Abstract
Background
Atrial fibrillation (AF) causes a continuum of atrial anatomical remodeling.
Methods
Using a library of perfusion-fixed human hearts, specimens with AF were compared to controls. During this preliminary assessment study, direct measurements were taken of atrial volume, pulmonary vein (PV) circumference, and left atrial (LA) wall thicknesses.
Results
Hearts with AF typically had larger atrial volumes, as well as a much larger variation in volume compared to controls (range of 59.6–227.1 mL in AF hearts compared to 65.1–115.9 mL in controls). For all hearts, right PVs were larger than left PVs (mean: 171.4 ± 84.6 mm[2] for right and 118.2 ± 50.1 mm[2] for left, P < 0.005). LA wall thicknesses ranged from 0.7 mm to 3.1 mm for both AF and control hearts.
Conclusions
Hearts with AF had a large range of sizes which is consistent with the progression of atrial remodeling during AF. The large range of thicknesses will influence the amount of energy needed to create transmural lesions during ablation procedures.
Keywords: left atrial dimensions, volumes, pulmonary vein ostia, atrial fibrillation
1. Introduction
Atrial fibrillation (AF) is the most prevalent tachyarrhythmia, with an occurrence of 1% in the general population.[1] One of the particular clinical features of this arrhythmia is its self-perpetuating nature. Paroxysmal AF (self-terminating episodes) may eventually turn into persistent (> 7 days) and perhaps even permanent AF.[2]–[4] This progression is considered to be, in part, due to both structural and electrophysiological remodeling of the tissue that provides substrates for maintenance of such arrhythmias. Structural remodeling associated with AF refers to physical changes, such as dilation of the atria and interstitial fibrosis. The electrophysiologic remodeling results in shortening of atrial effective refractory periods, thus aiding the perpetuation of an arrhythmia and/or limiting the ability to terminate fibrillations. More specifically, the self-perpetuation is a particularly disturbing symptom when patient long-term prognosis is taken into account; mortality rate doubles and stroke occurrence averages up to 5% per year in patients with AF, which is 2 to 7 times the rate of individuals of similar ages without AF.[4]
1.1. The prevalence of atrial fibrillation in relation to age and associated risks
The clinical treatment of AF is an especially relevant topic in geriatric cardiology due to the well documented increasing prevalence with age. When considering an overall general population, the relative prevalence of AF is 1%, yet in persons over 40 years of age the prevalence reaches 2.3% and then climbs to 5.9% for individuals over 65.[5] The geriatric population, defined by the World Health Organization as persons with age greater than 65, includes over 75% of people suffering from AF.[1] It should also be noted that AF has been found to be more common in men than that in women (1.1% vs. 0.8%).[1]
AF is well known to be associated with decreases in quality of life, as reported by 68% of patients with paroxysmal AF.[6] Interestingly the psychological, rather than physical, quality of life may be impacted more.[7] In addition to stroke,[4] AF has also been associated with many other health problems that would cause decreased quality of life such as depression, professional and sex life complications.[8] However, it is debated whether or not AF is associated with cognitive decline.[9]–[11] This relationship is highly probable; AF causes an increased risk of stroke and emboli, thus cognitive decline may partially be linked to the potential for multiple small and transient cerebral infarcts, yet this will also be dependent on the given patient and his/her anticoagulation management.[12]
In addition to the above detrimental effects of AF, anatomical remodeling of the atria can occur within the heart. Patients who have AF tend to have larger left atria and larger pulmonary veins which could potentially lead to the further propagation of AF.[13] Alternatively, the reverse has also been shown; upon return to sinus rhythm after radiofrequency ablation, there is a measureable reduction in left atrial (LA) size.[14],[15] Depending on the length of AF and the stage of remodeling, patients with the arrhythmia may have large variations in their anatomy.
1.2. LA volume measurements
LA volumes have been selected for measurement in this sample of perfusion-fixed human hearts, due to reported AF-related atrial remodeling (dilations). For example, Leung et al.[16] found that increases in LA volume could be used to independently predict increased risks of cardiovascular death, heart failure, AF, stroke, or myocardial infarction. Further, subsequent reductions in LA volume (reverse remodeling) have been found to be a strong predictor of successful treatment of AF using either catheter-based[17] or surgical[18] ablations. Relative LA volumes for a given patient have also been found to predict the potential for recurrence of AF after cardioversion[19] and conversion of atrial flutter to AF after successful ablation.[20] More specifically, the probability of relapse after catheter ablation was found to be significantly higher for LA volumes greater than 145 mL.[17] An LA volume index greater than 135 mL/m2 was found to have 100% specificity, and LA diameter greater than 60 mm was found to have 100% sensitivity for prediction of surgical Maze failure.[18] Not surprisingly, normal LA volumes have been found to be associated with absence of thrombus[21] and relative left atrial appendage (LAA) dimensions have been found to be a positive predictor for stroke and transient ischemic attacks in patients with AF.[22]
1.3. Pulmonary vein sizes
Since the clinical discovery that the pulmonary veins (PVs) can be substantial site-sources of ectopic beats,[23] PV anatomy has attracted increasing attention. A patient's pulmonary vein sizes are useful measurements when planning for an ablation since some procedures, such as cryoballoon ablation, offer multiple sized devices to optimize PV isolation. The PV circumference was chosen for this study because even though the PVs (especially the left PVs) are typically oval in shape,[24] they are also considered as compliant, and a balloon pushed against the ostium will slightly change its shape. In other words, using simply the long or short axis for planning may lead to the decision to use a balloon that is too big or small, respectively.
1.4. LA wall thicknesses
LA wall thicknesses are also commonly assessed. In the current study, the thickness of the posterior wall near each of the PVs was measured as well as the center of the posterior wall. These locations were selected not only because they are smooth in comparison to the LAA, but they are often areas of therapeutic focus during AF ablation procedures. Variation in LA wall thicknesses is also important to consider during radiofrequency ablation procedures, since it should influence the amount of energy to be applied.[25]
One of the primary goals of this preliminary assessment study is to present our ongoing investigations related to human cardiac anatomy. Our Visible Heart® laboratory has a current library of over 200 human heart perfusion-fixed specimens that we can uniquely employ for such investigations. In addition, many of our images and videos of functional cardiac anatomy are available to the general public via a free-access web site, “The Atlas of Human Cardiac Anatomy” (http://www.vhlab.umn.edu/atlas).
2. Methods
Human hearts deemed not viable for transplantation were donated for educational and research purposes. Following similar guidelines for transplantation, the hearts were stopped, cooled, and transported to our lab. Within 24 hours of being excised, each specimen was weighed and the aorta, superior vena cava, pulmonary artery, pulmonary veins and the inferior vena cava (when possible on a given specimen) were cannulated and attached to a perfusion fixation chamber as described previously.[26] This approach preserves the hearts in a modified end-diastolic state (fully expanded atria and ventricles). These hearts were then fixed with 10% formalin in PBS solution for at least 24 hours under normal physiologic pressure and stored in formalin.
Heart specimens have been collected and added to our library since 1997 and, to date, the library has grown to over 200 in the collection. Anatomical studies can be performed on this large collection to better understand the variation in anatomy between different hearts. For most specimens, pertinent clinical histories are also available, thus allowing us to assess the variation in anatomy with respect to the relative disease state of the heart. In the present study, we identified and used hearts that had a clinical diagnosis of AF and compared them to hearts with no indication of AF or mitral/tricuspid regurgitation (normal anatomies). The AF hearts were sex, age, weight, and height matched to control hearts. For all but two of this sample of ten AF hearts, acceptable control hearts were found and/or measureable (Table 1).
Table 1. Patient information from AF and corresponding control hearts.
| AF | Control | |
| n | 10 | 8 |
| Age (SD) | 69.9 ± 11.6 | 63.9 ± 12.5 |
| Male (%) | 6 (60) | 4 (50) |
| Weight, kg | 80.8 ± 21.8 | 93.5 ± 31.5 |
| CAD (%) | 2 (20) | 0(0) |
| HTN (%) | 4 (40) | 5 (62.5) |
| Valve insufficiencies | 0 | 0 |
For all but two AF hearts, suitable matches were found as controls based on subject gender, age, weight and height. CAD: coronary artery disease; HTN: hypertension; AF: atrial fibrillation.
To measure the volumes of the atria, each heart was oriented such that the annulus of the atrioventricular valves would be parallel to the ground while measuring the respective side. To measure the volume of the LA, the aorta and all but one PV were clamped off, and the LA and left ventricle were filled with deionized water. After the LA was completely filled, the water was drawn out and collected until the water level reached the level of the annulus of the mitral valve, which was determined by visualization using a fiberscope. The weight of the water was recorded and this process was repeated a minimum of three times. The right atrium (RA) was measured in the same way, however the pulmonary artery, inferior vena cava, coronary sinus, and posterior interventricular vein were closed up and the water was drawn out to the level of the tricuspid valve. The siphoned water was weighed and repeated three times. Note that since deionized water was used, the weight of the fluid is equivalent to the volume of the chamber.
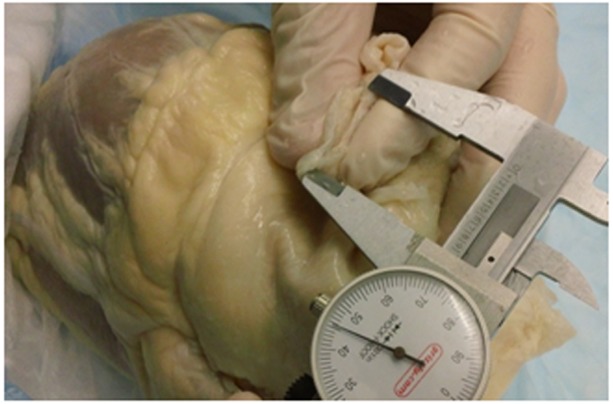
All other anatomical measurements were obtained using standard calipers or a C-clamp micrometer. The thicknesses of the LA were determined using the C-clamp at five distinct locations: the center of the posterior LA wall as well as the junction of the right superior, right inferior, left inferior, and left superior PVs. The junction of a PV was defined as the location in which the vein transitioned into the wall of the left atrium. To determine the relative areas of the PVs, the opening of each vessel was pinched together to measure half of the circumference (Figure 1) and obtain an area assuming the vessel was circular. Although it has been found in the literature that PVs are generally oval in shape, it was decided that comparing the vessels as circular would alleviate the problem of re-approximating the oval shape of the pulmonary veins. Furthermore, in some of these cases, the PV was slightly distorted due to the nature of the fixation process.
Figure 1. Illustration of the half circumference measurements taken to obtain diameters and area measurements of the various pulmonary veins.
The size of the fossa ovalis (FO) in each specimen was also measured; it should be noted that since the FOs were not collapsed during the fixation process, we were able to obtain accurate assessment of these structures. These areas were determined by measuring the diameters along the superior/inferior and the anterior/posterior direction of each FO.
Values presented are mean with standard error unless otherwise noted. For comparisons of continuous variables between two groups, a Student's t-test was used.
3. Results
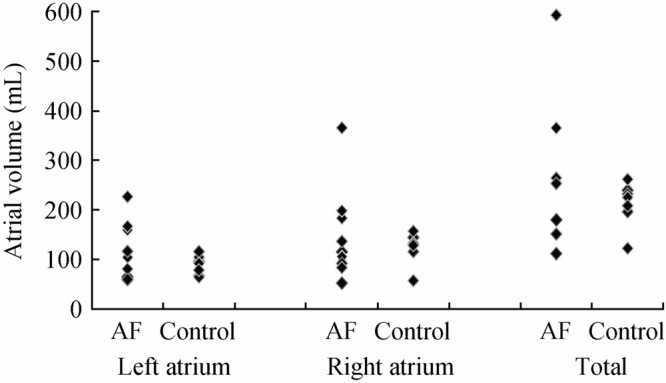
Within these specimens, the AF hearts had larger LA, RA and total atrial volumes compared to the control hearts (Figure 2). However when we compared individual AF hearts to their specific sex, age, weight and height matched controls, there was no clear correlation as to which types of heart had larger atrial chamber sizes. Yet, the data did suggest that there was a higher variability within the hearts from the AF patients compared to controls, with a range of 59.6 mL to 227.1 mL in the AF hearts compared to 65.1 mL to 115.9 mL in the control hearts. Note that the differences in range for both atria are over three times larger in the AF group than that in the control group.
Figure 2. Comparisons of the paired left, right, and total atrial volumes between the matched atrial fibrillation (AF) and control hearts. Note that the AF hearts had greater variability of volumes compared to the controls.
As noted above, the sizes of the PVs were assessed by assuming they were circular openings (Figure 3). Between the two groups, the control hearts had smaller PV ostia for both the left and right veins. One can observe two different peaks on these histograms, with the higher peak associated with data from the AF hearts. This finding was also consistent when the right and left PVs were compared separately (see Figure 3A and 3B respectively). Interestingly, we observed the trend that the left PVs were smaller than right PVs in both the control and AF specimens (Table 2). Further, while looking at the entire population of hearts, there was a significant difference between the right and left PVs (mean: 171.4 ± 84.6 mm2 for right and 118.2 ± 50.1 mm2 for left, P < 0.005).
Figure 3. The relative variation in pulmonary vein ostia sizes between the atrial fibrillation (AF) and control heart specimens. The histograms provide the corresponding areas of these ostia. (A): Relative distributions of pulmonary vein sizes of the right pulmonary veins, with means of 24.2 mm2 and 21.6 mm2 and medians of 23.1 mm2 and 19.9 mm2 respectively; (B): Left pulmonary vein areas, with means of 18.7 mm2 and 19.0 mm2 and medians of 18.4 mm2 and 17.7 mm2 respectively. The trend lines depict the two point moving averages of the distributions and illustrate the relative differences between these two heart populations.

Table 2. The mean and median values of the pulmonary vein (PV) ostia between atrial fibrillation (AF) and control heart specimens.
| All PVs |
Right PVs |
Left PVs |
||||
| Mean (mm2) | Median (mm2) | Mean (mm2) | Median (mm2) | Mean (mm2) | Median (mm2) | |
| AF | 152 | 139 | 193 | 170 | 116 | 109 |
| Control | 138 | 120 | 156 | 126 | 121 | 100 |
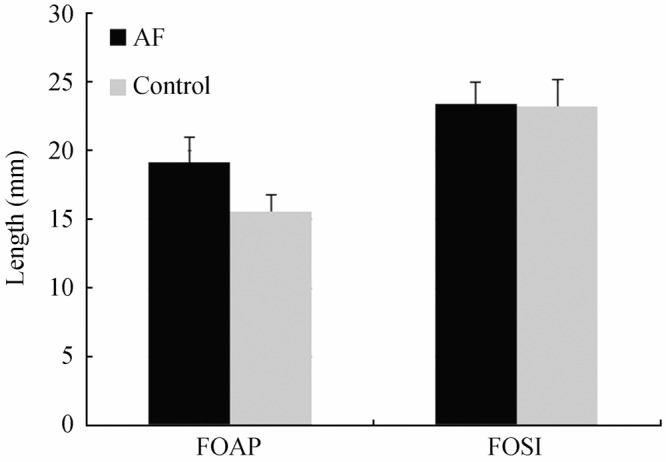
Although there were observed trends between the relative areas of the PV ostia of these two groups, in contrast there were minimal differences in the thicknesses of the LA wall within our samples; the means and medians were 1.19 mm and 1.24 mm for the AF population and 1.26 mm and 1.21 mm for the control population, respectively. The histograms and population spreads between the AF and control groups appear to elicit a fair amount of overlap; in other words, they appeared similar between the groups and were not dependent upon the diagnoses of AF (Figure 4). Similar findings were also observed when examining the relative sizes of the FO in these hearts. The FO dimensions only had slightly larger sizes in the anterior-posterior vectors, whereas they elicited minimal or no differences in the superior-inferior vectors (Figure 5).
Figure 4. Relative distribution of thicknesses of the left atrium between atrial fibrillation (AF) and control hearts. These thickness values were determined by taking measurements from each of the four pulmonary vein junctions in each heart, as well as the center of the posterior walls of the left atria. The trend lines depict the two point moving averages of the population distributions.

Figure 5. Average width and height measurements of fossa ovalis sizes between the two populations of hearts. The relative widths of the fossa ovalis were designated as the fossa ovalis anterior-posterior (FOAP) measurements, and the heights were designated as the fossa ovalis superior-inferior (FOSI) measurements (error bars represent SE). It was observed that there were no significant differences between the calculated averages of the measurements taken; distributions of the sizes showed similar trends between the atrial fibrillation (AF) and control hearts (data not shown).
4. Discussion
In the present study, we describe novel measurements of relevant cardiac anatomies obtained from our unique library of perfusion-fixed human hearts. We describe approaches that allow for the detailed quantification of specific atrial anatomies. The information presented provides useful insight regarding the changes in structure that may occur when the human heart remodels following the development of AF. We plan to continue these studies as our relevant library of specimens grows.
Current imaging modalities, including magnetic resonance imaging (MRI), ultrasound, and computed tomography (CT), allow for relative approximation of the volumes and sizes of the LA and RA using algorithms available in various software packages or by simply estimating diameters. Yet, it is considered that measurements of LA volumes are more accurate than diameter data, especially as a given heart dilates, i.e., small increases in diameter will provide for larger increases in derived LA volumes.[27],[28] Our volume measurements took into account the variability of chamber anatomy from heart to heart, and thus resulted in a more accurate representation of the total chamber sizes. Therefore, these chamber volumes, along with thickness measurements of the LA, were considered to better assess variations in anatomy due to a given pathological state. However, based on our data on this somewhat small sample size, we could not make correlations as to the clinical state based solely on these measurements. Yet, it should be noted that even in the absence of AF, large atria are often indicative of other cardiovascular issues,[29] thus in these hearts we studied from an elderly population of subjects it was likely that the non-AF hearts, or so-called controls, may not have been truly normal.
Our initial characterization of these AF hearts confirmed various trends that have been reported within current literature, such as the increased size of right PVs compared to left PVs.[13] However, as with most anatomical studies, a large sample set is required to potentially produce statistically significant results. With our growing library of these perfusion-fixed human hearts, we will be able to perform ongoing anatomical studies to better assess the differences within various populations of heart disease states or demographics. Our collection of specimens to date has allowed us to obtain useful insights regarding common anatomical features of the atria from AF patients with a high degree of accuracy. The variety of physical specimens also allows for physician education and therapeutic device feedback, as any number of devices can be placed within the hearts. The continuing study of human cardiac anatomy will provide further insights as to the changes in structure that may occur when the human heart remodels or reverse remodels due the presence of disease or following treatment, respectively. Though this study shows that PV measurements are not sufficient to diagnose AF, it illuminates the variety of anatomies that accompany the arrhythmia within the population. This large variation observed in diseased patients is important for physicians who treat AF, as well as educators and engineers who design medical devices to fit the whole range of anatomies. Using perfusion-fixed hearts, we plan to continue to expand these studies to further build an anatomical database, which we hope to compare to MRI, CT and 3D models derived from each.
4.1. Conclusions
Atrial fibrillation is associated with a great deal of anatomical and physiological changes. Anatomically, the size of the atria can vary greatly, but the pulmonary veins and vena cavae do not remodel significantly. The variation in anatomy has a great importance and should be considered when treating and designing devices for AF.
Acknowledgments
The authors would like to thank Krista Rothstein and Scott Pearson for their help in collecting measurements, as well as LifeSource, the donors, and their families for contributing to the collection of human hearts at the University of Minnesota.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. JAMA. 2001;285:2370. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wijffels M, Kirchof C, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 3.Rostock T, Steven D, Lutomsky B, et al. Atrial fibrillation begets atrial fibrillation in the pulmonary veins: On the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J Am Coll Cardiol. 2008;51:2153–2160. doi: 10.1016/j.jacc.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg W, Blackshear J, Laupacis A, et al. Prevalence, Age distribution, and gender of patients with atrial fibrillation. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 6.Hamer ME, Blumenthal JA, McCarthy EA, et al. Quality-of-life assessment in patients with paroxysmal atrial fibrillation or paroxysmal supraventricular tachycardia. Am J Cardiol. 1994;74:826–829. doi: 10.1016/0002-9149(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 7.Perret-Guillaume C, Briancon S, Wahl D, et al. Quality of Life in elderly inpatients with atrial fibrillation as compared with controlled subjects. J Nutr Health Aging. 2010;14:161–166. doi: 10.1007/s12603-009-0188-5. [DOI] [PubMed] [Google Scholar]
- 8.Dąbrowski R, Smolis-Bąk E, Kowalik I, et al. Quality of life and depression in patients with different patterns of atrial fibrillation. Kardiol Pol. 2010;68:1133–1139. [PubMed] [Google Scholar]
- 9.Park H, Hildreth A, Thomson R, et al. Non-valvular atrial fibrillation and cognitive decline: a longitudinal cohort study. Age Ageing. 2007;36:157. doi: 10.1093/ageing/afl164. [DOI] [PubMed] [Google Scholar]
- 10.Puccio D, Novo G, Baiamonte V, et al. Atrial fibrillation and mild cognitive impairment: what correlation. Minerva Cardioangiol. 2009;57:143–150. [PubMed] [Google Scholar]
- 11.Jozwiak A, Guzik P, Mathew A, et al. Association of atrial fibrillation and focal neurologic deficits with impaired cognitive function in hospitalized patients > or = 65 years of age. Am J Cardiol. 2006;98:1238–1241. doi: 10.1016/j.amjcard.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Rastas S, Verkkoniemi A, Polvikoski T, et al. Atrial fibrillation, stroke, and cognition: A longitudinal population-based study of people aged 85 and older. Stroke. 2007;38:1454–1460. doi: 10.1161/STROKEAHA.106.477299. [DOI] [PubMed] [Google Scholar]
- 13.Kato R, Lickfett L, Meininger G, et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003;107:2004–2010. doi: 10.1161/01.CIR.0000061951.81767.4E. [DOI] [PubMed] [Google Scholar]
- 14.Reant P, Lafitte S, Jaïs P, et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. 2005;112:2896–2903. doi: 10.1161/CIRCULATIONAHA.104.523928. [DOI] [PubMed] [Google Scholar]
- 15.Beukema WP, Elvan A, Sie HT, et al. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation. 2005;112:2089–2095. doi: 10.1161/CIRCULATIONAHA.104.484766. [DOI] [PubMed] [Google Scholar]
- 16.Leung DY, Chi C, Allman C, et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol. 2010;105:1635–1639. doi: 10.1016/j.amjcard.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Abecasis J, Dourado R, Ferreira A, et al. Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace. 2009;11:1289. doi: 10.1093/europace/eup198. [DOI] [PubMed] [Google Scholar]
- 18.Sunderland N, Maruthappu M, Nagendran M. What size of left atrium significantly impairs the success of maze surgery for atrial fibrillation? Interact Cardiovasc Thorac Surg. 2011;13:332–338. doi: 10.1510/icvts.2011.271999. [DOI] [PubMed] [Google Scholar]
- 19.Marchese P, Bursi F, Delle Donne G, et al. Indexed left atrial volume predicts the recurrence of non-valvular atrial fibrillation after successful cardioversion. Eur J Echocardiogr. 2011;12:214–221. doi: 10.1093/ejechocard/jeq176. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y, Hyun DW, Jung BC, et al. Left atrial volume index as a predictor for occurrence of atrial fibrillation after ablation of typical atrial flutter. J Cardiol. 2010;56:348–353. doi: 10.1016/j.jjcc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Ayirala S, Kumar S, O'Sullivan David M, et al. Echocardiographic predictors of left atrial appendage thrombus formation. J Am Soc Echocardiogr. 2011;24:499–505. doi: 10.1016/j.echo.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Beinart R, Heist EK, Newell JB, et al. Left atrial appendage dimensions predict the risk of stroke/TIA in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:10–15. doi: 10.1111/j.1540-8167.2010.01854.x. [DOI] [PubMed] [Google Scholar]
- 23.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 24.Wittkampf FHM, Vonken E, Derksen R, et al. Pulmonary vein ostium geometry: analysis by magnetic resonance angiography. Circulation. 2003;107:21–23. doi: 10.1161/01.cir.0000047065.49852.8f. [DOI] [PubMed] [Google Scholar]
- 25.Beinart R, Abbara S, Blum A, et al. Left atrial wall thickness variability measured by CT Scans in patients undergoing pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011 doi: 10.1111/j.1540-8167.2011.02100.x. In press. [DOI] [PubMed] [Google Scholar]
- 26.Anderson SE, Hill AJ, Iaizzo PA. Microanatomy of human left ventricular coronary veins. Anat Rec (Hoboken) 2009;292:23–28. doi: 10.1002/ar.20766. [DOI] [PubMed] [Google Scholar]
- 27.Lester SJ, Ryan EW, Schiller NB, et al. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 28.Nikitin NP, Witte KKA, Thackray SDR, et al. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr. 2003;4:36–42. doi: 10.1053/euje.2002.0611. [DOI] [PubMed] [Google Scholar]
- 29.Thomas L, Levett K, Boyd A, et al. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol. 2002;40:1630–1635. doi: 10.1016/s0735-1097(02)02371-9. [DOI] [PubMed] [Google Scholar]


