Abstract
Objective
To report Medtronic experiences with the development of animal models for atrial fibrillation (AF) and chronic heart failure (CHF) using high-rate pacing for AF and microemboli for CHF.
Methods
For the AF model, an atrial lead was attached to a Medtronic Synergy™ neurostimulator, which was programmed to stimulate at 50 Hz in an on-off duty cycle. Atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and N-terminal pro brain natriuretic peptide (NT-proBNP) were assayed at select time points. For CHF model, a serial injection of 90 µm polystyrene microspheres at 62,400 beads/mL (Polybead, Polysciences, Inc.) was performed to induce global ischemia, either with weekly monitoring and embolization schedule (group 1, n = 25) or with biweekly monitoring and emboliation schedule (group 2, n = 36 ). Echocardiograms were used along with ventriculograms and magnetic resonance imaging scans weekly to assess cardiac function and ANP, BNP and NT-proBNP were monitored.
Results
For the AF model, the days to sustained AF for four animals following surgery were 7, 25, 21 and 19, respectively; For the CHF model, the days to meet CHF endpoints were 116 in group 1 and 89 in group 2. For both AF and CHF models, NT-proBNP correlated well with the development of disease states.
Conclusion
Our experience for the development and assessment of AF and CHF dog models may help researchers who are in search for animal model for assessing the safety and efficacy of a device-based therapy.
Keywords: atrial fibrillation, congestive heart failure, biological markers, dog models, microembolism
1. Introduction
Atrial fibrillation (AF) and congestive heart failure (CHF) are two distinct diseases disproportionately prevalent in the geriatric population and responsible for significant morbidity and mortality in the Western world, previously estimated to affect 1% to 2% of the general population.[1]–[4] Studies that account for aging of the population and increasing obesity project large increases in their rates of occurrence.[5] AF and CHF are also frequent comorbidities. In the Framingham population, 41% of CHF subjects developed AF and 42% of AF subjects developed CHF at some time in their life respectively. AF preceded CHF approximately as often as CHF preceded AF.[6] This can be explained in part by their shared underlying pathologies such as coronary artery disease, hypertension, and nonischemic cardiomyopathy, but AF and CHF also reinforce each other.[7],[8] The presence of CHF can facilitate AF induction via increased atrial pressure and volume, increased catecholamine activity, conduction heterogeneity, and interstitial fibrosis, which act as triggers to AF and also increase the critical mass required to sustain fibrillation. AF can induce CHF through onset of rapid and irregular ventricular contractions, loss of atrial systolic activity, reduced cardiac output, and triggering of the renin-angiotensin-aldosterone system.
Various medical device technologies have been applied to treat both AF and CHF, respectively.[9]–[14] Animal models can provide a valuable means of assessing the safety and efficacy of a device-based therapy prior to human clinical trials, providing a simulation of human disease. For medical devices, large animals are typically required because of the need to model the size and anatomy of humans, with a plateau in growth at a reasonably young age and modest size. Also, for chronic studies an animal must have suitable behavior for frequent handling (e.g., device downloads, blood draws). For these reasons, healthy and diseased dogs have been used extensively to evaluate cardiac stimulation technology in chronic studies.
AF can be induced in large animals via high-rate pacing, hemodynamic overload, ischemic injury, or neurohormonal modulation.[15] Paroxysmal AF has been induced by infusion of adrenergic and cholinergic drugs[16] and neural growth factors.[17] Persistent or permanent AF has been induced in dogs by high-rate pacing of the atrium[18] and sometimes combined with induction of mitral valve regurgitation.[19] Older dogs have been used occasionally and have shown a greater susceptibility to AF,[20] but while advantageous in more closely matching the geriatric patient population, the availability and cost of older dogs is a barrier to their large-scale use. CHF is commonly induced in large animal models by ventricular pacing and/or ischemic myocardial injury.[21]–[25]
Electrophysiological and hemodynamic monitoring is important to track the development of arrhythmias for both AF and CHF. Clinical biomarkers are useful for screening or to track development, progression, and prognosis of AF and CHF.[26]–[32] Neurohormonal imbalance plays a role in the pathogenesis of heart failure.[33] Both epinephrine and norepinephrine have been assayed in canine models of heart failure with ambiguous success.
In this paper, Medtronic experiences with the development of animal models for AF and CHF are presented, focusing on unique refinements to model development using high-rate pacing for AF and microemboli for CHF.
2. Methods
The study protocols were approved by the Institutional Animal Care and Use Committee at Medtronic physiologic research laboratories and all animals received humane care in accordance with the guide for the care and use of laboratory animals published by the National Institutes of Health (National Institutes of Health Publication 85–23, revised 1996). Animals were quarantined for a minimum of 10 days before surgery and preoperative assessments, including laboratory tests and transthoracic echocardiography, were performed on all surgical candidates.
2.1. AF Animals
For AF animal models, four dogs were given an analgesic (morphine, im) and sedative (Acepromazine, im). The animals were induced with a hypnotic (propofol, to effect). Isoflurane (to effect) was used to maintain the animal at an appropriate plane of anesthesia.
An atrial lead was attached to a Medtronic Synergy™ neurostimulator, which was programmed to stimulate at 50 Hz in an on-off duty cycle. The stimulation “on” phase was fixed at five seconds. The no stimulation “off” phase was originally set to two minutes and adjusted down to as low as 30 seconds until AF was consistently observed.
To monitor electrogram (EGM) in AF animals, one Medtronic pacing lead was implanted in the right ventricle and another in the right atrium. Both were connected to an EnRhythm® pacing generator loaded with research software to record EGMs at programmed intervals. The devices were monitored throughout the study using the Medtronic 2090 programmer. The EGM data was downloaded and used to adjust the stimulation from longer to shorter off cycles until the longest off cycle that would reliably produce AF was found.
Dogs were put on aspirin and dipyridamole the day prior to surgery and continued throughout the duration of the study to minimize the chance of embolic events (e.g., stroke). Atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and N-terminal pro brain natriuretic peptide (NT-proBNP) were drawn and assayed at selected time points.
2.2. CHF Animals
CHF models included two groups of dogs: group 1 (n = 25) with weekly monitoring and embolization schedule and group 2 (n = 36) with biweekly monitoring and embolization schedule. Time points included: pre-embolization, post-embolization (and prior to device implantation), therapy baseline (4 weeks post-implantation), and weeks 6 and 12 past therapy baseline.
For all CHF animals, analgesic (morphine, im) and a sedative (Acepromazine, im) were given to the dogs. The animals were induced with a hypnotic (propofol, to effect). Isoflurane (to effect) was used to maintain the animal at an appropriate plane of anesthesia. Target vessel selection was determined prior to each surgical intervention with the aid of echocardiographic imaging. Access to the coronary arteries was obtained by femoral arteriotomy. Once the desired coronary artery was cannulated a serial injection of 90 µm polystyrene microspheres at 62,400 beads/mL (Polybead, Polysciences, Inc.) was performed to induce global ischemia in either the left anterior descending or left circumflex coronary artery. Microsphere injections were performed during diastole with the aid of fluoroscopy until sustained ST segment changes were observed on electrocardiogram (ECG) or up to a maximum of 3 mL. Heart rate and blood pressure were monitored closely during injections. Any sudden increases in heart rate combined with drops in blood pressure that were slow to rebound indicated that embolizations were discontinued. ECG changes were tracked on more than one lead (I, II, III, and a precordial lead were used). Low blood pressure was treated with dobutamine and/or epinephrine administration for as long as it was required. ECG monitoring post-operatively allowed appropriate decisions on which animals were treated with anti-arrhythmic drugs.
Immediately following embolization surgery, animals were admitted into the post anesthesia care unit (PACU). If the animal experienced numerous arrhythmias (tachyarrhythmia with heart rate > 150 bpm (beats per minutes) or if the ECG appeared polymorphic) the animals were treated with lidocaine and/or procainamide. If animals remained in the PACU for more than 24 hours postoperatively, they were not eligible for embolization the following week. Regardless of whether an animal underwent embolization, an awake echocardiograph was completed weekly. Echocardiography results and recovery from previous embolizations were utilized to select animals for subsequent embolization.
In early CHF animals, both awake and anesthetized echocardiograms were used along with ventriculograms and magnetic resonance imaging scans weekly to assess cardiac function. In early animals, two of the following three independent measurements of left ventricular triplane ejection fraction (EF) were used to define CHF in the canines: (1) An awake echocardiogram with a triplane EF of 30% ± 5%; (2) An anesthetized echocardiogram with a triplane EF of 25% ± 5%; and (3) An anesthetized ventriculogram with an EF of 25% ± 5%.
In group 2, CHF embolizations were performed every other week and animals received a diagnostic ultrasound during the week in between. If analysis of the ultrasound showed that the EF was 35% or lower, the animal was not embolized and reexamined by ultrasound the next week. If the second ultrasound analysis confirmed that the dog's EF was still at 35% or lower, the dog was transferred to a therapy study, otherwise it was scheduled for embolization during the next week.
A series of inflammatory, hemodynamic, necrosis, and kidney function biomarkers selected from Table 1, were collected for group 1. C-reactive protein (CRP) was performed using a canine specific ELISA (KMI Diagnostics, Inc.). Epinephrine and norepinephrine were assayed by a radioimmunoassay (RIA) method (KMI Diagnostics, Inc.) that included an extraction process. An enzyme immunoassay method designed to measure immunoreactive NT-proBNP specific for the dog used two immunoaffinity purified sheep antibodies specific for canine NT-proBNP (IDEXX Laboratories). ANP and BNP were analyzed using RIA kits (Mayo Clinic, Rochester, MN). The BNP method was canine specific, while the ANP used anti-human ANP. Blood urea nitrogen (BUN) and creatinine concentrations were measured using an automated chemistry analyzer (Roche Cobas Integra 400+). Cardiac troponin I (cTnI) was determined by use of the Abbott i-STAT ELISA method.
Table 1. Examples of biomarkers in canine heart failure.
| Category | Name |
| Inflammatory | TNF-α, IL-6, TGF-β, IL-10, CRP |
| Hemodynamic | Catecholamines (epinephrine & norepinephrine),BNP, NT-proBNP, ANP |
| Necrosis | CK, CK-MB, Troponin-I |
| Kidney | BUN, creatinine |
TNF: tumor necrosis factor; IL: interleukin; TGF: transforming growth factor β; CRP: C-reactive protein; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro brain natriuretic peptide; ANP: atrial natriuretic peptide; CK-MB: creatine kinase MB; CK: creatine kinase; BUN: blood urea nitrogen.
3. Results
3.1. AF Model
Figure 1 shows EGM samples at different time points for a representative AF dog. Burst stimulation was applied to induce AF, which would sustain for varied lengths of time before returning to sinus rhythm. Eventually, the AF remained persistent. The ventricular rates for the on-off burst pacing rarely exceeded 200 bpm.
Figure 1. Electrogram 15 seconds samples for dog C at (A) initiation of burst pacing, (B) three days after burst pacing, and (C) 38 days after initiation of burst pacing, and seven days after stimulation has been turned off.
The days to sustained AF for four animals A, B, C, and D following surgery were 7, 25, 21 and 19, respectively, with stimulation turned on between two days and one week post-surgery. In all cases, once AF was observed for the full 15 seconds of EGM sample it was observed for all subsequent samples. For two of the animals stimulation was later turned off and AF remained. In all cases, a final terminal procedure was performed to evaluate therapies without continuous stimulation where AF was sustained without interruption for between 6 and 8 hours. No embolic events were observed and heart failure symptoms only observed several weeks after AF was observed.
Figures 2, 3, and 4 respectively show NT-proBNP, BNP, and ANP values for the AF dogs. Dog C reached AF before the two week blood draw, which was reflected in elevated measurements for all three biomarkers. ANP and BNP values with and without AF showed a statistically significant difference with a non-paired t-test (Minitab, Minitab Inc.). NT-proBNP values with and without AF were not compared statistically because the test values hit an assay maximum at 3,000 pmol/L. However, upon inspection, NT-proBNP tracked AF better than ANP and BNP; all dogs were in AF by week 4 and only NT-proBNP was consistent in exhibiting both no elevation at week 2 and elevation at week 4.
Figure 2. NT-proBNP for AF dogs over time (top diagram) and with and without AF (bottom dagram), (A, B, C and D indicate dogs A, B, C and D, respectively, Ref: reference value). NT-proBNP: N-terminal pro brain natriuretic peptide; AF: atrial fibrillation.
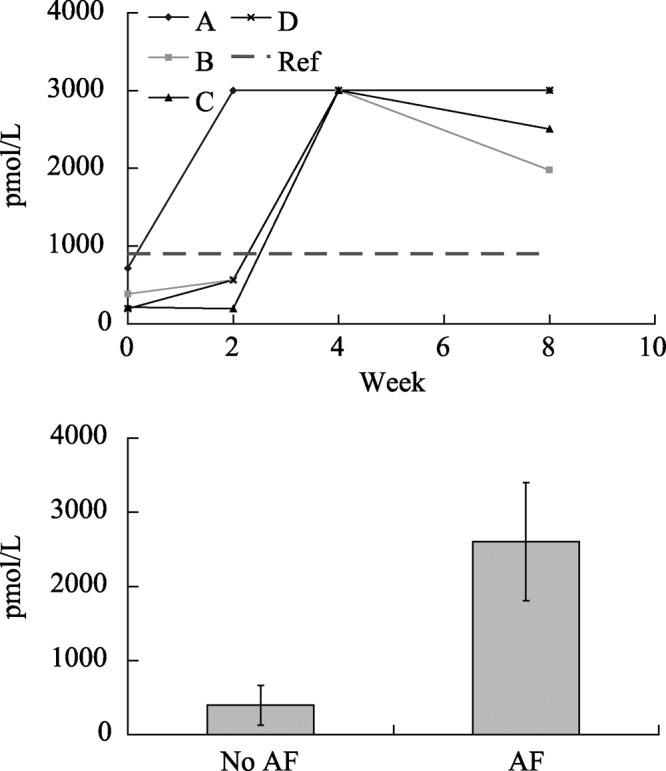
Figure 3. ANP for AF dogs over time (top diagram) and with and without AF (bottom diagram), (High: high end of reference range; Low: low end of reference range, A, B, C and D indicate dogs A, B, C and D, respectively). ANP: atrial natriuretic peptide; AF: atrial fibrillation.

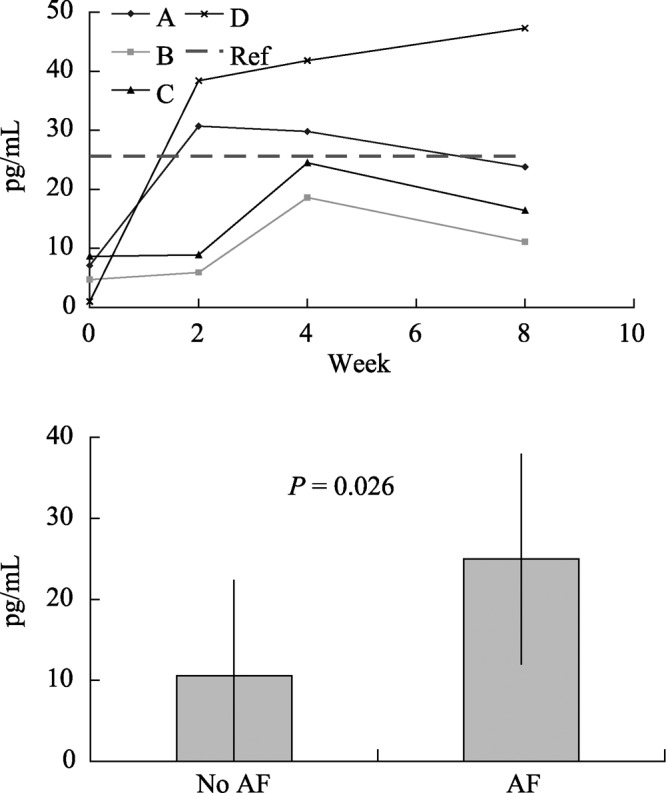
Figure 4. BNP for AF dogs over time (top diagram) and (bottom dagram) with and without AF (A, B, C and D indicate dogs A, B, C and D, respectively, Ref: reference value). BNP: brain natriuretic peptide; AF: atrial fibrillation.
3.2. CHF Model
The two cohorts of CHF animals resulted in successful endpoints for the microembolization protocols despite slightly differing embolization and monitoring regimens. In Table 2, the comparison of results between group 1 and group 2 CHF groups show the effect of more frequent embolizations. While the mortality increased from 16% to 22% in group 2, the average time to meet the endpoint dropped from 116 days to 89 days in this cohort. Echocardiographic monitoring of EF and other measurements were used in the follow-up period after the CHF animals were transferred to the therapy protocol.
Table 2. Comparison between group 1 and 2 CHF model.
| Groups | Total number of embolizations | Microspheres injected | Days to CHF | Original EF (%) | Final EF (%) | Change in EF (%) | Mortality (%) |
| Group 1 | 7.2 | 860,600 | 116 | 45.9 | 34.7 | −11.2 | 16.0 |
| Group 2 | 5.9 | 748,800 | 89 | 48.9 | 34.3 | −14.6 | 22.0 |
CHF: congestive heart failure; EF: ejection fraction.
Early model development required monitoring in multiple imaging modalities to explore their respective associations with heart failure. Figure 5 plots the EF for a subset of 6 animals in group 1 CHF animals at the beginning and end of model development for awake echocardiogram, anesthetized echocardiogram, ventriculography, and magnetic resonance imaging (MRI). Both ventriculography and MRI reported higher EF results at the end of model development than echocardiography. It was observed that animals diagnosed in CHF using clinical signs including decreased appetite and decreased activity levels consistently presented with EF measurements below 35% when measured via awake echocardiography. Thus, the awake echo became the preferred method of measuring EF. Both cohorts exhibited degradation of awake echocardiographic EF over time, as shown in Figure 6. Moreover, the EF results show that both cohorts maintained their hemodynamic status even after microembolizations had ceased.
Figure 5. Ejection fraction for different imaging modes for a subset of six animals in group 1.
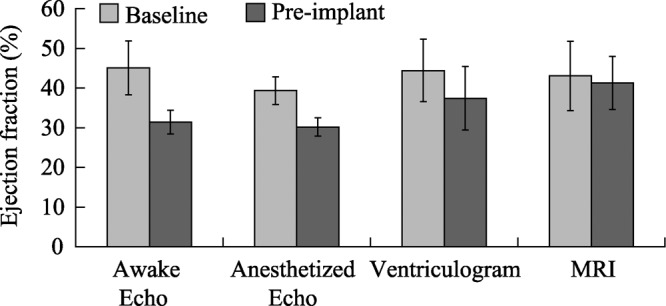
Figure 6. Awake echocardiographic ejection fraction (EF) for (A) group 1 and (B) group 2 showing progressive decay into heart failure and stability of the heart failure well beyond the embolization stage.
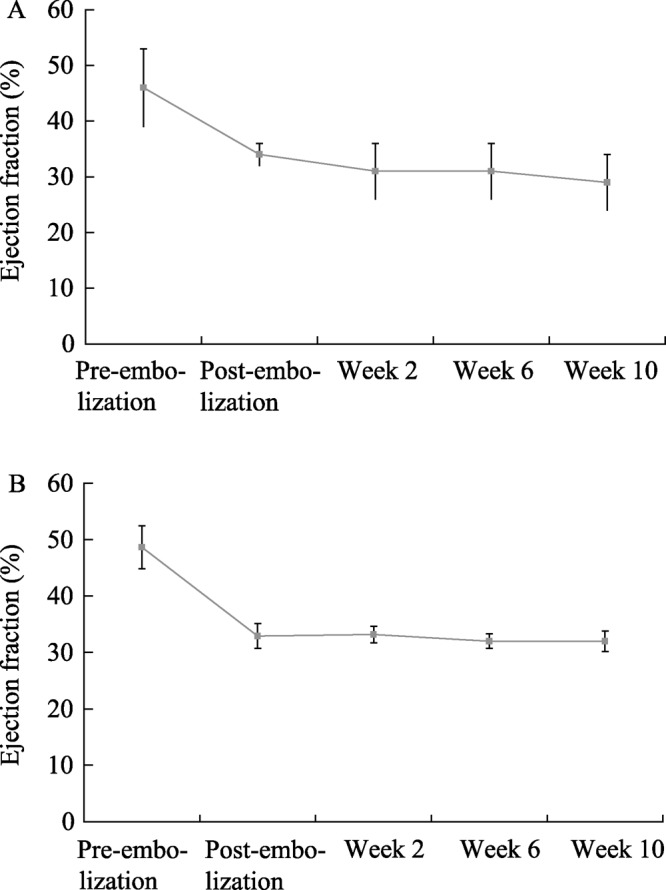
Table 3 shows the biomarkers tested for indications of heart failure, cardiac tissue damage, or necrosis. The most compelling evidence for CHF was demonstrated by increased NT-proBNP levels. The reference cut-off value for canine NT-proBNP measured in this group was < 566 pmol/mL, with any values above this range indicated some level of compromised hemodynamic function. Overall compromise of cardiac function was confirmed with increased NT-proBNP concentrations as a function of time and number of embolizations. As shown in Table 3, levels of BUN and creatinine, markers of renal function, remained within their respective reference intervals at all time points. Concentrations of epinephrine and norepinephrine in this dog model failed to show any increases during CHF development. This observation is in contrast to humans with CHF and differs from a previous report of increased catecholamines in an ischemic canine model.[25] In addition to the analytes listed in this table, TGF-β, TNF-α, and IL-6 were measured, but levels fell below their respective lower reference limits at all time points. For NT-proBNP, significant increases above the upper reference limit were attained at the four post-embolization time points.
Table 3. Biological markers taken in group 1 CHF dogs.
| Analyte | Reference Interval | Pre-embolization | Post-embolization | Post-device implant, week 0) | Week 6 | Week 12 |
| BUN (mg/dL) | 8–22 | 14 ± 3 (n = 16) | 14 ± 4 (n = 16) | 13 ± 3 (n = 16) | 12 ± 3 (n = 8) | 12 ± 2 (n = 8) |
| Creatinine (mg/dL) | 0.6–1.1 | 0.9 ± 0.1 (n = 16) | 0.9 ± 0.1 (n = 16) | 0.8 ± 0.1 (n = 16) | 0.9 ± 0.1 (n = 8) | 0.9 ± 0.1 (n = 8) |
| Epinephrine (ng/mL) | < 3 | 0.095 ± 0.115 (n = 6) | 0.069 ± 0.083 (n = 9) | 0.044 ± 0.115 (n = 16) | 0.039 ± 0.062 (n = 8) | 0.007 ± 0.010 (n = 8) |
| Norepinephrine (ng/mL) | < 3 | 0.077 ± 0.054 (n = 6) | 0.158 ± 0.189 (n = 9) | 0.046 ± 0.035 (n = 16) | 0.018 ± 0.020 (n = 8) | 0.011 ± 0.018 (n = 8) |
CHF: congestive heart failure; BUN: blood urea nitrogen. Data are presented as mean ± SD, n: number of samples.
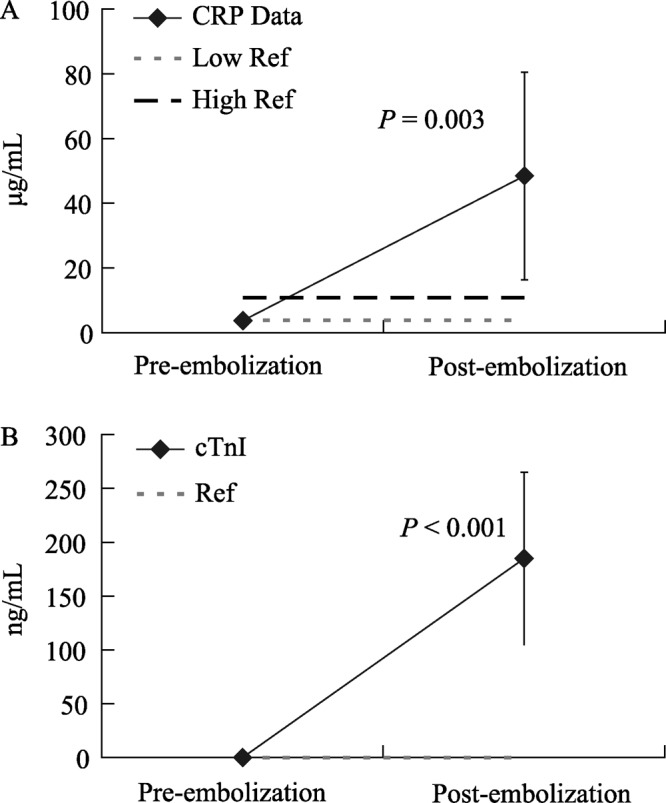
The temporal levels of biomarkers are useful at different milestones of model development. While NT-proBNP reflected compromised hemodynamic function after induction of CHF, CRP and cTnI levels were used to assess inflammation and myocyte damage incurred within 24 hours following each embolization event. Figure 7A shows a comparison of CRP levels immediately before embolization and 24 hours after embolization surgery and Figure 7B shows cTnI levels at the same time points. For both CRP and cTnI the levels before and after embolization were compared with a paired t-test (Minitab, Minitab Inc.) and found to be statistically different.
Figure 7. Biomarkers before and after embolization for group 1, with reference intervals in dotted lines, including (A) CRP (n = 9) and (B) cTnI (n = 10) to exhibit a sharp increase reflecting myocardial damage. CRP: C-reactive protein; cTnI: cardiac troponin I.
4. Discussion
In order to develop and refine AF and CHF models at Medtronic, significant attention was paid to clinical management without utilizing clinical therapies (e.g., beta blockers) that could confound the evaluation of the test device, as well as characterization of changes seen. Electrophysiological monitoring, hemodynamic monitoring, and biomarker data were all key aspects of characterizing both AF and CHF study animals.
For the development of an AF model, it is highly preferable for AF to precede CHF, once an animal is in CHF it becomes more challenging to manage clinically. Because of its compromised clinical state, any induction methodology (e.g., high-rate pacing) may have to be restricted. On-off burst pacing yielded better results than continuous high-rate pacing (data not reported), because ventricular rate could be controlled better in the early stages of model development. Although once in AF the fast intrinsic atrial rate will drive a fast ventricular rate, on-off cycles ensure that the high-rate pacing itself does not drive a rapid ventricular rate. Induction of mitral valve regurgitation via snaring of the chordae tendinea has been used in the development of AF models.[34] This method can dramatically increase the dilation of the atrium. However, it is technically challenging and may facilitate CHF prior to development of AF. For the microembolism model AF was not observed in the cohort reported here, although it has been observed in our other studies.
With model refinements, CHF was induced more rapidly in group 2 dogs as compared to group 1 (89 days as compared to 116) without increasing mortality above the reported value of 30% (Table 3).[24] It should be noted that in the Medtronic experiences, ischemic models are relatively insensitive to therapies that exhibit success in the literature (e.g., beta blockers). It is possible based on comparison of the echocardiogram and ventriculogram results that a more severe and stable pathology is being induced in the methods described by the Medtronic experiences than in the literature.
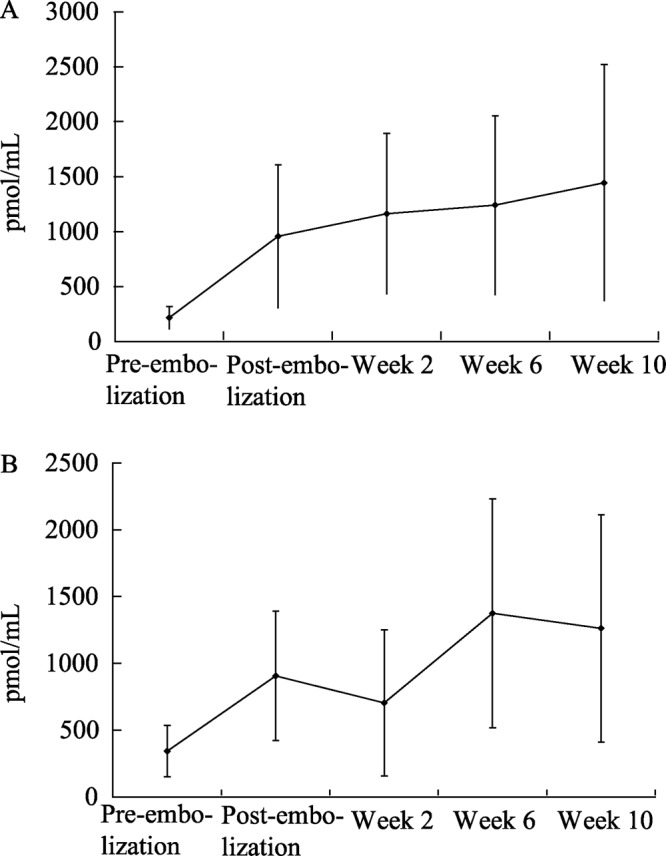
For both AF/CHF models, NT-proBNP correlated well with the development of disease states. Elevated NT-proBNP values were compared to echocardiography data, and overall compromise of cardiac function was confirmed (Figure 8). This relationship could result because NT-proBNP is more stable in plasma and serum and fluctuates less compared to BNP and is therefore a better marker of the long-term status of heart disease. In canine plasma, BNP has an extremely short half-life of 90 seconds and is inferior to NT-proBNP in its ability to track CHF.[35] Despite achievement of a CHF state, adequate kidney function was preserved as reflected by normal BUN and creatinine levels. Many biomarkers are used for diagnosis, prognosis, and assessment of therapeutic response in humans afflicted with AF and CHF. Several of these biomarkers tested in our animal studies have failed to correlate with clinical symptoms, physiological functions, or diagnostic imaging. Care must be exercised before translating human tests to animal species.
Figure 8. NT-proBNP for (A) group 1 and (B) group 2 showing increased values with progressive heart failure. NT-proBNP: N-terminal pro brain natriuretic peptide.
4.1. Limitations
A distinct difference between the animal model and the human clinical course is the timeline of disease progression Human cardiac pathology is driven by underlying systemic disease coupled with the aging process over many years. These models are being induced in young animals that often lack comorbidities over a brief period of time. However, age significantly changes many of the cellular and physiological processes affected by therapies. This fact may become even more of a factor for delivery of biological therapies, whose mechanisms of action are so dependent on cellular level processes.
Acknowledgments
The authors thank Dr. Hani Sabbah and Dr. Ruth Klepfer of Medtronic for initial assistance in developing the microembolism model, Dr. Vinod Sharma for initial assistance in developing an AF animal model, and Michael Kopcak for editorial comments on the manuscript.
References
- 1.Feinberg W, Blackshear J, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation: Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 2.Furgerg C, Psaty B, Manolio T, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 3.Cowie M, Wood D, Coats A, et al. Incidence and aetiology of heart failure: A population-based study. Eur Heart J. 1999;20:421–428. doi: 10.1053/euhj.1998.1280. [DOI] [PubMed] [Google Scholar]
- 4.Kannel W, Abbott R, Savage D, et al. Epidemiologic feature of chronic atrial fibrillation: The Framinham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 5.Miyasaka Y, Barnes M, Gersh B, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections of future prevelance. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Larson M, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 7.Gronefeld G, Hohnloser S. Heart failure complicated by atrial fibrillation: mechanistic, prognostic, and therapeutic implications. J Cardiovasc Pharmacol Ther. 2003;8:107–113. doi: 10.1177/107424840300800203. [DOI] [PubMed] [Google Scholar]
- 8.Savelieva I, Camm A. Atrial fibrillation and heart failure: natural history and pharmacological treatment. Europace. 2004;5(Suppl 1):S5–S19. doi: 10.1016/j.eupc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Cleland J, Daugert J, Erdmann E, et al. Longer-term efects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz P. Vagal stimulation for heart failure. Curr Opin Cardiol. 2010;26:51–54. doi: 10.1097/HCO.0b013e3283413961. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaisis L, Elahi D, Hentosz T, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 12.Luca Botto G, Boriani G, Favale S, et al. Treatment of atrial fibrillation with a dual defibrillator in heart failure patients (TRADE HF): protocol for a randomized clinical trial. Trials. 2011;12:44. doi: 10.1186/1745-6215-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Yamada H, Bibevski S, et al. Chronic atrioventricular nodal vagal stimulation: first evidence for long-term ventricular rate control in canine atrial fibrillation model. Circulation. 2005;112:2904–2911. doi: 10.1161/CIRCULATIONAHA.105.568832. [DOI] [PubMed] [Google Scholar]
- 14.Sigg D, Hiniduma-Lokuge P, Coles J, et al. Focal pharmacological modulation of atrioventricular nodal conduction via implantable catheter. A novel therapy for atrial fibrillation? Circulation. 2006;113:2373. doi: 10.1161/CIRCULATIONAHA.105.609420. [DOI] [PubMed] [Google Scholar]
- 15.Nattel S, Shiroshita-Takeshita A, Brundel BJ, et al. Mechanisms of atrial fibrillation: lessons from animal models. Prog Cardiovasc Dis. 2005;48:9–28. doi: 10.1016/j.pcad.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Sharifov O, Fedorov V, Beloshapko G, et al. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Swissa M, Zhou S, Paz O, et al. Canine model of paroxysmal atrial fibrillation and paroxysmal atrial tachcardia. Am J Physiol Heart Circ Physiol. 2005;289:1851–1857. doi: 10.1152/ajpheart.00083.2005. [DOI] [PubMed] [Google Scholar]
- 18.Wijffels M, Kirchhof C, Dorland R, et al. Atrial fibrillation begets atrial fibrillation: A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Leung T, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 20.Anyukhovsky E, Sosunov E, Plotnikov A, et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res. 2002;54:462–469. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 21.Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39:60–76. doi: 10.1016/s0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Lahtinen S, Lentz L, et al. Feasibility of using an implantable system to measure thoracic congestion in an ambulatory chronic heart failure canine model. Pacing Clin Electrophysiol. 2005;28:404–411. doi: 10.1111/j.1540-8159.2005.40009.x. [DOI] [PubMed] [Google Scholar]
- 23.Shainbane J, Wood M, Jensen D, et al. Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah H, Stein P, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;(4 Pt 2):H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 25.Lopshire JC, Zhou X, Dusa C, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine post-infarction heart failure model. Circulation. 2009;120:286–294. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
- 26.Apple F. Tissue specificity of cardiac troponin I, cardiac troponin T and creatine kinase-MB. Clin Chim Acta. 1999;284:151–159. doi: 10.1016/s0009-8981(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 27.Apple F, Murakami M, Ler R, et al. Analytical characteristics of commercial cardiac troponin I and T immunoassays in serum from rats, dogs, and monkeys with induced acute myocardial injury. Clin Chem. 2008;54:1982–1989. doi: 10.1373/clinchem.2007.097568. [DOI] [PubMed] [Google Scholar]
- 28.Oyama M, Rush J, Rozanski E, et al. Clinical utility of serum N-terminal pro-B-type natriuretic peptide concentration for identifying cardiac disease in dogs and assessing disease severity. J Am Vet Med Assoc. 2008;232:1496–1503. doi: 10.2460/javma.232.10.1496. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald K, Kittleson M, Munro C, et al. Brain natriuretic peptide concentration in dogs with heart disease and congestive heart failure. J Vet Intern Med. 2003;17:172–177. doi: 10.1111/j.1939-1676.2003.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 30.Beardow A. Canine N-terminal pro-B-type natriuretic peptide and echocardiographic measurements in small breed dogs with and without cardiac disease. American College of Veterinary Internal Medicine Proceedings, Montreal, Quebec, 2009.
- 31.Boomsma F, van den Meiracker A. Plasma A- and B-type natriuretic peptides; physiology, methodology and clinical use. Cardiovasc Res. 2001;51:442–449. doi: 10.1016/s0008-6363(01)00195-x. [DOI] [PubMed] [Google Scholar]
- 32.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 33.Chidsey C, Kaiser G, Sonnenblick E, et al. Cardiac norepinephrine stores in experimental heart failure in the dog. J Clin Invest. 1964;43:2386–2393. doi: 10.1172/JCI105113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everett T, Li H, Mangrum M, et al. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation. 2000;102:1454–1460. doi: 10.1161/01.cir.102.12.1454. [DOI] [PubMed] [Google Scholar]
- 35.Woods RL JM. Atrial, B-type, and C-type natriuretic peptides cause mesenteric vasoconstriction in conscious dogs. Am J Physiol. 1999;276:1143–1452. doi: 10.1152/ajpregu.1999.276.5.R1443. [DOI] [PubMed] [Google Scholar]



