Abstract
Human aging is a global issue with important implications for current and future incidence and prevalence of health conditions and disability. Cardiac arrhythmias, including atrial fibrillation, sudden cardiac death, and bradycardia requiring pacemaker placement, all increase exponentially after the age of 60. It is important to distinguish between the normal, physiological consequences of aging on cardiac electrophysiology and the abnormal, pathological alterations. The age-related cardiac changes include ventricular hypertrophy, senile amyloidosis, cardiac valvular degenerative changes and annular calcification, fibrous infiltration of the conduction system, and loss of natural pacemaker cells and these changes could have a profound effect on the development of arrhythmias. The age-related cardiac electrophysiological changes include up- and down-regulation of specific ion channel expression and intracellular Ca2+ overload which promote the development of cardiac arrhythmias. As ion channels are the substrates of antiarrhythmic drugs, it follows that the pharmacokinetics and pharmacodynamics of these drugs will also change with age. Aging alters the absorption, distribution, metabolism, and elimination of antiarrhythmic drugs, so liver and kidney function must be monitored to avoid potential adverse drug effects, and antiarrhythmic dosing may need to be adjusted for age. Elderly patients are also more susceptible to the side effects of many antiarrhythmics, including bradycardia, orthostatic hypotension, urinary retention, and falls. Moreover, the choice of antiarrhythmic drugs in the elderly patient is frequently complicated by the presence of co-morbid conditions and by polypharmacy, and the astute physician must pay careful attention to potential drug-drug interactions. Finally, it is important to remember that the use of antiarrhythmic drugs in elderly patients must be individualized and tailored to each patient's physiology, disease processes, and medication regimen.
Keywords: aging, antiarrhythmic drugs, pharmacokinetics, pharmacodynamics, polypharmacy, cardiac electrophysiology, ion channels
1. Issues of global aging
Elderly people constitute a larger segment of the population today than at any other time in history. In 1900, only 4% of the population in the United States was 65 or older. In 2000, more than 40 million people were in that age group, representing nearly 13% of the population. By 2030, when the last wave of baby boomers reaches the age of 65, elderly people are expected to constitute over 20% of the U.S. population.[1]
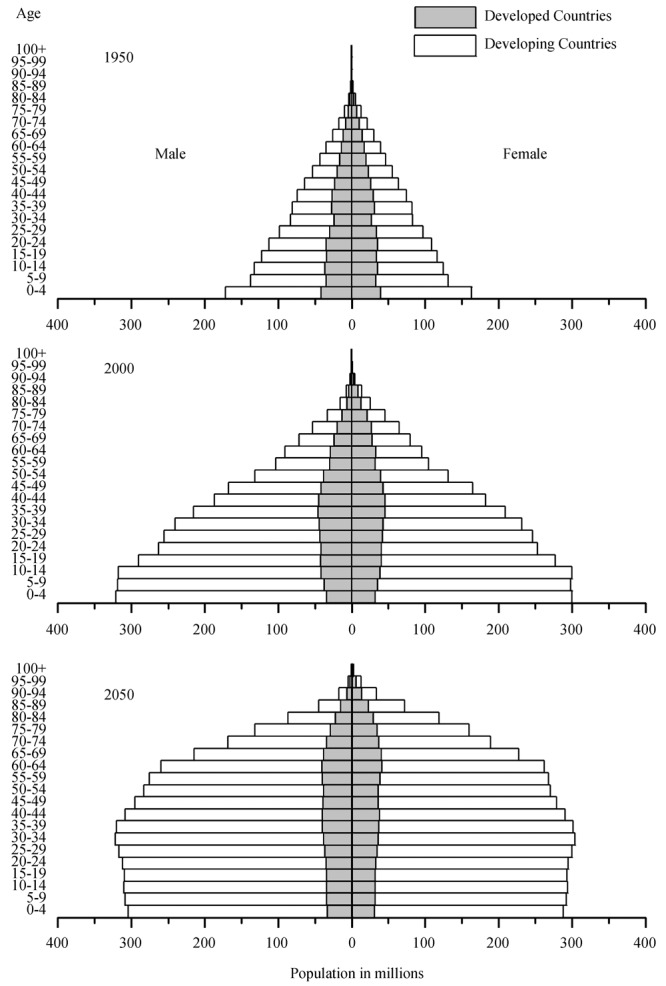
Moreover, human aging is a global issue. Based on census and population projections from 1950 to 2050, there has been a worldwide transformation of the distribution of the population by age, from a population pyramid to a population dome (Figure 1). This trend applies to both male and females, in developing countries as well as in developed countries.[2] In fact, the large population in the developing world contributes significantly to the transformation in demographics. These trends in the global patterns of aging have important implications for the current and future incidence and prevalence of many health conditions. With the advance in overall age comes an increase in the incidence of cardiovascular diseases, including cardiac arrhythmias. Global aging has important implications for health care delivery, as age-related physiological changes are frequently overlooked when treatment options are considered and may result in unnecessary adverse outcomes. This review will focus on issues pertaining to the pharmacologic treatment of cardiac arrhythmias in the elderly population.
Figure 1. World population by age and sex: 1950–2050. Distribution of the world population by age and sex from 1950 (top panel) to 2000 (middle panel) and 2050 (bottom panel). With global aging, the world population is being transformed from a population pyramid to a population dome. These changes occur not only in developed countries (grey) but also in developing countries (white). Adopted from United Nations and United States Census Bureau.[1],[2].
2. Aging and cardiac arrhythmias
The prevalence of cardiac diseases, including cardiac arrhythmias, increases with age.[3],[4] Increased frequencies of supraventricular and ventricular ectopies have been reported in elderly patients.[4] Additionally, elderly patients have a heightened propensity to develop certain arrhythmias, including atrial fibrillation,[5] sudden cardiac death,[6] and bradyarrhythmias requiring pacemaker placement.[7] Results from the ATRIA (AnTicoagulation and Risk Factors in Atrial fibrillation) study showed that there is an exponential increase in the prevalence of atrial fibrillation after the age of 60 for both men and women, with over 10% of people older than 80 years of age affected.[8] Based on these results, it is projected that by 2050, the majority of patients with atrial fibrillation will be 80 years or older. Similarly, the incidence of sudden cardiac death in the general population shows an exponential increase after 55 years of age, peaking in the seventies and eighties. This affects both men and women, with women accounting for 43% of sudden cardiac death cases.[9],[10] There is also a remarkable age-related increase in bradyarrhythmias, both in sinus nodal dysfunction and in atrioventricular (AV) blocks. The incidence of first time pacemaker implantation increases substantially after the age of 60, peaking in the seventies and leveling off in the eighties.[11],[12]
3. Changes in cardiac electrophysiology with aging
Cardiac electrophysiological properties change with age, and it is essential to differentiate between normal, physiologic consequences of aging versus abnormal, pathological transformations. First, intrinsic heart rate decreases with age.[13] Heart rate response is blunted with advanced age, though excessive sinus pauses are not considered physiologic.[14] Second, the PR interval becomes prolonged with age. First degree AV block is common among elderly people, but second and third degree AV blocks are abnormal. These changes are associated with AH prolongation without alteration of the HV interval.[15] Third, conduction system changes in the form of right bundle branch block (RBBB) are more common in elderly patients and are not associated with cardiac morbidity or mortality, but the presence of left bundle branch block (LBBB) in older patients is frequently an indication of the presence of underlying cardiac disease[4] and is associated with adverse outcomes.[16] Fourth, atrial and ventricular ectopies are known to increase with age, but atrial fibrillation and ventricular tachycardia should not be considered normal.[17],[18] Fifth, age-related non-specific ST-T wave changes are common, but the presence of Q waves is seldom normal. Sixth, the QT interval increases with age, and this is true for both men and women.[19] Usually, men have shorter QT intervals compared to women at the same age, but QT intervals are similar between men and women in the post-menopausal years.[19]
Normal aging is associated with a reduced autonomic response to stress, including decreased baroreflex buffering[20] and depressed heart rate variability.[21] There is an increase in the prevalence of orthostatic hypotension and supine hypertension with age; however, the presence of baroreceptor and autonomic failure is abnormal.[22] Because of these alterations, the cardiac electrophysiological changes that occur with age reduce the margin of functional reserve and render the aging heart more susceptible to the development of electrophysiological abnormalities. In addition, the age-related cardiac changes which include ventricular hypertrophy, senile amyloidosis, cardiac valvular degenerative changes and annular calcification, fibrous infiltration of the conduction system, and loss of natural pacemaker cells could also have profound direct and indirect effects on the development of arrhythmias.
4. Changes in cardiac ion channels with aging
The age-related electrocardiogram (ECG) and action potential changes are associated with changes in the properties and activities of specific ion channels (Table 1).[23],[24] Among the inward currents, the voltage-gated sodium channel is unaltered with age,[25] but the L-type Ca2+ currents (ICaL) are increased with delayed inactivation in the ventricles,[26] while becoming significantly reduced by more than 40% in the atria.[27] On the other hand, among the repolarizing currents in the ventricles, the transient outward K+ currents (Ito)[24],[27] and the ATP-sensitive K+ currents (IKATP) are reduced.[28] These, together with the enhanced ICaL, result in lengthened action potential durations and prolonged QT intervals. Meanwhile, the strong inward rectifier K+ currents (IK1) are unchanged.[29]
Table 1. Change in cardiac ionic currents with old age.
| Currents | Change in aging |
| INa | Atria: ↔, Sinus node: ↓ |
| ICaL | Ventricles: ↑, delayed inactivation Atria: ↓ by 40%, Sinus node: ↓ |
| Ito | ↓ |
| IKATP | ↓ |
| IKur | Atria: ↑ |
| IK1 | ↔ |
| INCX | ↑ |
| SERCA-2 | ↓ |
| Cx-43 | Sinus node: ↓ |
| HCN4 | Sinus node: ↓ mRNA |
| APD | Atria and ventricles: ↑ |
INa: voltage-gated sodium currents; ICaL: L-type calcium currents; Ito: transient outward potassium currents; IKATP: ATP-sensitive potassium currents; IKur: ultra-rapid delayed rectifier potassium currents; IK1: strong inward rectifier potassium currents; INCX: sodium-calcium exchange currents; SERCA-2: sarcoplasmic reticulum calcium pump; Cx-43: connexin-43; HCN4: hyperpolarization-activated cyclic nucleotide-gated channel 4; APD: action potential duration; ↔: unalter; ↑: increase; ↓: decrease.
In the atria, the ultra-rapid delayed rectifier K+ currents (IKur) are enhanced.[27] Since IKur is atrial-specific, the upregulation in IKur may render the aging atria more susceptible to the development of atrial fibrillation.[30] In the sinus node, there is significant age-related electrical and structural remodeling in the form of downregulation of sodium channels,[31] L-type calcium channels,[32] and connexin-43,[33] as well as an increase in fibrosis.[34] These ionic changes may lead to the development of sinus node dysfunction in the elderly patient.
Aging has also been shown to be associated with a downregulation in the mRNA expression of HCN4, which encodes If,[35] but reduced If current has not been demonstrated. In addition, the aging myocardium is associated with abnormal intracellular Ca2+ regulation as expression of the SERCA-2 Ca2+ pump is reduced [36],[37] while that of the sodium-calcium exchanger is increased.[38] This is very important because the electrical and structural remodeling of the aging heart suggest that the therapeutic targets of antiarrhythmic drugs, i.e., the cardiac ion channels, change with age. In addition to these age-related alterations in the ionic channels, the pharmacokinetics and pharmacodynamics of antiarrhythmic drugs also vary with age.
5. Changes in antiarrhythmic drug pharmacokinetics with age: Drug-body interactions
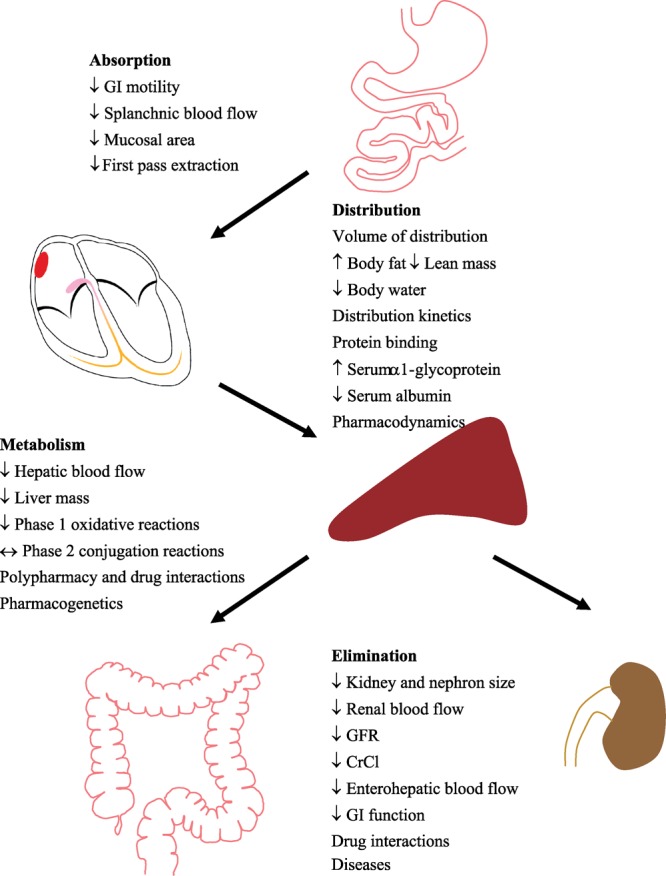
Pharmacokinetics is defined as the handling of a drug within the body, including its absorption, distribution, metabolism, and elimination. A number of age-related physiological changes that occur with aging may affect drug disposition in the body (Figure 2).
Figure 2. Age-related changes in pharmacokinetics of antiarrhythmic drugs. Diagrammatic representation of age-related changes in the absorption, distribution, metabolism, and elimination of antiarrhythmic drugs. GI: gastrointestinal; GFR: glomerular filtration rate; CrCl: creatinine clearance; ↔: unalter; ↑: increase; ↓: decrease.
5.1. Absorption
With age, there is a decrease in gastric acid secretion that may reduce the dissolution of tablets and the solubility of basic drugs.[39] Antiarrhythmic drugs are weak bases, so there may be reduced gastric solubility with age, but the effects are small. The gastric emptying rate is somewhat delayed as there is reduced gastrointestinal motility, decreased mucosal surface area, and reduced splanchnic blood flow, which decreases by 30%–40% between ages 20 and 70.[40],[41] Despite these changes, the oral absorption of antiarrhythmic drugs is not significantly affected by aging, probably because most of these drugs are absorbed passively.[39],[42] However, bioavailability can be significantly affected by the decrease in first-pass intestinal wall/hepatic extraction with aging, resulting in enhanced systemic bioavailability for drugs such as propranolol and lidocaine.[43],[44]
5.2. Distribution
Drug distribution can be profoundly affected by some of the physiological changes that occur as patients get older. Between ages 20 and 70, body fat as a proportion of body weight rises from 18% to 36% in males and from 33% to 45% in females, while there is an overall reduction in lean body mass of 20% in men and 12% in women during those years.[39],[45],[46] These changes significantly increase the volume of distribution of fat soluble drugs like amiodarone. At the same time, there is a reduction of 8% in total body water, thereby reducing the volume of distribution for water soluble drugs like digoxin.[47]
In addition, the serum level of α-1-glycoprotein, which binds weak bases such as antiarrhythmic drugs, increases in the elderly, causing a reduction in the free fraction of the drugs and in the effective volume of distribution while simultaneously increasing drug plasma concentration.[48] Plasma albumin tends to be reduced in the elderly.[49],[50] Weakly acidic drugs, such as warfarin, normally bind to albumin. Thus, the reduced plasma albumin may result in a more intense drug effect with age, making the elderly patient more susceptible to bleeding complications.[42]
5.3. Metabolism
Drugs are metabolized in the liver by two types of reactions, namely phase I oxidative reactions and phase II conjugative/synthetic reactions.[51] Most phase I reactions are mediated by cytochrome P450 (CYP) monooxygenases.[52] Table 2 shows the five CYP isozymes that are important for the metabolism of clinically relevant antiarrhythmic drugs. CYP2D6 and CYP3A4 in particular are two of the most important isozymes for the metabolism of drugs, including antiarrhythmics.[42],[52],[53] Quinidine, disopyramide, lidocaine, mexiletine, flecainide, propafenone, amiodarone, verapamil and metoprolol are all dependent on the activities of these enzymes for metabolism. After the age of 30, there is a 1% per year decline in liver blood flow and liver mass, correlating with a significant age-elated reduction in phase I reactions.[54]–[56] In contrast, the activities of phase II reactions, through which an acetyl group or a sugar is conjugated to the drug to enhance its polarity, water solubility and hence excretion via the kidneys, are relatively unaffected by age.[54],[57] Nevertheless, the reduction in liver function may reduce the clearance and increase the half-ives of antiarrhythmic drugs metabolized by the liver, such as quinidine, propafenone, amiodarone. The reduction in hepatic blood flow with age is also known to significantly reduce the clearance of high hepatic extraction ratio drugs, such as lidocaine and propranolol, which require dose adjustments according to age to minimize the risk of adverse effects.[58]–[62]
Table 2. Clinically important cytochrome P450 (CYP) isozymes and substrates.
| CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4 |
| Mexiletine | Phenytoin | Phenytoin | Flecainide | Quinidine |
| Caffeine | Warfarin | Diazepam | Encainide | Disopyramide |
| Theophylline | Propafenone | Lidocaine | ||
| Mexiletine | Amiodarone | |||
| Metoprolol | Verapamil |
5.4. Elimination
Normal aging is associated with a significant reduction in kidney size as well as in the number and size of nephrons, while the proportion of sclerotic glomeruli increases. With age, glomerular filtration rate, tubular secretion, and renal blood flow decrease approximately by 0.5%, 0.5% and 1% per year, respectively, after the age of 20.[42],[63],[64] Hence, in an 80-year-old patient, the loss of renal function leads to a significant accumulation of renally-cleared antiarrhythmic drugs such as digoxin, procainamide, N-acetyl procainamide (NAPA), sotalol, and dofetilide. In addition, because of the reduction in muscle mass with advancing age, serum creatinine should not be used as a marker for estimation of renal function.[65] A serum creatinine of 1.0 is associated with a normal creatinine clearance (CrCl) for a 35-year-old patient, an adequate but reduced CrCl in a 65-year-old patient, and an abnormally low CrCl in an 85-year-old patient.[63] An equation such as the following should be used to estimate CrCl[66],[67]: CrCl = ((140−age) × (wt in kg)) / (72 × sCr).
For women, CrCl as calculated in the equation above should be multiplied by a factor of 0.85.
The age-related pharmacokinetic changes associated with antiarrhythmic drugs are summarized in Table 3 and show several general features. Aging is associated with reduced clearance of many antiarrhythmic drugs, resulting in an increase in drug half-lives. Maximum drug concentration may also increase, potentially augmenting the risk of drug toxicity. The volume of drug distribution also changes with age and depends on whether the drug is lipophilic or hydrophilic. Thus, most antiarrhythmic drugs will need dosage adjustments in the elderly patient.
Table 3. Age-related pharmacokinetic changes with antiarrhythmic drugs.
| Drug | Pharmacokinetics |
||||
| tmax | Cmax | t1/2 | Vd | CL | |
| Digoxin | ↑ | NA | ↑ | ↓ | ↓↓ |
| Disopyramide | ↔ | ↑ | ↑ ↔ | NA | ↓ |
| Quinidine | NA | NA | ↑ | ↔ | ↓ |
| Lidocaine | ↔ | ↑ | ↑ | ↑ | ↔ ↓ |
| Mexiletine | ↔ | ↔ ↑ | ↔ ↑ | NA | ↔ ↓ |
| Flecainide | NA | NA | ↑ | ↑ | ↓ |
| Propafenone | NA | ↔ ↑ | ↑ | NA | ↓ |
| Sotalol | ↔ | ↑ | ↑ | ↔ | ↓↓ |
| Dofetilide | ↔ | ↑ | ↑ | ↔ | ↓↓ |
| Amiodarone | NA | NA | ↑ | ↑ | ↓ |
| Dronedarone | ↔ | ↑ | ↑ | ↑ | ↓ |
| Verapamil | ↔ ↑ | ↔ ↑ | ↑ | ↔ | ↓ |
tmax: time to maximum plasma concentration; Cmax: maximum plasma concentration; t1/2: half life; Vd: volume of distribution; CL: clearance; NA: not available; ↔: unalter; ↑: increase; ↓: decrease.
6. Changes in pharmacodynamics of antiarrhythmic drugs with aging: Drug-disease interactions
Pharmacodynamics is defined as the effects of the drugs on the body. The physiological changes associated with aging can profoundly affect drug pharmacodynamics and make the elderly patient more susceptible to potential adverse effects of antiarrhythmic drugs. Aging is associated with reduced cardiac reserve, rendering the heart more susceptible to heart failure.[68]–[70] Baroreceptor sensitivity is blunted, increasing the risk of orthostatic hypotension.[71],[72] There is reduced sensitivity to agonists and antagonists, causing a decreased response to adrenergic drugs.[73]–[75] Deterioration in sinus and AV nodal function in the elderly increases the propensity for developing significant bradycardia and heart blocks.[4],[76] Also, the increase in sensitivity to anticoagulating agents heightens the risk of bleeding in the elderly patient.[77]–[80]
Specifically, pertaining to antiarrhythmic drugs, the age-related changes in pharmacodynamics include reduced sensitivity to blockers[73]–[75] as well as increased sensitivity to Class I antiarrhythmic drugs in terms of sinus nodal function and electrical impulse conduction.[51] Also, antiarrhythmic drugs have greater avidity in binding to cardiac tissue in patients with ischemic heart disease and cardiomyopathy. Finally, impaired homeostatic mechanisms increase the risks of potential adverse drug effects such as orthostasis, urinary retention, constipation, falls, and bleeding.[71],[77],[78],[80]–[83]
Some of the important antiarrhythmic drug-disease interactions in geriatric patients are listed in Table 4. Most antiarrhythmic drugs are negative inotropic agents. Disopyramide, flecainide, and sotalol are known to cause acute decompensation in patients with poor ventricular function.[84] Class I antiarrhythmic drugs, blockers, and Ca2+ channel blockers can precipitate heart block or sinus bradycardia in the elderly. Sotalol and propafenone are blockers and can exacerbate bronchospasm.[85] Since lung function declines throughout adult life even in healthy persons and may accelerate after age 70, elderly patients are more vulnerable to such adverse effects.[86] Disopyramide has anticholinergic properties and is known to worsen symptoms of prostatism, which is prevalent in elderly men.[81] In the presence of hypokalemia, digoxin can exacerbate the development of life-threatening ventricular arrhythmias. Due to reduced renal function, elderly patients are more susceptible to developing digitalis toxicity.[87],[88] Class I antiarrhythmic drugs in patients with coronary artery disease and myocardial infarction are pro-arrhythmic and may cause sudden death.[89],[90] These conditions are more common with advanced age, precluding the use of these medications in many elderly patients. Lastly, antiarrhythmics with vasodilator properties, such as procainamide, quinidine, and sotalol, can exacerbate orthostatic hypotension and precipitate falls in the elderly.[82],[83]
Table 4. Important antiarrhythmic drug-drug interactions in elderly patients.
| Underlying disease | Drugs | Adverse effects |
| CHF | Disopyramide, flecainide, sotalol | Decompensation |
| Cardiac conduction disorders | Class 1 drugs, β and Ca2+ blockers | Heart block |
| COPD | Sotalol | Bronchospasm |
| Prostate hypertrophy | Disopryramide | Urinary retention |
| Hypokalemia | Digoxin | Arrhythmias |
| CAD | Class 1 drugs | Proarrhythmia |
| Postural hypotension | β and Ca2+ blockers, quinidine, procainamide | Falls |
CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CAD: coronary artery disease.
7. Issues with polypharmacy: Drug-drug interactions
In addition to the above, polypharmacy is an important problem in the elderly. Both the prevalence of adults with prescriptions and the number of prescriptions per person increase with age. More than 75% of elderly people in the community use one or more medications, with an average of eight prescriptions per elderly patient.[91]
The pervasiveness of polypharmacy makes the elderly vulnerable to adverse drug-drug interactions, including those involving antiarrhythmic drugs. Class I antiarrhythmic drugs are all sodium channel blockers, but many of them, especially the Class IA drugs, also have potassium channel blocker effects, including blockade of Ito, IKr, and IKS.[84],[92] The potassium channel blocker effects confer the QT prolonging properties of these drugs, which are known to be associated with torsade de pointes.[93] The Class IC drugs have also been shown to block IKur, which may account for their comparative effectiveness in treating atrial arrhythmias.[84],[92] In addition, the Class IA drugs have vagolytic effects as well as adrenergic receptor blocking effects. Propafenone is a propranolol analog and has weak blocker properties. All of these “off-target” effects of Class I antiarrhythmic drugs may cause important adverse consequences when these drugs accumulate.
All Class I antiarrhythmics except procainamide are metabolized in the liver by CYP3A4 or CYP2D6.[94] Procainamide is acetylated in the liver to form NAPA and is subsequently excreted by the kidneys through active tubular secretion, which can be inhibited by cimetidine and trimethoprim. Quinidine is metabolized by CYP3A4, but it is also a potent inhibitor of CYP2D6 and has complex interactions with propafenone metabolism.[95] Drugs that inhibit the CYP system, such as cimetidine, ketoconazole, and macrolide antibiotics, also inhibit clearance of Class I antiarrhythmic drugs. Grapefruit juice inhibits intestinal wall CYP3A4 and CYP1A2 and potentiates the effects of drugs that are cleared by these enzymes.[96] On the other hand, rifampin, glucocorticoids, phenytoin, ethanol, and phenobarbital induce CYP3A4, which increases quinidine, disopyramide, amiodarone, lidocaine, and verapamil clearance. Quinidine, amiodarone, and dronedarone are inhibitors of P-glycoprotein, which is required for renal excretion of digoxin, thereby augmenting digoxin levels.[97]
Class III antiarrhythmic drugs are potassium channel blockers, but amiodarone and dronedarone are promiscuous, inhibiting the voltage-gated sodium channel, the L-type Ca2+ channel, and β-adrenergic receptors.[84],[92] Amiodarone and dronedarone are hepatically metabolized, while sotalol and NAPA are eliminated by the kidney. Although dofetilide is mostly cleared by the kidney, 20% is metabolized by CYP3A4, so its level can be altered by drugs, such as ketoconazole, that modulate CYP3A4 activities. Also, cimetidine inhibits renal cationic secretion of dofetilide and prolongs its half-life.[98] Other agents that inhibit renal cationic secretion may have similar effects. Amiodarone is a potent inhibitor to a number of drug metabolizing enzymes and drug transporters, including CYP3A4, CYP2C9, and P-glycoprotein. Hence, the substrate of these enzymes, which include a variety of drugs commonly used in the geriatric population, will need appropriate dose adjustment when used concomitantly with amiodarone.[99]
Adenosine has a very short half-life and is removed by cellular uptake. Its effects are augmented by dipyridamole, which blocks adenosine deaminase, allowing adenosine to accumulate extracellularly. Theophylline counteracts the effects of adenosine by potentiating intracellular cAMP. Hence, when using adenosine, it is crucial that the patient is not taking dipyridamole or theophylline, which will profoundly alter the effects of adenosine.[100]–[102] Digoxin is a Na/K pump inhibitor and is an inotropic agent that activates the Na/Ca exchanger, leading to an increase in intracellular Ca2+.[87] Digoxin is removed via the kidney and its secretion is mediated by P-glycoprotein. Thus, drugs such as quinidine that inhibit P-glycoprotein cause an increase in digoxin levels.[97] The dose of digoxin should be adjusted based on the other drugs that the elderly patient is taking and plasma levels of digoxin should be monitored. A recent prospective cohort study of 2030 elderly patients showed that the risk of digoxin toxicity requiring hospitalization is increased more than four-fold in those with a history of recent hospitalization (within two months).[88] This underscores the prevalence of medication misadventures in the elderly population, which imposes a significant burden on the health care system,[103] and the importance of paying close attention to evaluate adherence, prescribing, and adverse drug reactions in the management of elderly patients' medical care.
8. Summary
To conclude, what do we need to know about antiarrhythmic drug therapy in elderly patients? The first thing to know is the patient population. Elderly patients are avid consumers of medications, which increases the risk of drug-drug interactions. Moreover, the elderly are subject to significant age-related physiological changes that may alter the effects of individual drugs. The second thing to know is the antiarrhythmic drugs. Age-related alterations in drug pharmacokinetics, in hepatic metabolism, and in renal elimination can be pronounced for some drugs and are often under-appreciated. The third thing to know is that the use of antiarrhythmic drugs in elderly patients must be individualized. Potential drug-drug and drug-disease interactions are common and must be scrutinized for each patient. All these issues may have a significant impact on the health-related quality of life in the elderly.
References
- 1.Day JC. Population projections of the United States, by age, sex, race, and hispanic origin: 1993 to 2050. U.S. Bureau of the Census, U.S Government Printing Office, Current Population Reports; Washington DC: 1996. pp. 25–1130. [Google Scholar]
- 2.DESA . World population prospect: The 2008 revision, population database. United Nations; New York: 2009. [Google Scholar]
- 3.Mittelmark MB, Psaty BM, Rautaharju PM, et al. Prevalence of cardiovascular diseases among older adults. The Cardiovascular health study. Am J Epidemiol. 1993;137:311–317. doi: 10.1093/oxfordjournals.aje.a116678. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Mann DL, Zipes DP, et al. Braunwald's Heart Disease: A textbook of cardiovascular medicine. 9th Edition. Saunders; 2011. Cardiovascular disease in the elderly; pp. 1727–1756. [Google Scholar]
- 5.Fang MC, Chen J, Rich MW. Atrial fibrillation in the elderly. Am J Med. 2007;120:481–487. doi: 10.1016/j.amjmed.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Buxton AE. Implantable cardioverter-defibrillators should be used routinely in the elderly. Am J Geriatr Cardiol. 2006;15:361–364; quiz 365–366. doi: 10.1111/j.1076-7460.2006.06029.x. [DOI] [PubMed] [Google Scholar]
- 7.Kusumoto FM, Phillips R, Goldschlager N. Pacing therapy in the elderly. Am J Geriatr Cardiol. 2002;11:305–316. doi: 10.1111/j.1076-7460.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 9.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaszala K, Kalahasty G, Ellenbogen KA. Cardiac pacing in the elderly. Am J Geriatr Cardiol. 2006;15:77–81. doi: 10.1111/j.1076-7460.2006.04817.x. [DOI] [PubMed] [Google Scholar]
- 12.Samartin RC, Ferrer JM, de Carranza MJ, et al. Spanish pacemaker registry. 6th official report of the Spanish Society of Cardiology Working Group on Cardiac Pacing (2008) Rev Esp Cardiol. 2009;62:1450–1463. doi: 10.1016/s1885-5857(09)73538-8. [DOI] [PubMed] [Google Scholar]
- 13.Joyner MJ. Not so fast: intrinsic heart rate vs. beta-adrenergic responsiveness in the aging human heart. J Appl Physiol. 2008;105:3–4. doi: 10.1152/japplphysiol.90645.2008. [DOI] [PubMed] [Google Scholar]
- 14.Christou DD, Seals DR. Decreased maximal heart rate with aging is related to reduced {beta}-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate. J Appl Physiol. 2008;105:24–29. doi: 10.1152/japplphysiol.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleg JL, Das DN, Wright J, et al. Age-associated changes in the components of atrioventricular conduction in apparently healthy volunteers. J Gerontol. 1990;45:M95–M100. doi: 10.1093/geronj/45.3.m95. [DOI] [PubMed] [Google Scholar]
- 16.Miller WL, Ballman KV, Hodge DO, et al. Risk factor implications of incidentally discovered uncomplicated bundle branch block. Mayo Clin Proc. 2005;80:1585–1590. doi: 10.4065/80.12.1585. [DOI] [PubMed] [Google Scholar]
- 17.Fleg JL, Kennedy HL. Cardiac arrhythmias in a healthy elderly population: detection by 24-hour ambulatory electrocardiography. Chest. 1982;81:302–307. doi: 10.1378/chest.81.3.302. [DOI] [PubMed] [Google Scholar]
- 18.Manolio TA, Furberg CD, Rautaharju PM, et al. Cardiac arrhythmias on 24-h ambulatory electrocardiography in older women and men: the cardiovascular health study. J Am Coll Cardiol. 1994;23:916–925. doi: 10.1016/0735-1097(94)90638-6. [DOI] [PubMed] [Google Scholar]
- 19.Mangoni AA, Kinirons MT, Swift CG, et al. Impact of age on QT interval and QT dispersion in healthy subjects: a regression analysis. Age Ageing. 2003;32:326–331. doi: 10.1093/ageing/32.3.326. [DOI] [PubMed] [Google Scholar]
- 20.Jones PP, Christou DD, Jordan J, et al. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- 21.Reardon M, Malik M. Changes in heart rate variability with age. Pacing Clin Electrophysiol. 1996;19(11Pt2):1863–1866. doi: 10.1111/j.1540-8159.1996.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 22.Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults. The cardiovascular health study. CHS collaborative research group. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 23.Janczewski AM, Spurgeon HA, Lakatta EG. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J Mol Cell Cardiol. 2002;34:641–648. doi: 10.1006/jmcc.2002.2004. [DOI] [PubMed] [Google Scholar]
- 24.Walker KE, Lakatta EG, Houser SR. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc Res. 1993;27:1968–1977. doi: 10.1093/cvr/27.11.1968. [DOI] [PubMed] [Google Scholar]
- 25.Baba S, Dun W, Hirose M, et al. Sodium current function in adult and aged canine atrial cells. Am J Physiol Heart Circ Physiol. 2006;291:H756–H761. doi: 10.1152/ajpheart.00063.2006. [DOI] [PubMed] [Google Scholar]
- 26.Josephson IR, Guia A, Stern MD, et al. Alterations in properties of L-type Ca channels in aging rat heart. J Mol Cell Cardiol. 2002;34:297–308. doi: 10.1006/jmcc.2001.1512. [DOI] [PubMed] [Google Scholar]
- 27.Dun W, Yagi T, Rosen MR, et al. Calcium and potassium currents in cells from adult and aged canine right atria. Cardiovasc Res. 2003;58:526–534. doi: 10.1016/s0008-6363(03)00288-8. [DOI] [PubMed] [Google Scholar]
- 28.Jovanovic S, Jovanovic A. Sarcolemmal K (ATP) channels in ageing. Ageing Res Rev. 2004;3:199–214. doi: 10.1016/j.arr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Cerbai E, Barbieri M, Li Q, et al. Ionic basis of action potential prolongation of hypertrophied cardiac myocytes isolated from hypertensive rats of different ages. Cardiovasc Res. 1994;28:1180–1187. doi: 10.1093/cvr/28.8.1180. [DOI] [PubMed] [Google Scholar]
- 30.Dun W, Boyden PA. Aged atria: electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol. 2009;25:9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanni J, Tellez JO, Sutyagin PV, et al. Structural remodelling of the sinoatrial node in obese old rats. J Mol Cell Cardiol. 2010;48:653–662. doi: 10.1016/j.yjmcc.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones SA, Boyett MR, Lancaster MK. Declining into failure: the age-dependent loss of the L-type calcium channel within the sinoatrial node. Circulation. 2007;115:1183–1190. doi: 10.1161/CIRCULATIONAHA.106.663070. [DOI] [PubMed] [Google Scholar]
- 33.Jones SA, Lancaster MK, Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol. 2004;560:429–437. doi: 10.1113/jphysiol.2004.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monfredi O, Dobrzynski H, Mondal T, et al. The anatomy and physiology of the sinoatrial node—a contemporary review. Pacing Clin Electrophysiol. 2010;33:1392–1406. doi: 10.1111/j.1540-8159.2010.02838.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Yang P, Du Y, et al. Age-related down-regulation of HCN channels in rat sinoatrial node. Basic Res Cardiol. 2007;102:429–435. doi: 10.1007/s00395-007-0660-5. [DOI] [PubMed] [Google Scholar]
- 36.Lompre AM, Lambert F, Lakatta EG, et al. Expression of sarcoplasmic reticulum Ca(2+)-ATPase and calsequestrin genes in rat heart during ontogenic development and aging. Circ Res. 1991;69:1380–1388. doi: 10.1161/01.res.69.5.1380. [DOI] [PubMed] [Google Scholar]
- 37.Taffet GE, Tate CA. CaATPase content is lower in cardiac sarcoplasmic reticulum isolated from old rats. Am J Physiol. 1993;264:H1609–H1614. doi: 10.1152/ajpheart.1993.264.5.H1609. [DOI] [PubMed] [Google Scholar]
- 38.Koban MU, Moorman AF, Holtz J, et al. Expressional analysis of the cardiac Na-Ca exchanger in rat development and senescence. Cardiovasc Res. 1998;37:405–423. doi: 10.1016/s0008-6363(97)00276-9. [DOI] [PubMed] [Google Scholar]
- 39.Williams BR, Kim J. Cardiovascular drug therapy in the elderly: theoretical and practical considerations. Drugs & aging. 2003;20:445–463. doi: 10.2165/00002512-200320060-00004. [DOI] [PubMed] [Google Scholar]
- 40.Bender AD. The effect of increasing age on the distribution of peripheral blood flow in man. J Am Geriatr Soc. 1965;13:192–198. doi: 10.1111/j.1532-5415.1965.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 41.Evans MA, Triggs EJ, Cheung M, et al. Gastric emptying rate in the elderly: implications for drug therapy. J Am Geriatr Soc. 1981;29:201–205. doi: 10.1111/j.1532-5415.1981.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 42.Podrazik PM, Schwartz JB. Cardiovascular pharmacology of aging. Cardiol Clin. 1999;17:17–34. doi: 10.1016/s0733-8651(05)70054-0. [DOI] [PubMed] [Google Scholar]
- 43.Castleden CM, George CF. The effect of ageing on the hepatic clearance of propranolol. Br J Clin Pharmacol. 1979;7:49–54. doi: 10.1111/j.1365-2125.1979.tb00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamy PP. Comparative pharmacokinetic changes and drug therapy in an older population. J Am Geriatr Soc. 1982;30:S11–S19. doi: 10.1111/j.1532-5415.1982.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 45.Novak LP. Aging, total body potassium, fat-free mass, and cell mass in males and females between ages 18 and 85 years. J Gerontol. 1972;27:438–443. doi: 10.1093/geronj/27.4.438. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82:87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 47.Cusack B, Kelly J, O'Malley K, et al. Digoxin in the elderly: pharmacokinetic consequences of old age. Clin Pharmacol Ther. 1979;25:772–776. doi: 10.1002/cpt1979256772. [DOI] [PubMed] [Google Scholar]
- 48.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 49.Campion EW, deLabry LO, Glynn RJ. The effect of age on serum albumin in healthy males: report from the normative aging study. J Gerontol. 1988;43:M18–M20. doi: 10.1093/geronj/43.1.m18. [DOI] [PubMed] [Google Scholar]
- 50.Greenblatt DJ. Reduced serum albumin concentration in the elderly: a report from the Boston collaborative drug surveillance program. J Am Geriatr Soc. 1979;27:20–22. doi: 10.1111/j.1532-5415.1979.tb01715.x. [DOI] [PubMed] [Google Scholar]
- 51.Buxton ILO, Benet LZ. Pharmacokinetics: The dynamics of drug absorption, distribution, metabolism, and elimination. In: Brunton LL, Chabner BA, Knollman BC, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th Edition. McGraw-Hill; 2010. pp. 17–40. [Google Scholar]
- 52.Klotz U. Antiarrhythmics: elimination and dosage considerations in hepatic impairment. Clin Pharmacokinet. 2007;46:985–996. doi: 10.2165/00003088-200746120-00002. [DOI] [PubMed] [Google Scholar]
- 53.Cheng JW, Frishman WH, Aronow WS. Updates on cytochrome p450-mediated cardiovascular drug interactions. Dis Mon. 2010;56:163–179. doi: 10.1016/j.disamonth.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Kinirons MT, O'Mahony MS. Drug metabolism and ageing. J Br Menopause Soc. 2004;57:540–544. doi: 10.1111/j.1365-2125.2004.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka E. In vivo age-related changes in hepatic drug-oxidizing capacity in humans. J Clin Pharm Ther. 1998;23:247–255. doi: 10.1046/j.1365-2710.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 56.Tateishi T, Fujimura A, Shiga T, et al. Influence of aging on the oxidative and conjugative metabolism of propranolol. Int J Clin Pharmacol Res. 1995;15:95–101. [PubMed] [Google Scholar]
- 57.Miners JO, Penhall R, Robson RA, et al. Comparison of paracetamol metabolism in young adult and elderly males. Eur J Clin Pharmacol. 1988;35:157–160. doi: 10.1007/BF00609245. [DOI] [PubMed] [Google Scholar]
- 58.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 59.Serste T, Bourgeois N. Ageing and the liver. Acta Gastroenterol Belg. 2006;69:296–298. [PubMed] [Google Scholar]
- 60.Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab. 2011 doi: 10.2174/138920011796504527. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Vestal RE. Aging and determinants of hepatic drug clearance. Hepatology. 1989;9:331–334. doi: 10.1002/hep.1840090228. [DOI] [PubMed] [Google Scholar]
- 62.Wynne H. Drug metabolism and ageing. J Br Menopause Soc. 2005;11:51–56. doi: 10.1258/136218005775544589. [DOI] [PubMed] [Google Scholar]
- 63.Aymanns C, Keller F, Maus S, et al. Review on pharmacokinetics and harmacodynamics and the aging kidney. Clin J Am Soc Nephrol. 2010;5:314–327. doi: 10.2215/CJN.03960609. [DOI] [PubMed] [Google Scholar]
- 64.Muhlberg W, Platt D. Age-dependent changes of the kidneys: pharmacological implications. Gerontology. 1999;45:243–253. doi: 10.1159/000022097. [DOI] [PubMed] [Google Scholar]
- 65.Lindeman RD. Changes in renal function with aging. Implications for treatment. Drugs & aging. 1992;2:423–431. doi: 10.2165/00002512-199202050-00006. [DOI] [PubMed] [Google Scholar]
- 66.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 67.Hu KT, Matayoshi A, Stevenson FT. Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patient. Am J Med Sci. 2001;322:133–136. doi: 10.1097/00000441-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Bowie MW, Slattum PW. Pharmacodynamics in older adults: a review. Am J Geriatr Pharmacother. 2007;5:263–303. doi: 10.1016/j.amjopharm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 71.Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989;321:952–957. doi: 10.1056/NEJM198910053211407. [DOI] [PubMed] [Google Scholar]
- 72.Parati G, Frattola A, Di Rienzo M, et al. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol. 1995;268:H1606–H1612. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]
- 73.ElDesoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther. 2007;14:488–498. doi: 10.1097/01.mjt.0000183719.84390.4d. [DOI] [PubMed] [Google Scholar]
- 74.Vestal RE, Wood AJ, Shand DG. Reduced beta-adrenoceptor sensitivity in the elderly. Clin Pharmacol Ther. 1979;26:181–186. doi: 10.1002/cpt1979262181. [DOI] [PubMed] [Google Scholar]
- 75.White M, Roden R, Minobe W, et al. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation. 1994;90:1225–1238. doi: 10.1161/01.cir.90.3.1225. [DOI] [PubMed] [Google Scholar]
- 76.Saksena S, Reddy VJ. Cardiac arrhythmias in elderly people:advances in diagnosis and management. In: Harzzard WR, Blass JP, Halter JB, et al., editors. Principles of Geriatric Medicine and Gerontology. 5th edition. McGraw-Hill; 2003. pp. 475–490. [Google Scholar]
- 77.Ali Raza J, Movahed A. Use of cardiovascular medications in the elderly. Int J Cardiol. 2002;85:203–215. doi: 10.1016/s0167-5273(02)00193-6. [DOI] [PubMed] [Google Scholar]
- 78.Beyth RJ, Quinn L, Landefeld CS. A multicomponent intervention to prevent major bleeding complications in older patients receiving warfarin. A randomized, controlled trial. Ann Intern Med. 2000;133:687–695. doi: 10.7326/0003-4819-133-9-200011070-00010. [DOI] [PubMed] [Google Scholar]
- 79.Fihn SD, Callahan CM, Martin DC, et al. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996;124:970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 80.Palareti G, Hirsh J, Legnani C, et al. Oral anticoagulation treatment in the elderly: a nested, prospective, case-control study. Arch Intern Med. 2000;160:470–478. doi: 10.1001/archinte.160.4.470. [DOI] [PubMed] [Google Scholar]
- 81.Alves LE, Rose EP, Cahill TB, Jr, et al. Disopyramide- induced urinary retention. Arch Intern Med. 1984;144:2099. [PubMed] [Google Scholar]
- 82.Davidson E, Fuchs J, Rotenberg Z, et al. Drug-related syncope. Clin Cardiol. 1989;12:577–580. doi: 10.1002/clc.4960121006. [DOI] [PubMed] [Google Scholar]
- 83.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 84.Sampson KJ, Kass RS. Anti-arrhythmic drugs. In: Brunton LL, Chabner BA, Knollman BC, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th edition. McGraw-Hill; 2010. [Google Scholar]
- 85.Torres D, Parrinello G, Paterna S, et al. Severe brochostenosis by oral propafenone immediately after commencing treatment. Am J Ther. 2011 doi: 10.1097/MJT.0b013e3181fc2fab. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 86.Griffith KA, Sherrill DL, Siegel EM, et al. Predictors of loss of lung function in the elderly: the cardiovascular health study. Am J Respir Crit Care Med. 2001;163:61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 87.Eichhorn EJ, Gheorghiade M. Digoxin. Prog Cardiovasc Dis. 2002;44:251–266. doi: 10.1053/pcad.2002.31591. [DOI] [PubMed] [Google Scholar]
- 88.Haynes K, Hennessy S, Localio AR, et al. Increased risk of digoxin toxicity following hospitalization. Pharmacoepidemiol Drug Saf. 2009;18:28–35. doi: 10.1002/pds.1680. [DOI] [PubMed] [Google Scholar]
- 89.CAST-II-Investigators Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. The cardiac arrhythmia suppression trial II Investigators. N Engl J Med. 1992;327:227–233. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- 90.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 91.Hayes BD, Klein-Schwartz W, Barrueto F., Jr Polypharmacy and the geriatric patient. Clin Geriatr Med. 2007;23:371–390. doi: 10.1016/j.cger.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Darbar D. Standard Antiarrhythmic Drugs. In: Zipes DP, Jalife J, editors. Cardiac Electrophsuiology: From Cell to Bedside. 5th edition. Saunders; 2009. pp. 959–973. [Google Scholar]
- 93.Taira CA, Opezzo JA, Mayer MA, et al. Cardiovascular drugs inducing QT prolongation: facts and evidence. Curr Drug Saf. 2010;5:65–72. doi: 10.2174/157488610789869229. [DOI] [PubMed] [Google Scholar]
- 94.Trujillo TC, Nolan PE. Antiarrhythmic agents: drug interactions of clinical significance. Drug Saf. 2000;23:509–532. doi: 10.2165/00002018-200023060-00003. [DOI] [PubMed] [Google Scholar]
- 95.Caporaso NE, Shields PG, Landi MT, et al. The debrisoquine metabolic phenotype and DNA-based assays: implications of misclassification for the association of lung cancer and the debrisoquine metabolic phenotype. Environ Health Perspect. 1992;98:101–105. doi: 10.1289/ehp.9298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bailey DG, Dresser GK. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004;4:281–297. doi: 10.2165/00129784-200404050-00002. [DOI] [PubMed] [Google Scholar]
- 97.Fromm MF, Kim RB, Stein CM, et al. Inhibition of P-glycoprotein-mediated drug transport: A unifying mechanism to explain the interaction between digoxin and quinidine [see comments] Circulation. 1999;99:552–557. doi: 10.1161/01.cir.99.4.552. [DOI] [PubMed] [Google Scholar]
- 98.Abel S, Nichols DJ, Brearley CJ, et al. Effect of cimetidine and ranitidine on pharmacokinetics and pharmacodynamics of a single dose of dofetilide. Br J Clin Pharmacol. 2000;49:64–71. doi: 10.1046/j.1365-2125.2000.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–941. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]
- 100.Mustafa SJ, Morrison RR, Teng B, et al. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009:161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen WK, Kurachi Y. Mechanisms of adenosine-mediated actions on cellular and clinical cardiac electrophysiology. Mayo Clin Proc. 1995;70:274–291. doi: 10.4065/70.3.274. [DOI] [PubMed] [Google Scholar]
- 102.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 103.Marcum ZA, Handler SM, Boyce R, et al. Medication misadventures in the elderly: a year in review. Am J Geriatr Pharmacother. 2010;8:77–83. doi: 10.1016/j.amjopharm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


