on recent USA statistics, the prevalence of HF among older women (> 75 years) is 10.9% and in men of the same age group is 9.8%.[1] Overall prevalence rates of 1%–7% have been reported in Europe, Australia, USA and Canada.[2]
Following the establishment of a clinical diagnosis, the etiology of HF influences both the selection of treatment and outcome. The associated risk factors for developing HF can influence mortality and morbidity, and contribute to the differences in clinical outcomes between genders.[3],[4] For instance, myocardial infarction (MI) as an etiologic factor for HF is detrimental for both genders. However in women within six years of acute MI, 46% of women are disabled because of HF, as compared to a 22% rate of disability in men.[1] Estrogen affects collagen synthesis and degradation, inhibits the renin-angiotensin system and the loss of its protective mechanisms may render the heart of postmenopausal women more vulnerable. Other gender differences in underlying mechanisms identified include calcium handling, the NO system, and natriuretic peptides.[5]
One of the limitations in reaching any conclusions about gender differences in the etiology of HF is that many major clinical studies do not include a gender analysis of factors involved with the development of HF. Women are only represented at about 21% of heart failure intervention trials[6] and data on patients over the age of 80 years is very limited. Knowledge on relevant gender specific risk factors for HF can assist with appropriate targeted preventative interventions, diagnosis and therapeutics for each gender.
The objective of this study was to examine the strength of evidence available for gender differences in the etiology of chronic heart failure—specifically focusing on coronary artery disease, hypertension, diabetes mellitus and left ventricular (LV) dysfunction.
2. Method
Computer-assisted searches for gender differences in the primary etiology of heart failure were performed, beginning with Medline (1996–2009), followed by EMBASE (1980–2009) and PubMed. The search terms used for Medline, and adapted for use in other databases, were #1 Heart Failure, Congestive; #2 Heart Failure, Congestive/et (Etiology); #3 Gender; #4 (#1 and #3); #5 (#2 and #3).
In addition, references from review articles were hand searched for further relevant articles. No language limitations were applied. The electronic database searches yielded 2347 abstracts. Inclusion criteria for review were studies with explicit definition for HF and provided gender-based original data in etiology and risk factors in developing heart failure. All article abstracts identified through our searches were assessed by two independent reviewers. An over inclusive list from the articles based on abstracts reviewed were contrasted and discrepancies were discussed. The protocol permitted a 3rd party reviewer if agreement could not be reached. Independent data extraction was performed only from the original articles and in the areas of disagreement, reviewers discussed and reached consensus. Data extraction was based on observational studies (prospective/retrospective cohort or cross sectional) with a mean follow up of 3 months on human adults. Pilot studies were excluded. An analysis and summary for each etiology was independently collated and then critically appraised by the research team members.
The process of article selection based on PRISMA guidelines[7] is presented in Figure 1.
Figure 1. Process of article selection based on PRISMA guidelines.
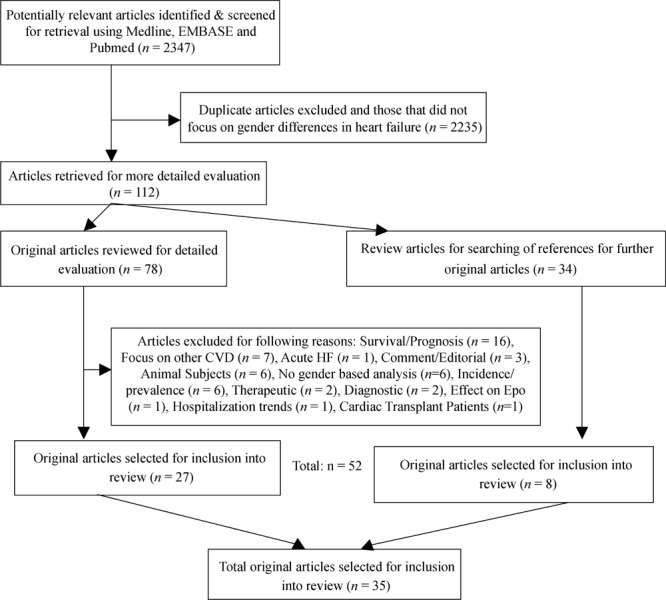
3. Result
From 2347 abstracts reviewed that met inclusion criteria, 35 original articles that provided data for both genders were chosen for detailed analysis. The baseline characteristics of included studies (10 prospective, 15 retrospective, 8 cross-sectional and 2 baseline data from clinical trials) published during 1996–2009 relevant to the inclusion criteria are presented in Table 1.
Table 1. Baseline characteristics of included studies.
| Study | Year of Publication | Method | Subjects Analyzed | Male (%) | Female (%) | Average Age | Ethnicity | Follow Up |
| Ghali, et al[8] | 2003 | 1 | 2708 | 2115 (78) | 593 (22) | 58.8 ± 14.1 | 30% Blacks | |
| Deswal and Bozkurt[9] | 2006 | 1 | 719 | 378 (52) | 341 (48) | 68.5 | 85% White | |
| Adams, et al[10] | 1996 | 2 | 557 | 380 (68) | 177 (32) | 51 ± 14 | 28.8 mo | |
| Rywik, et al[11] | 2000 | 2 | 10 579 | 3901 (37) | 6678 (63) | 75 ± 10 | 5 mo | |
| Crabbe, et al[12] | 2003 | 2 | 100 | 72 (72) | 28 (28) | 53 ± 3 | 38 mo | |
| Klapholz, et al[13] | 2004 | 2 | 619 | 170 (27) | 449 (73) | 71.7 ± 14.1 | 30% Black | 30 mo |
| Gottdiener, et al[14] | 2000 | 2 | 5625 | 2368 (42) | 3257 (58) | 72.8 ± 5.2 | 66 mo | |
| He, et al[15] | 2001 | 2 | 13 643 | 5545 (40) | 8098 (60) | 50 ± 17 | 14% Blacks | 228 mo |
| Cowie, et al[16] | 1999 | 2 | 220 | 118 (54) | 102 (46) | 76 | 20 mo | |
| Kenchaiah, et al[17] | 2002 | 2 | 5881 | 2704 (46) | 3177 (54) | 56 ± 15 | MajorityWhite | 168 mo |
| Lloyd-Jones, et al[18] | 2002 | 2 | 8229 | 3757 (46) | 4472 (54) | 59 ± 25 | MajorityWhite | 1971–1996 |
| Levy, et al[19] | 1996 | 2 | 5143 | 2334 (45) | 2809 (55) | 59 ± 15 | MajorityWhite | Mean followup 14 yr |
| Hussey and Hardin[20] | 2005 | 3 | 206 | 128 (62) | 78 (38) | 70.4 ± 12.9 | 21% Blacks | Unclear |
| Samuel, et al[21] | 1999 | 3 | 695 | 336 (48) | 359 (52) | 70.2 ± 13.0 | 10 yr | |
| Mendes, et al[22] | 1997 | 3 | 1667 | 1081 (65) | 586 (35) | 61.5 ± 1.5 | 24 mo | |
| Agvall and Dahlstrom[23] | 2001 | 3 | 256 | 148 (58) | 108 (42) | 78 | Unclear | |
| Varela-Roman, et al[24] | 2005 | 3 | 1252 | 767 (61) | 485 (39) | 69.4 ± 11.7 | 144 mo | |
| Peyster, et al[25] | 2004 | 3 | 247 | 98 (40) | 149 (60) | 76.3 | Majoritynon-White | Unclear |
| Lee, et al[26] | 2004 | 3 | 1591 | 839 (53) | 752 (47) | 72 ± 13 | 12 mo | |
| Sheppard, et al[27] | 2005 | 3 | 32 639 | 16 017 (49) | 16 622 (51) | 75.5 ± 13.5 | 48 mo | |
| Martinez-Selles, et al[28] | 2003 | 3 | 1065 | 443 (41) | 622 (59) | 73 ± 14.4 | median19 mo | |
| Hellermann, et al[29] | 2003 | 3 | 395 | 159 (40) | 236 (60) | 70 ± 13 | 1979–1994 | |
| Vaccarino, et al[30] | 1999 | 3 | 2445 | 1019(42) | 1426 (58) | 78 ± 1.5 | 12 mo | |
| Mejhert, et al[31] | 1999 | 3 | 379 | 187(49) | 192 (51) | 79.5 ± 1.5 | 6 mo | |
| Lenzen, et al[32] | 2004 | 3 | 6806 | 4016 (59) | 2791 (41) | 68.5 ± 14.5 | 14 mo | |
| Gustafsson, et al[33] | 2004 | 3 | 5491 | 3285 (59) | 2206 (41) | 69.5 ± 17.5 | 60–96 mo | |
| Bener, et al[34] | 2005 | 3 | 3617 | 2411 (67) | 1206 (33) | 59 ± 16 | Qatari & Asian | 120 mo |
| Masoudi, et al[35] | 2003 | 4 | 19 710 | 8475 (43) | 11 235 (57) | 78.7 ± 7.5 | ||
| Dunlap, et al[36] | 1999 | 4 | 680 | 469 (70) | 211 (30) | 51 ± 0.5 | 44% Blacks | |
| Aronow, et al[17] | 1998 | 4 | 572 | 177 (31) | 395 (69) | 82 ± 8 | ||
| Ahmed, et al[38] | 2003 | 4 | 394 | 171 (43) | 223 (57) | 78 ± 7 | ||
| Oyati, et al[39] | 2004 | 4 | 95 | 60 (63) | 35 (37) | 54 ± 11.15 | Nigerians | |
| Mosterd, et al[40] | 1999 | 4 | 5540 | 2251 (40) | 3289 (60) | 68.9 ± 8.7 | ||
| Abhayaratna, et al[41] | 2006 | 4 | 1275 | 638 (50) | 637 (50) | 69.4 | ||
| O'Mahony et a[42] | 2003 | 4 | 351 | 126 (36) | 126 (64) | 77.9 |
Method: 1= baseline data from clinical trials, 2= prospective, 3 = retrospective, 4 = cross section
The baseline characteristics of 2 clinical trials—Best and DIG trials[8],[9]—included in the analysis had a total of 3427 subjects with an age range of 45–73 years old and 27.2% being female.
In the 10 prospective studies[10]-[19], the total number of subjects reported was 13863. Female representation was 54% in these studies with a range of 40%-68%; and the age range were 35–90 years old. The follow up duration in these studies were reported from 5 months to 25 years.
In the 15 retrospective studies[20]-[34] the total number of subjects analysed was 32 845. Female representation was 52.5% with a range of 33%–72%; and the age range were 41–81 years old. The study period were reported from 5 months to 15 years. In three of the retrospective studies[20],[23],[25] the precise duration of follow up was not specified.
In the 8 cross sectional studies[35]–[42] included the total number of subjects analyzed was 19805; with 45.5% female population in these studies ranging from 22% to 69%. The age range was from 43 years old to 90 years old.
Quality of included studies and their potential biases is reported in table 2. Sex-based differences reported by these studies on risk factors [hypertension (HTN), coronary heart diseasel (CHD), diabetes mellitus (DM)] and LV function are presented in Figures 2–5.
Table 2. Methodological characteristics of studies.
| Study | Are study participants well defined and inclusion criteria explicit? | Were losses to follow up mentioned? | Were clear definitions used to deter-mine presence of HF? | Were clear definitions used to determine the presence of risk factors? | Are outcomes measured in a standard and reliable way for majority of patients (>75%)? | |
| Ghali, et al[8] | yes | yes | yes | yes | ||
| Deswal and Bozkurt[9] | yes | yes | ||||
| Adams, et al[10] | yes | yes | yes | yes | yes | |
| Rywik, et al[11] | yes | ? | yes | |||
| Crabbe, et al[12] | yes | yes | ||||
| Klapholz, et al[13] | yes | yes | yes | |||
| Gottdiener, et al[14] | Yes | ? | yes | yes | ||
| He, et al[15] | yes | yes | yes | yes | yes | |
| Cowie, et al[16] | yes | yes | yes | yes | ||
| Kenchaiah, et al[17] | yes | yes | yes | yes | ||
| Lloyd-Jones, et al[18] | yes | ? | yes | yes | yes | |
| Levy, et al[19] | yes | ? | yes | yes | yes | |
| Hussey and Hardin[20] | yes | ? | Yes | |||
| Samuel, et al[21] | yes | ? | yes | |||
| Mendes, et al[22] | yes | yes | yes | yes | ||
| Agvall and Dahlstrom[23] | yes | yes | yes | |||
| Varela-Roman, et al[24] | yes | yes | yes | yes | ||
| Peyster, et al[25] | yes | yes | yes | |||
| Lee, et al[26] | yes | yes | yes | yes | yes | |
| Sheppard, et al[27] | yes | yes | yes | yes | ||
| Martínez-Sellés M[28] | yes | yes | yes | yes | yes | |
| Hellermann, et al[29] | yes | yes | yes | |||
| Vaccarino, et al[30] | yes | yes | ||||
| Mejhert, et al[31] | yes | yes | yes | |||
| Lenzen, et al[32] | yes | yes | ||||
| Gustafsson, et al[33] | yes | yes | yes | yes | yes | |
| Bener, et al[34] | yes | yes | yes | |||
| Masoudi, et al[35] | yes | yes | ||||
| Dunlap, et al[36] | yes | ? | yes | yes | yes | |
| Aronow, et al[37] | yes | yes | yes | yes | ||
| Ahmed, et al[38] | yes | yes | ||||
| Oyati, et al[39] | yes | yes | yes | yes | ||
| Mosterd, et al[40] | yes | yes | yes | yes | ||
| Abhayaratna, et al[41] | yes | yes | yes | |||
| O'Mahony et al[42] | yes | yes | Yes | yes | ||
Figure 2. Heart failure subjects with preserved left ventricular.
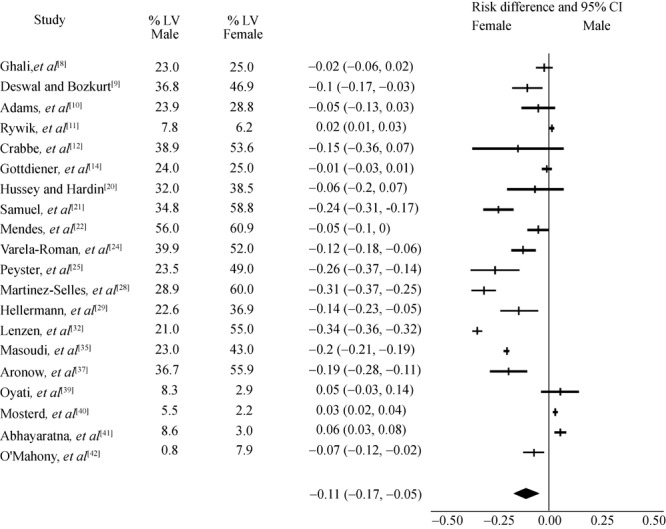
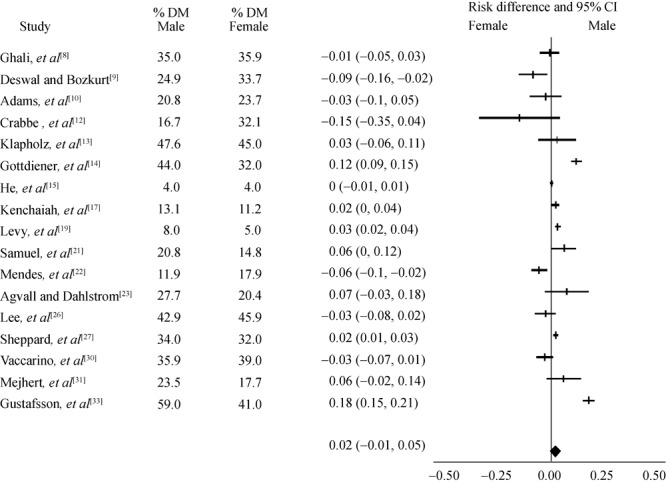
Figure 5. Heart failure subjects with diabetes mellitus (DM).
Twenty of the included studies reported LV function measurement by echocardiogram for risk analysis. Figure 2 demonstrate differences between male and female with regards to systolic dysfunction (lower ejection fraction) being more associated with male sex, but female sex were more reported to be associated with preserved LV function, although there were varied levels of LV measurements reported in these studies ranging from 20% to 87%. Rywik et al.[11] reported less than 20% of their subjects had echocardiogram and did not show the same correlation. Gottdiener et al.[14] based on 2D echocardiography of only 553 patients found slightly more women than men with preserved systolic function (95% vs. 88%). In a small study on 95 hypertensive Nigerians with HF, it was found women more likely to have combined diastolic and systolic LV dysfunction.[26] In another cross sectional echocardiography survey in the Euro study, of patients who had clinical HF, 77% of women had preserved LV systolic function, compared to 52% of men.[31] Only one study (baseline data from DIG trial) with 85% white population reported ischemic etiology in HF with preserved systolic function being more prevalent in men.[12]
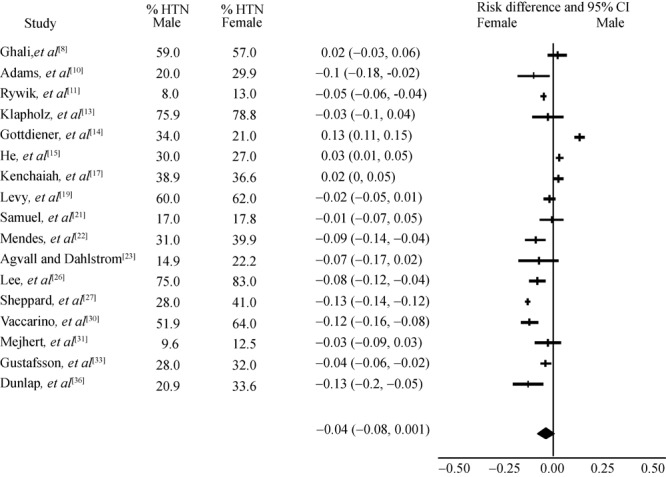
Figure 3 demonstrates risk differences for hypertension (HTN) between male and female. In eight studies analysed (two prospective, five retrospective, one cross sectional), etiology of HF was more likely to be HTN for females than in males. In contrast, the prospective Cardiovascular Health Study reported HF incidence rates for those with HTN being 21.2% for females and 33.6% for males.[22]
Figure 3. Heart failure subjects with hypertension.
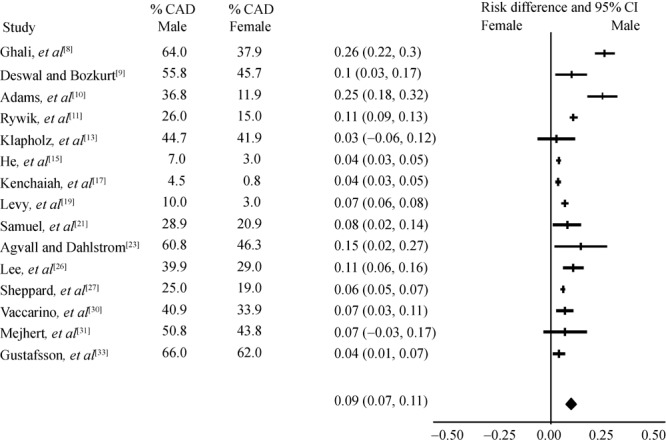
Ischemic etiology among 15 studies analysed (6 prospective, 7 retrospective, 2 baseline data from clinical trials) showed associated coronary heart disease were strongly correlated with male gender, Figure 4. The association for DM as the etiology of HF among 17 studies analysed was somewhat equal between males and females, Figure 5. Among other etiologies reported in these studies, valvular heart disease, high BMI, and idiopathic cardiomyopathy were mainly observed in women. Alcohol related cardiomyopathy and atrial fibrillation associated HF were more often seen in men.
Figure 4. Heart failure subjects with coronary heart disease (CND).
4. Discussion
The prevalence and incidence of HF are increasing in Western countries, particularly in individuals older than 80 years of age.[43] The life time risk for developing HF according to data from Framingham Heart Study (FHS), independent of gender, is one in five for those less than 40 years old. In the same cohort, at the age of 40, the lifetime risk of HF without myocardial infarction is 1 in 9 in men and 1 in 6 for women.[18] Over the past 20 years in this mainly white cohort, incidence of HF was rising faster in women than in men (9% vs. 6%).
Information on gender differences in heart failure outcomes is limited and not consistent. Among epidemiologic studies, women appear to have better survival rates than men. In FHS, the one year mortality rate was 36% in women compared with 43% in men (HR 0.64; 95% CI 0.54–0.77).[44] In contrast, women in the Studies of Left Ventricular Dysfunction (SOLVD) registry had a higher one year mortality rate (22%) than men (17%).[30] This difference has been attributed to hypertension being the most common etiology for HF in women, whereas myocardial infarction is the most common factor causing HF in men. Women with HF tend to be older than men with HF and may have higher level of co-morbidities at baseline. Hypertension and MI together account for about three quarters of the population-attributable risk of HF. According to FHS, the hazard for developing HF in hypertensive compared with normotensive subjects was about 2-fold in men and 3-fold in women. There was a significant association between HTN and HF in both men and women—the risk doubled with blood pressure (BP) ≥ 160/90 mmHg compared to BP < 140/90.[16] HTN had the highest population attributable risk (PAR) of all risk factors; 39% for males and 59% for females. The PAR for MI was 34% for men, 13% for females and for DM was 6% for men and 12% for females.
This implies that efforts to reduce the incidence of HF for both men and women, require targeted strategies to control major associated risk factors based on gender related data. For instance it has been shown that a 10-mm reduction in systolic BP can reduce the incidence of congestive HF by 50%.[19] If women are particularly at risk for hypertension associated HF, the role of early detection and management of HTN is even greater in them.
In this review, we found convergence of findings between prospective, retrospective and cross sectional analysis; demonstrating there is positive correlation between female gender and preserved LV function and non-ischemic etiologies (Figure 2 and Figure 4). Out of 10 prospective studies reviewed only 3 reported LV function measurement and they found significant positive correlation between female gender with HF and preserved LV function.[10],[13],[14] Of the 15 retrospective studies reviewed, the majority reported positive association between HF with preserved LV and female gender.[20]-[34] Martínez-Sellés M[28] reported a difference in HF and preserved LV function rate of 60% vs. 29% between women and men, and Lenzen et al.[32] reported a difference of 55% vs. 29%. Hellerman et al.[29] reported an OR =1.97 for women developing HF with preserved LV function. From 8 cross-sectional studies reviewed, 5 reported HF with preserved LV was independently associated with female gender,[13],[17],[25],[31],[36] while Oyati et al.[39] found similar rates between two genders. Of note, these studies had varied levels of echocardiography measurements of LV in their subjects which may account for different results.
It is important to note that most HF clinical trials have focused on patients with decreased left-ventricular ejection fraction, whereas women are more likely to have preserved ventricular systolic function. Further research is required to evaluate the effectiveness of therapies in diastolic dysfunctio—the predominant presentation in elderly women.
In relation to HTN (Figure 3), there was significantly higher association between female gender and HTN. However, the prospective Cardiovascular Health Study with 66 months follow up on a large population with an average age of 72 years old reported HF incidence rates for those with HTN as being 21.2% for females and 33.6% for males.[14]
Male gender had a significantly higher correlation with CAD and ischemic etiology (Figure 4). The life time risk of HF in males free of MI was one in nine (compared to one in five for all men), indicating the importance of antecedent MI in men.[16]
The association for DM as the etiology of HF among 17 studies analysed was similar between males and females (Figure 5). The population-attributable risk (PAR) for DM was reported as 6% for men and 12% for women in Framingham Heart Study,[19] in contrast in Cardiovascular Health Study[14] the HF incidence was reported higher in diabetic men than in diabetic women (44.6% vs. 32.5%). In addition, NHANES 1 data on 19 years follow up of a large population (14% blacks) found risk of DM for HF was comparable between genders.[15]
The clinical implications for gender differences in HF are significant. It impacts the risk factor screening and targeting gender-specific intervention. Clinical guidelines should reflect on these gender-based data and provide needed evidence and recommendations regarding appropriate approach and management. Clearly, the researchers need also become more aware of the importance of balanced recruitment of both men and women in clinical trials, offering gender-specific data in their analysis and in assisting clinicians with tailoring the optimum intervention for each gender.
References
- 1.AHA Statistics Update. Heart Disease and Stroke Statistics-2006 Update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Yu DS, Lee DT, Kwong AN, et al. Living with chronic heart failure: a review of qualitative studies of older people. J Adv Nurs. 2008;5:474–483. doi: 10.1111/j.1365-2648.2007.04553.x. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed A. Clinical manifestations, diagnostic assessment, and etiology of heart failure in older adults. Clin Geriatr Med. 2007;23:11–30. doi: 10.1016/j.cger.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Majahalme S. Gender and Congestive Heart Failure. Geriatrics & Aging. 2006;9:551–554. [Google Scholar]
- 5.Regitz-Zagrosek V, Broket S, Tschope C. Role of Gender in Heart Failure with Normal Left Ventricular Ejection Fraction. Prog Cardiovasc Dis. 2007;49:241–251. doi: 10.1016/j.pcad.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 8.Ghali JK, Krause-Steinrauf HJ, Adams KF, et al. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–2134. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Deswal A, Bozkurt B. Comparison of morbidity in women versus men with heart failure and preserved ejection fraction. Am J Cardiol. 2006;97:1228–1231. doi: 10.1016/j.amjcard.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Adams KF, Jr, Dunlap SH, Sueta CA, et al. Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol. 1996;28:1781–1788. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 11.Rywik SL, Wagrowska H, Broda G, et al. Heart failure in patients seeking medical help at outpatients clinics. Part I. General characteristics. Eur J Heart Fail. 2000;2:413–421. doi: 10.1016/s1388-9842(00)00106-9. [DOI] [PubMed] [Google Scholar]
- 12.Crabbe DL, Dipla K, Ambati S, et al. Gender differences in post-infarction hypertrophy in end-stage failing hearts. J Am Coll Cardiol. 2003;41:300–306. doi: 10.1016/s0735-1097(02)02710-9. [DOI] [PubMed] [Google Scholar]
- 13.Klapholz M, Maurer M, Lowe AM, et al. New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–1438. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 15.He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in us men and women. NHANCES 1 Epidemiologic Follow-up Study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 16.Cowie MR, Wood DA, Coats AJ, et al. Incidence and aetiology of heart failure: a population-based study. Eur Heart J. 1999;20:421–428. doi: 10.1053/euhj.1998.1280. [DOI] [PubMed] [Google Scholar]
- 17.Kenchaiah S, Evand JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure–the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 19.Levy D, Larson MG, Vasan RS, et al. The Progression From Hypertension to Congestive Heart Failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 20.Hussey LC, Hardin S. Comparison of characteristics of heart failure by race and gender. Dimens Crit Care Nurs. 2005;24:41–46. doi: 10.1097/00003465-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Samuel RS, Hausdorff JM, Wei JY. Congestive heart failure with preserved systolic function: is it a woman's disease? Women’s Health Issues. 1999;9:219–222. doi: 10.1016/s1049-3867(99)00008-0. [DOI] [PubMed] [Google Scholar]
- 22.Mendes LA, Davidoff R, Cupples LA, et al. Congestive heart failure in patients with coronary artery disease: the gender paradox. Am Heart J. 1997;134:207–212. doi: 10.1016/s0002-8703(97)70126-1. [DOI] [PubMed] [Google Scholar]
- 23.Agvall B, Dahlstrom U. Patients in primary health care diagnosed and treated as heart failure, with special reference to gender differences. Scand J Prim Health Care. 2001;19:14–19. doi: 10.1080/028134301300034549. [DOI] [PubMed] [Google Scholar]
- 24.Varela-Roman A, Grigorian L, Barge E, et al. Heart failure in patients with preserved and deteriorated left ventricular ejection fraction. Heart. 2005;91:489–494. doi: 10.1136/hrt.2003.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyster E, Norman J, Domanski M. Prevalence and predictors of heart failure with preserved systolic function: community hospital admissions of a racially and gender diverse elderly population. J Card Fail. 2004;10:49–54. doi: 10.1016/s1071-9164(03)00579-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee WY, Capra AM, Jensvold NG, et al. Epidemiology, Practice, Outcomes, and Cost of Heart Failure (EPOCH) Study. Gender and risk of adverse outcomes in heart failure. Am J Cardiol. 2004;94:1147–1152. doi: 10.1016/j.amjcard.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 27.Sheppard R, Behlouli H, Richard H, et al. Effect of gender on treatment, resource utilization, and outcomes in congestive heart failure in Quebec, Canada. Am J Cardiol. 2005;95:955–959. doi: 10.1016/j.amjcard.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Sellés M, García Robles JA, Prieto L, et al. Systolic dysfunction is a predictor of long term mortality in men but not in women with heart failure. Eur Heart J. 2003;24:2046–2053. doi: 10.1016/j.ehj.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Hellermann JP, Jacobsen SJ, Reeder GS, et al. Heart failure after myocardial infarction: prevalence of preserved left ventricular systolic function in the community. Am Heart J. 2003;145:742–748. doi: 10.1067/mhj.2003.187. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarino V, Chen YT, Wang Y, et al. Sex differences in the clinical care and outcomes of congestive heart failure in the elderly. Am Heart J. 1999;138:835–842. doi: 10.1016/s0002-8703(99)70007-4. [DOI] [PubMed] [Google Scholar]
- 31.Mejhert M, Holmgren J, Wändell P, et al. Diagnostic testes, treatment and follow up in heart failure patients–is there a gender bias in the coherence to guidelines? Eur J Heart Fail. 1999;1:407–410. doi: 10.1016/s1388-9842(99)00053-7. [DOI] [PubMed] [Google Scholar]
- 32.Lenzen MJ, Scholte op Reimer WJ, Boersma E, et al. Differences between patients with a preserved and a depressed left ventricular function: a report from the Euro Heart Failure Survey. Eur Heart J. 2004;25:1214–1220. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Gustafsson I, Brendorp B, Seibaek M, et al. Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. J Am Coll Cardiol. 2004;43:771–777. doi: 10.1016/j.jacc.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Bener A, Al Suwaidi J, Ghaffar A. Is hypertension a predictor for heart failure? A cross cultural comparison over a 10-year period. Eur J Heart Fail. 2005;7:784–786. doi: 10.1016/j.ejheart.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 36.Dunlap SH, Sueta CA, Tomasko L, et al. Association of body mass, gender and race with heart failure primarily due to hypertension. J Am Coll Cardiol. 1999;34:1602–1608. doi: 10.1016/s0735-1097(99)00374-5. [DOI] [PubMed] [Google Scholar]
- 37.Aronow WS, Ahn C, Kronzon I. Normal left ventricular ejection fraction in older persons with congestive heart failure. Chest. 1998;113:867–869. doi: 10.1378/chest.113.4.867. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed A, Nanda NC, Weaver MT, et al. Clinical correlates of isolated left ventricular diastolic dysfunction among hospitalized older heart failure patients. Am J Geriatr Cardiol. 2003;12:82–89. doi: 10.1111/j.1076-7460.2003.01617.x. [DOI] [PubMed] [Google Scholar]
- 39.Oyati IA, Danbauchi SS, Alhassan MA, et al. Diastolic dysfunction in persons with hypertensive heart failure. J Natl Med Assoc. 2004;96:68–73. [PMC free article] [PubMed] [Google Scholar]
- 40.Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population. The Rotterdam Study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 41.Abhayaratna WP, Smith WT, Becker NG, et al. Prevalence of heart failure and systolic ventricular dysfunction in older Australians: The Canberra heart study. Med J Aust. 2006;184:151–154. doi: 10.5694/j.1326-5377.2006.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 42.O'Mahony MS, Sim MF, Ho SF, et al. Diastolic heart failure in older people. Age Ageing. 2003;32:519–524. doi: 10.1093/ageing/afg090. [DOI] [PubMed] [Google Scholar]
- 43.McCullough PA, Philbin EF, Spertus JA, et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 44.Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]


