Abstract
Multislice CT angiography represents one of the most exciting technological revolutions in cardiac imaging and it has been increasingly used in the diagnosis of coronary artery disease. Rapid improvements in multislice CT scanners over the last decade have allowed this technique to become a potentially effective alternative to invasive coronary angiography in patients with suspected coronary artery disease. High diagnostic value has been achieved with multislice CT angiography with use of 64- and more slice CT scanners. In addition, multislice CT angiography shows accurate detection and analysis of coronary calcium, characterization of coronary plaques, as well as prediction of the disease progression and major cardiac events. Thus, patients can benefit from multislice CT angiography that provides a rapid and accurate diagnosis while avoiding unnecessary invasive coronary angiography procedures. The aim of this article is present an overview of the clinical applications of multislice CT angiography in coronary artery disease with a focus on the diagnostic accuracy of coronary artery disease; prognostic value of coronary artery disease with regard to the prediction of major cardiac events; detection and quantification of coronary calcium and characterization of coronary plaques. Limitations of multislice CT angiography in coronary artery disease are also briefly discussed, and future directions are highlighted.
Keywords: coronary artery disease, plaque, diagnosis, multislice computed tomography, angiography
1. Introduction
Coronary artery disease (CAD) is the leading cause of death in advanced countries and its prevalence is increasing among developing countries.[1],[2] In 2001, CAD was reported to be responsible for 7.3 million deaths and 58 million disability-adjusted life years lost worldwide.[3] According to recent World Health Organization statistics for 2007, cardiovascular deaths account for 33.7% of all deaths worldwide, whereas cancer represents 29.5%, other chronic diseases, injury and communicable diseases contribute to 26.5%, 7%, and 4.6%, respectively.[4] Cardiovascular disease costs more than any other diagnostic group.[1] The total direct and indirect cost of cardiovascular disease and stroke in the United States for 2010 is estimated to be $503.2 billion. In comparison the estimated cost of all cancer and benign neoplasms was $228 billion in 2008. Due to the current global focus on healthcare utilization, costs, and quality, it is essential to monitor and understand the magnitude of healthcare delivery and costs, as well as the quality of healthcare delivery in relation to the cardiovascular disease.
Traditionally, diagnosis of CAD is performed by invasive coronary angiography which is recognized as the gold standard technique, since it has superior spatial and temporal resolution leading to excellent diagnostic accuracy. However, it is an invasive and expensive procedure associated with a small but distinct procedure-related morbidity (1.5%) and mortality (0.2%).[5] Furthermore, invasive coronary angiography usually requires patients to stay for a short period in the hospital after the examination and this causes discomfort for the patients. Moreover, not all of the invasive angiography examinations are performed for both diagnostic and therapeutic purposes, and some of the invasive angiography examinations are performed only for diagnostic purposes, which is only for verification of the presence and extent of CAD. Thus, a non-invasive technique for imaging and diagnosis of CAD is highly desirable. Over the last decades, non-invasive imaging modalities have undergone rapid developments, such as multislice CT (MSCT), magnetic resonance imaging (MRI), radionuclide imaging such as single photon emission computed tomography (SPECT) and positron emission tomography (PET).[6],[7] Although promising results have been achieved with cardiac MRI, especially with the technical improvements of MRI scanners such as 3T MRI,[8],[9] cardiac MRI has a number of limitations including variable protocols, long scanning time and lack of wide availability. These limitations prevent cardiac MRI from becoming a widely acceptable non-invasive imaging modality in the diagnosis of CAD. Myocardial perfusion imaging with SPECT is a widely established method for non-invasively evaluating the myocardial viability, left ventricular function and coronary artery stenosis.[10],[11] PET has been reported to be valuable in the diagnosis of CAD and is more sensitive and specific than SPECT in the detection and localization of coronary stenoses.[12],[13] However, these nuclear medicine imaging modalities have an inherent disadvantage of poor anatomical details, thus, providing little information about coronary lumen changes as well as characterization of coronary plaques. SPECT and PET are not recommended as a routine imaging modality for the detection and diagnosis of CAD, despite excellent cardiac functional information can be acquired with these techniques.
In comparison, MSCT angiography allows for excellent visualization of anatomical details of coronary artery and its branches, and is the only imaging modality which has been widely used in the diagnosis of CAD with high diagnostic accuracy being achieved.[14],[15] MSCT angiography is a relatively simple procedure that does not require arterial access or hospital admission. After intravenous injection of contrast medium and elective pre-medication, e.g., use of beta-blocker to slow down heart rate in some patients, the entire procedure does not require more than 15 min. Images can be post-processed and reconstructed during different cardiac phases to allow retrospective selection of the phases with the least motion artifacts, while in the meantime to assess ventricular performance. In addition to the detection and diagnosis of coronary artery stenosis, the benefits of MSCT angiography compared to invasive coronary angiography are to quantify and characterize atherosclerotic plaques, provide independent prognostic information for predicting cardiac events and mortality in patients with known or suspected CAD.[16],[17] This article reviews the clinical applications of MSCT angiography in cardiac imaging with a focus on the diagnostic accuracy and prognostic value of the MSCT angiography in CAD. MSCT angiography in the detection and quantification of coronary calcium as well as characterization of coronary plaques is also explored. Limitations and future directions of MSCT angiography are highlighted.
2. Multislice CT angiography-technical developments
Imaging of the heart has always been technically challenging due to the heart's continuous movement. Coronary artery has a diameter which ranges from 3 mm to 5 mm in the main segments, and 1 mm to 1.5 mm in the distal segments. A normal heart rate ranges from 60 to 80 beats per minute in a healthy adult. In order to adequately visualize the coronary artery tree with clear demonstration of the normal coronary lumen and detection and quantification of coronary artery stenosis with a minimal 20% change in the coronary diameter, the CT scanners need to provide a spatial resolution of at least 0.5 mm and a temporal resolution of between 200 ms and 250 ms. It is impossible to achieve this goal with a single slice CT scanner as it has limited spatial resolution with 1.0 mm along the longitudinal axis, and inferior temporal resolution which is reflected in the long gantry rotation time (1 s per rotation). Imaging of the heart has moved into the diagnostic era with the introduction of MSCT scanners in 1998, and this represents a significant technical improvement in the CT scanning technique.[18],[19]
The early generation of MSCT scanners enables simultaneous acquisition of four slices at a rotation time of 0.5 s, which is four times faster than the traditional single slice CT, providing significant improvement of scan speed and longitudinal resolution.[20],[21] Later technical developments such as 64- or more slice CT scanners allow for acquisition of large volume data in a very short time with a rotation time down to 0.165 s, and with high spatial and temporal resolution.[22]–[24] The developments of MSCT have been widely recognized as revolutionary improvements in the medical imaging field that eventually enable cardiac imaging to be performed with high diagnostic accuracy.
CT angiography has been widely applied to investigate vascular anatomy and diseases, and it has been regarded as one of the most valuable applications in CT imaging. Diagnostic value of CT angiography has been significantly enhanced with use of MSCT techniques, owing to its improved resolution, enabling excellent visualization of both main artery and side branches. MSCT angiography produces angiography-like images non-invasively with high diagnostic accuracy, thus it has replaced invasive angiography in many applications.[7] In particular, MSCT angiography has proved valuable in imaging the coronary artery tree for diagnosis of CAD. Studies have shown that in selected patients, MSCT angiography can be used as a reliable alternative to invasive coronary angiography.[15],[25]–[27] This is of clinical significance because the number of invasive coronary angiography examinations can be reduced or unnecessary invasive procedures can be avoided based on MSCT angiography findings.
3. Multislice CT angiography in coronary artery disease: coronary calcium scoring
Direct relationships between coronary artery calcification and the presence and to a modest degree, the extent and severity of atherosclerotic CAD have been demonstrated in comparisons based on histology, ultrasound and invasive angiography.[28]–[30] This correlation of coronary artery calcium (CAC) with the amount of coronary plaque has raised increasing interest in the non-invasive imaging detection and quantification of coronary calcium for diagnosis of CAD and estimation of the disease prognosis. Electron beam CT (EBCT) was the first accurate and sensitive non-invasive imaging technique to quantify CAC.[31] The main limitation of EBCT is its inferior spatial resolution which is between 1.5 mm and 3.0 mm. This restricts its diagnostic value to accurately evaluate the severity of coronary artery disease. EBCT has been replaced with MSCT since 2003 onwards.
Quantifying the amount of CAC with unenhanced CT scan has been widely accepted as a reliable non-invasive technique for screening risk of future cardiac events,[32],[33] and is usually quantified by using the Agatston score.[34] The purpose of the scan is to detect and calculate the calcium density, volume or mass. The total coronary calcium is used as a way of predicting and stratifying the risk of CAD. Clinical application of CAC scoring has been supported by evidence showing that absence of calcium reliably excludes obstructive coronary artery stenoses,[35] and that the amount of CAC is a strong predictor for risk assessment of myocardial infarction and sudden cardiac death, independent of conventional coronary risk factors.[36],[37] CAC scoring is regarded as a good predictor of major cardiac events and adds incremental prognostic value to risk factors in patients from different risk groups. However, its predictive value is determined by the patients' symptoms. In asymptomatic individuals, it has been reported that a zero CAC is associated with a very low (<1% per year) risk of major cardiac events over the next 3–5 years, whereas in asymptomatic patients with extensive coronary calcification, the major cardiac events have been reported to be increased by up to 11-fold.[38]–[40] Several large population-based studies have reported that in asymptomatic patients without known CAD, CAC is predictive of future cardiac events above and beyond traditional risk factors.[41]–[43]
In symptomatic patients, CAC scoring is considered only marginally related to the degree of coronary stenosis, as it is well known that both obstructive and non-obstructive CAD can occur in the absence of calcification.[44],[45] Coronary stenoses are frequently found to be non-calcified, and highly calcified plaques are frequently non-obstructive. Thus, the value of a zero or low calcium score in symptomatic patients remains unclear. Studies have shown that zero or low calcium score is present in up to 8.7% of symptomatic patients with obstructive non-calcified plaques.[46],[47] Cheng et al.[46] reported that low but detectable CAC scores are less reliable in predicting plaque burden due to their association with high overall non-calcified coronary artery plaque. According to these reports, low CAC scores are less valuable in the prediction of prevalence or severity of coronary artery disease caused by the non-calcified coronary plaques.
4. Multislice CT angiography in coronary artery disease: characterization of plaques
Since major adverse cardiac events are caused by plaque rupture, it is important to use imaging methods to detect, quantify and characterize coronary atherosclerotic plaque which enables risk stratification. As calcium comprises only one component of plaque and non-calcified structures, such as a large necrotic core and thin fibrous cap are usually considered to indicate high inclination towards plaque rupture,[48] an increasing interest has seen in the use of medical imaging to visualize and analyze the components of coronary atherosclerotic plaques.
Coronary plaques can be characterized into the following three types based on the CT attenuation[49]: non-calcified plaques are defined as lesions with a radiodensity greater than neighbouring soft tissue but lower density than the contrast-enhanced coronary lumen (Figure 1); calcified plaques indicate lesions with density higher than contrast-enhanced coronary artery lumen (Figure 2); mixed plaques refer to lesions with non-calcified and calcified components (calcium component between 20% and 80%) within a single lesion (Figure 3) or within a segment of the coronary artery (Figure 4).
Figure 1. A non-calcified coronary plaque (arrow) is visualized at the proximal segment of right coronary artery on a curved planar reformatted image in a 67-year-old woman presenting with the symptom of chest pain. The plaque results in more than 50% coronary lumen stenosis.
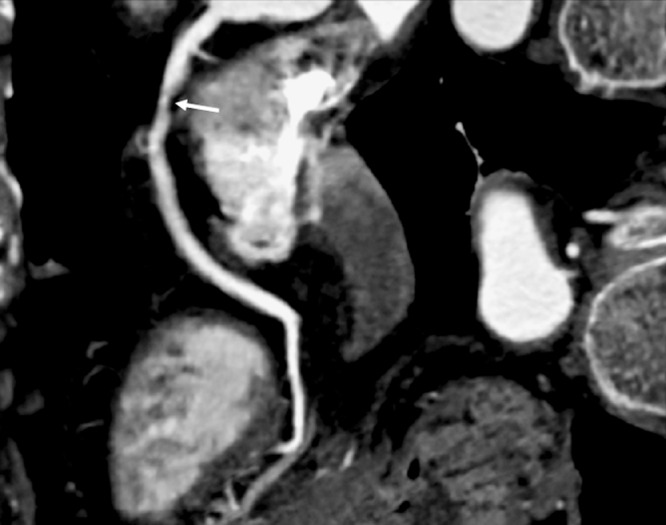
Figure 2. Calcified coronary plaques are shown at the proximal segment of left anterior descending (long arrow) and distal segment of left circumflex (short arrow) branches on a coronal maximum-intensity projection image in a 73-year old man with chest pain and history of hypertension.
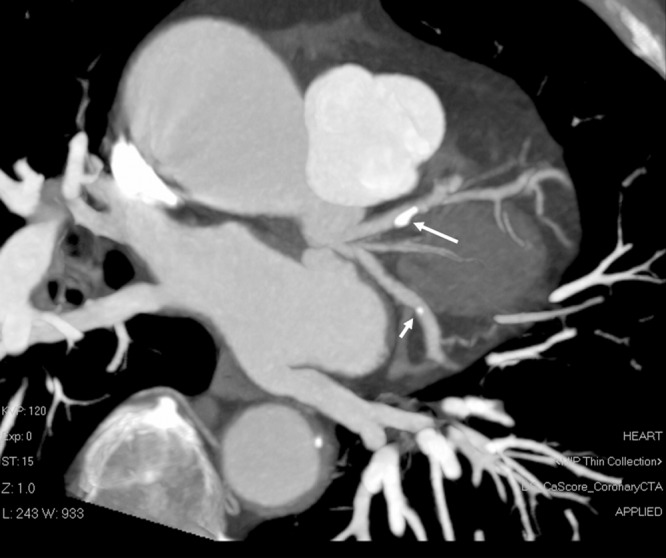
Figure 3. A mixed coronary plaque (long arrow in A) is present within a lesion at the proximal segment of left anterior descending artery as shown on a curved planar reformatted image in a 55-year-old man with symptoms of chest discomfort and epigastric pain. A calcified plaque is also noticed at the mid-segment of the same left coronary artery (short arrow in A). Three-dimensional (3D) volume rendering demonstrates significant stenosis in the left anterior descending due to the calcified plaque (arrows in B).
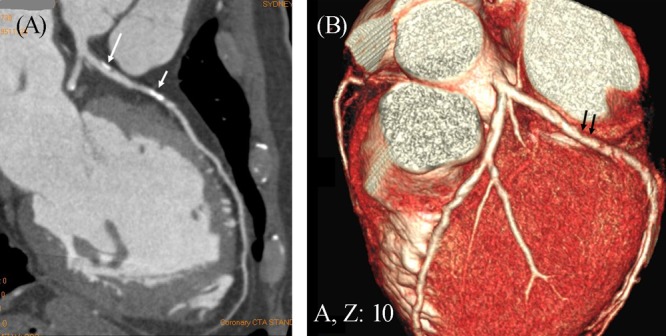
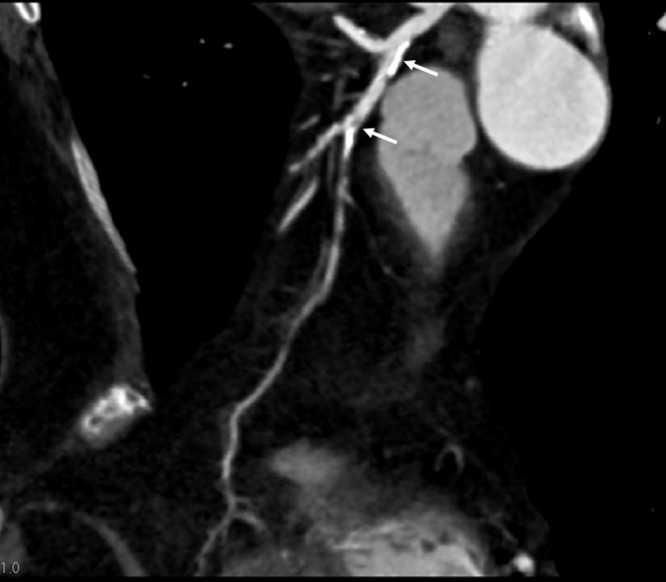
Figure 4. Curved planar reformatted image shows mixed coronary plaques (arrows) at the proximal segment of left anterior descending in a 67-year-old female with atypical chest pain, diabetes and hypertension.
It is generally believed that lipid-rich plaques have a higher risk of rupture with consequent thrombosis than fibrotic plaques, thus, differentiation of different plaques based on measurements of CT attenuation has attracted attention to researchers. Studies comparing MSCT angiography with intravascular ultrasound (IVUS) demonstrated that MSCT angiography is able to detect variable densities in the coronary atherosclerotic plaques.[50]–[52] However, a direct comparison between MSCT angiography and IVUS revealed general overestimation on MSCT for quantitative measurements of plaque areas and thickness.[53],[54] Moreover, MSCT angiography fails to detect unstable plaques, thus differenttiation of lipid-rich content from fibrous content with MSCT remaining challenging due to overlap in the attenuation values of lipid and fibrous tissue.[55]
5. Multislice CT angiography in coronary artery disease: diagnostic value
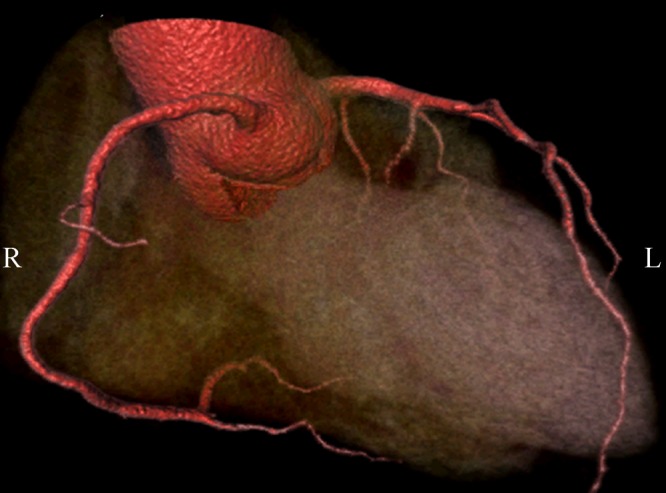
Diagnostic value of MSCT angiography in CAD has been significantly enhanced with the developments of MSCT scanners over the last decade. Early studies with use of 4- and 16-slice CT showed moderate diagnostic accuracy in the diagnosis of CAD due to technical limitations.[14] Image quality of coronary artery visualization was impaired and suboptimal in a number of cases with 4-slice CT as the unassessable coronary segments could be as high as more than 20%.[14] With 16-slice CT, thinner detector rows increased the spatial resolution and further shortened the total scan time. Therefore, image quality in 64-slice CT angiography has become more consistent with the reported sensitivities and specificities ranging from 83% to 98% and 96% to 98%, respectively.[56]–[58] Further increased diagnostic accuracy was achieved with 64-slice CT due to improved spatial and temporal resolution, thus leading to shorter examination times. Several meta-analyses of 64-slice CT studies in the diagnosis of CAD reported that the sensitivities were more than 90% and specificities more than 96% in most of the studies.[15],[25]–[27] Heart rate control with use of beta-blockers is necessary in single source 64-slice CT as image quality is affected by motion artifacts in patients with heart rates more than 65 beats per minute. This limitation has been overcome with the introduction of dual-source CT as the temporal resolution was shortened from 165 ms to 75 ms, thus heart rate dependence was eliminated. Studies performed with dual-source CT demonstrated high diagnostic accuracy for diagnosis of CAD, and most importantly the image quality is independent of heart rate (Figure 5).[59]–[61] In particular, MSCT angiography has demonstrated a very high negative predictive value (more than 95%), indicating that it can be used as a reliable technique to exclude patients suspected of CAD, thereby reducing the need for invasive coronary angiography.
Figure 5. Coronal maximum-intensity projection images acquired with a dual-source 64-slice CT angiography shows normal right (A) and left coronary artery branches (B) in a 47-year-old man with atypical chest pain. 3D volume rendering images (C and D) demonstrate right and left coronary arteries with excellent visualization of the main coronary and side branches.
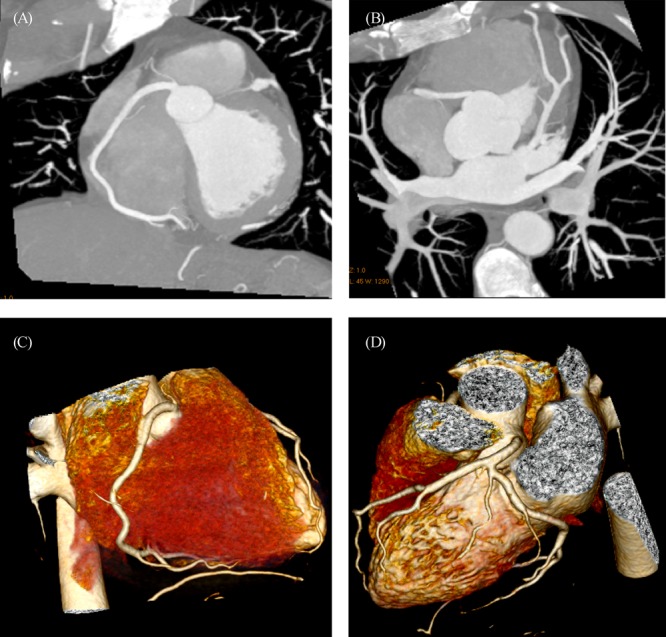
Expansion of MSCT systems from a prototype 64-slice to a 256-slice or 320-slice system has allowed for acquisition of whole-heart coverage in one gantry rotation with a slice thickness of 0.5 mm.[23],[24],[62]–[64] A maximum 4 cm (64 × 0.625 mm) longitudinal coverage can be achieved with 64-slice CT in one heart beat, thus, 3–5 heart beats are normally required to cover the entire heart volume with 64-slice CT scanners. In contrast, a 12.8-cm (256 × 0.5 mm) and 16-cm (320 × 0.5 mm) of craniocaudal coverage can be achieved with 256- and 320-slice CT in a single heart beat, with excellent image quality and demonstration of the entire coronary arteries (Figure 6). Very high diagnostic accuracy has been reported with 320-slice CT angiography for detection of significant coronary stenosis across all coronary segments, regardless of size, cardiac rhythm or image quality.[62],[63] Furthermore, 320-slice CT enables visualization of coronary artery and its segments with sufficient image quality in patents with atrial fibrillation.[64] 320-slice CT represents a promising technical improvement of MSCT angiography in cardiac imaging, although further studies based on large cohorts from multicenters are needed to confirm its diagnostic value.
Figure 6. 3D volume rendering of the coronary arteries and side branches are clearly demonstrated with use of 320-slice CT angiography in a 58-year-old man presenting with chest pain. Volumetric data are acquired within a single heart beat with excellent image quality.
6. Multislice CT angiography in coronary artery disease: prognostic value
The anatomy-based approach is a well established method for risk stratification of patients as demonstrated by invasive coronary angiography, which clearly delineates the severity and extent of significant coronary stenosis. High risk coronary anatomy (three-vessel CAD, stenosis of left main coronary artery) is directly related to poorer outcome,[65]–[67] while normal coronary artery is associated with an excellent prognosis. [68] Despite many reports showing the prognostic value of coronary calcifications detected on non-enhanced CT scans, it is not until very recently that the prognostic value of MSCT angiography has been made clear.
Early studies investigating the short to mid-term outcomes of patients undergoing 64-slice CT angiography reported that MSCT angiography is able to provide independent prognostic information for predicting cardiac events and mortality in patients with known or suspected CAD.[69],[16] Findings of MSCT angiography based on a single centre experience have been closely associated with the future cardiac events, with 0% or 1% cardiac events being reported in patients with normal cardiac CT or mild coronary artery disease, and up to 30% in patients with one or more vessel obstructive CAD.[70],[17] Recently, Abdulla et al.[71] conducted a meta-analysis of 10 relatively large studies evaluating the prognostic value of 64-slice CT angiography. The meta-analysis showed that cumulative cardiac event rates over a mean follow-up of 21 mo were 0.5% in patients with normal MSCT angiography, 3.5% in those with non-obstructive CAD and 16% in patients with obstructive CAD by 64-slice CT angiography. Compared to a normal MSCT angiography, non-obstructive CAD was associated with a significant increased risk of major adverse cardiac events, while obstructive CAD was associated with a greatly increased further significant risk. The prediction of excellent prognosis together with the high negative predictive value makes MSCT angiography the appropriate imaging modality to exclude CAD and prognosticate populations with different pre-test likelihoods for CAD.
7. Multislice CT angiography in coronary artery disease: future directions
MSCT angiography is one of the most exciting developments in recent years in the diagnosis of CAD. Although rapidly technical improvements and increased diagnostic accuracy with satisfactory results have been achieved with latest MSCT scanners, invasive coronary angiography still remains the gold standard technique in the diagnosis of CAD as it allows for quantitative assessment of the coronary artery lumen. The spatial resolution of the latest MSCT scanners is 0.5 mm, which is quite close to the 0.2 mm that is available with invasive coronary angiography. Thus detection of coronary wall changes with current MSCT scanners can be achieved with high accuracy. However, the temporal resolution of 75 ms that is available with MSCT angiography is still inferior to the 20 ms with invasive coronary angiography, therefore, aggressive heart rate control is a necessity in most of the MSCT angiography examinations. Further technical developments to improve the gantry rotation speed will enable MSCT angiography applicable to patients with high or irregular heart rates.
There is no doubt that MSCT angiography cannot replace invasive coronary angiography in the diagnosis of CAD in the near future, however, the judicious use of this non-invasive imaging technique could play an important role in the clinical workup and management of patients with suspected CAD. MSCT angiography should be used to aim for more effective risk stratification of patients, allowing identification of those patients who would be most likely to benefit from invasive coronary angiography and reduce the number of invasive procedure in patients who do not have obstructive coronary disease. Studies have shown that a reasonable number of patients with suspected CAD had normal coronary arteries or non-obstructive disease on invasive coronary angiography.[72]–[74] These reports suggest that many unnecessary invasive angiography examinations were performed in the clinical evaluation of patients with suspected CAD. MSCT angiography is an effective imaging modality of determining which patients should undergo invasive coronary angiography.
It is generally agreed that CT is an imaging modality with high radiation exposure, as it contributes up to 70% radiation dose of all radiological examinations, although it comprises only 15% of all radiological examinations.[75] Radiation-induced malignancy associated with cardiac CT imaging is a major issue which has raised serious concern in the literature. With increasing application of MSCT angiography in the diagnosis of CAD, the research focus has shifted from the previous emphasis on diagnostic value to the current focus on reduction of radiation dose with acceptable diagnostic images. This is reflected in the increasing publications on dose reduction through applying different dose-saving strategies in MSCT angiography.[76]–[79] Thus, the responsible use of MSCT angiography is absolutely necessary in terms of justifying and adjusting the MSCT scanning techniques.
8. Summary and conclusion
MSCT angiography represents the most rapidly developed imaging modality in cardiac imaging, with satisfactory results having been achieved in the diagnosis of CAD. MSCT angiography demonstrates high accuracy for detection of coronary calcium, characterization of atherosclerotic plaques and prediction of disease progression. Currently, MSCT angiography cannot be recommended as a reliable alternative to invasive coronary angiography in patients with suspected CAD. MSCT angiography serves as an effective and independent predictor for predicting coronary artery disease progress and major cardiac events. This has significant clinical value because a normal MSCT angiography suggests that patients have normal coronary arteries and can be safely reassured without undergoing further tests or invasive examinations such as invasive coronary angiography.
Radiation associated with MSCT angiography has increased significantly over the last decade and this should draw attention to the clinicians responsible for referring patients for CT examinations. Accurate risk stratification for appropriate selection of MSCT angiography is crucial, and both radiologists and referring physicians (mainly cardiologists) need to work together to develop better selection criteria for patients referred for MSCT angiography.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stoke statistics 2010 update: A report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Gaziano TA, Bitton A, Anand S, et al. Growing epidemic of coronary heart disease in low-and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Geneva: World Health Organization; 2002. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. [DOI] [PubMed] [Google Scholar]
- 4. http://www.who.int/whostat2007/en/index.html.(Accessed on April 15, 2011)
- 5.Noto TJ, Jr, Johnson LW, Krone R, et al. Cardiac catheterization 1990: a report of the registry of the Society for Cardiac Angiography and Interventions. (SCA&I) Cathet Cardiovasc Diagn. 1991;24:75–83. doi: 10.1002/ccd.1810240202. [DOI] [PubMed] [Google Scholar]
- 6.Sun Z, Ng KH. American Scientific Publishers; 2011. Cardiac computed tomography imaging: technical advances and clinical applications. in press. [Google Scholar]
- 7.Sun Z, Ng KH, Brennan P. American Scientific Publishers; 2011. Medical Imaging of heart and cardiovascular system. in press. [Google Scholar]
- 8.Sommer T, Hackenbroch M, Hofer U, et al. Coronary MR angiography at 3.0 T versus that at 1.5 T: initial results in patients suspected of having coronary artery disease. Radiology. 2005;234:718–725. doi: 10.1148/radiol.2343031784. [DOI] [PubMed] [Google Scholar]
- 9.Stuber M, Botner RM, Fischer SE, et al . Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med. 2002;48:425–529. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 10.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97(6):535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 11.Van der Vaart MG, Meerwaldt R, Slart RHJA, et al. Application of PET/SPECT imaging in vascular disease. Eur J Vasc Endovasc Surg. 2008;35(5):507–513. doi: 10.1016/j.ejvs.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Husmann L, Wiegand M, Valenta I, et al. Diagnostic accuracy of myocardial perfusion imaging with single photon emission computed tomography and positron emission tomography: a comparison with coronary angiography. Int J Cardiovasc Imaging. 2008;24:511–518. doi: 10.1007/s10554-007-9288-7. [DOI] [PubMed] [Google Scholar]
- 13.Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: Comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Sun Z, Jiang W. Diagnostic value of multislice CT angiography in coronary artery disease: A meta-analysis. Eur J Radiol. 2006;60:279–286. doi: 10.1016/j.ejrad.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Sun Z, Lin CH, Davidson R, et al. Diagnostic value of 64-slice CT angiography in coronary artery disease: A systematic review. Eur J Radiol. 2008;67:78–84. doi: 10.1016/j.ejrad.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Gilard M, Le Gal, Cornily JC, et al. Midterm prognosis of patients with suspected coronary artery disease and normal multislice computed tomographic findings: a prospective management outcome study. Arch Intern Med. 2007;167:1686–1689. doi: 10.1001/archinte.167.15.1686. [DOI] [PubMed] [Google Scholar]
- 17.Aldrovandi E, Maffei A, Palumbo S, et al. Prognostic value of computed tomography coronary angiography in patients with suspected coronary artery disease: a 24-month follow-up study. Eur Radiol. 2009;19:1653–1660. doi: 10.1007/s00330-009-1344-3. [DOI] [PubMed] [Google Scholar]
- 18.McCollough CH, Zink FE. Performance evaluation of a multi-slice CT system. Med Phys. 1999;26:2223–2230. doi: 10.1118/1.598777. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, He HD, Foley WD, et al. Four multidetector-row helical CT: image quality and volume coverage speed. Radiology. 2000;215:55–62. doi: 10.1148/radiology.215.1.r00ap3755. [DOI] [PubMed] [Google Scholar]
- 20.Nieman K, Oudkerk M, Rensing BJ, et al. Coronary angiography with multi-slice computed tomography. Lancet. 2001;357:599–603. doi: 10.1016/S0140-6736(00)04058-7. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach S, Giesler T, Ropers D, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice computed tomography. Circulation. 2001;103:2535–2538. doi: 10.1161/01.cir.103.21.2535. [DOI] [PubMed] [Google Scholar]
- 22.Raff GL, Gallagher MJ, O'Neill WW, et al. Diagnostic accuracy of non-invasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–557. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 23.Chao SP, Law WY, Kuo CJ, et al. The diagnostic accuracy of 256-row computed tomographic angiography compared with invasive coronary angiography in patients with suspected coronary artery disease. Eur Heart J. 2010;31:1916–1923. doi: 10.1093/eurheartj/ehq072. [DOI] [PubMed] [Google Scholar]
- 24.Rybicki F, Otero H, Steigner M, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 25.Abdulla J, Abildstrom Z, Gotzsche O, et al. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28:3042–3050. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 26.Mowatt G, Cook JA, Hillis GS, et al. 64-slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–1393. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 27.Vanhoenacker P, Heijenbrok-Kal M, Van Heste R, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244:419–428. doi: 10.1148/radiol.2442061218. [DOI] [PubMed] [Google Scholar]
- 28.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 29.Baumgart D, Schmermund A, Goerge G, et al. Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol. 1997;30:57–64. doi: 10.1016/s0735-1097(97)00147-2. [DOI] [PubMed] [Google Scholar]
- 30.Kajinami K, Seki H, Takekoshi N, Mabuchi H. Coronary calcification and coronary atherosclerosis: site by site comparative morphologic study of electron beam computed tomography and coronary angiography. J Am Coll Cardiol. 1997;29:1549–1556. doi: 10.1016/s0735-1097(97)00090-9. [DOI] [PubMed] [Google Scholar]
- 31.Wong ND, Hsu JC, Detrano RC, et al. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–498. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 32.Oudkerk M, Stillman AE, Halliburton SS, et al. Coronary artery calcium screening: current status and recommendations from the European Society of Cardiac Radiology and North American Society for Cardiovascular Imaging. Int J Cardiovasc Imaging. 2008;24:645–671. doi: 10.1007/s10554-008-9319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 34.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 35.Haberl R, Becker A, Leber A, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–457. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]
- 36.Keelan PC, Bielak LF, Ashai K, et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412–417. doi: 10.1161/hc2901.093112. [DOI] [PubMed] [Google Scholar]
- 37.Reddy GP, Chernoff DM, Adams JR, Higgins CB. Coronary artery stenoses: assessment with contrast-enhanced electron-beam CT and axial reconstructions. Radiology. 1998;208:167–172. doi: 10.1148/radiology.208.1.9646809. [DOI] [PubMed] [Google Scholar]
- 38.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/ AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 40.Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Budoff MJ, Nasir K, McClelland RL, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2009;53:345–352. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor AJ, Bindeman J, Feuerstein I, et al. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 43.LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010;55:627–634. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang SM, Nabi F, Xu J, et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009;54:1872–1882. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 46.Cheng VY, Lepor NE, Madyoon H, et al. Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183–1186. doi: 10.1016/j.amjcard.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 47.Hausleiter J, Meyer T, Hadamitzky M, et al. Prevalence of noncalcified coronary plaques by 64-slice computed tomography in patients with an intermediate risk for significant coronary artery disease. J Am Coll Cardiol. 2006;48:312–318. doi: 10.1016/j.jacc.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 48.Burke AP, Virmani R, Galis Z, et al. 34th Bethesda Conference: Task force #2–What is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41:1874–1886. doi: 10.1016/s0735-1097(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 49.Pundziute G, Schuijf J, Jukema J, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49:62–70. doi: 10.1016/j.jacc.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 50.Korosoglou G, Mueller D, Lehrke S, et al. Quantitative assessment of stenosis severity and atherosclerotic plaque composition using 256-slice computed tomgoraphy. Eur Radiol. 2010;20:1841–1850. doi: 10.1007/s00330-010-1753-3. [DOI] [PubMed] [Google Scholar]
- 51.Motoyama S, Sarai M, Harigay H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 52.Molewsky F, Ropers D, Pohle K, et al. Comparison of measurement of cross-sectional coronary atherosclerotic plaque and vessel areas by 16-slice computed tomography versus intravascular ultrasound. Am J Cardiol. 2004;94:1294–1297. doi: 10.1016/j.amjcard.2004.07.117. [DOI] [PubMed] [Google Scholar]
- 53.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive ad nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 54.Chopard R, Boussel L, Motreff P, et al. How reliable are 40 MHz IVUS and 64-slice MDCT in characterizing coronary plaque composition? An ex vivo study with histopathological comparison. Int J Cardiovasc Imaging. 2010;26:373–383. doi: 10.1007/s10554-009-9562-y. [DOI] [PubMed] [Google Scholar]
- 55.Matter CM, Stuber M, Nahrendorf M. Imaging of the unstable plaque: how far have we got? Eur Heart J. 2009;30:2566–2574. doi: 10.1093/eurheartj/ehp419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107:664–666. doi: 10.1161/01.cir.0000055738.31551.a9. [DOI] [PubMed] [Google Scholar]
- 57.Kuettner A, Trabold T, Schroeder S, et al. Noninvasive detection of coronary artery lesions using 16-detector row multislice spiral computed tomography technology: initial clinical results. J Am Coll Cardiol. 2004;44:1230–1237. doi: 10.1016/j.jacc.2004.05.079. [DOI] [PubMed] [Google Scholar]
- 58.Achenbach S, Ropers S, Pohle FK, et al. Detection of coronary artery stenoses using multi-detector CT with 16x0.75 collimation and 375ms rotation. Eur Heart J. 2005;26:1978–1986. doi: 10.1093/eurheartj/ehi326. [DOI] [PubMed] [Google Scholar]
- 59.Leber AW, Johnson T, Becker A, et al. Diagnostic accuracy of dual-source multi-slice CT coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J. 2007;28:2354–2360. doi: 10.1093/eurheartj/ehm294. [DOI] [PubMed] [Google Scholar]
- 60.Brodoefel H, Burgstahler C, Tsiflikas I, et al. Dual-source CT: Effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology. 2008;247:346–355. doi: 10.1148/radiol.2472070906. [DOI] [PubMed] [Google Scholar]
- 61.Johnson T, Nikolaou K, Busch S, et al. Diagnostic accuracy of dual-source computed tomography in the diagnosis of coronary artery disease. Invest Radiol. 2007;42:484–491. doi: 10.1097/RLI.0b013e31806907d0. [DOI] [PubMed] [Google Scholar]
- 62.de Graaf FR, Schuijf JD, van Velzen JE, et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography in the non-invasive evaluation of significant coronary artery disease. Eur Heart J. 2010;31(15):1908–1915. doi: 10.1093/eurheartj/ehp571. [DOI] [PubMed] [Google Scholar]
- 63.Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol. 2010;106:1429–1435. doi: 10.1016/j.amjcard.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 64.Pasricha SS, Nandurkar D, Seneviratne SK, et al. Image quality of coronary 320-MDCT in patients with atrial fibrillation: Initial experience. Am J Roentgenol. 2009;193:1514–1521. doi: 10.2214/AJR.09.2319. [DOI] [PubMed] [Google Scholar]
- 65.Bell MR, Gersh BJ, Schaff HV, et al. Effect of completeness of revascularization on long-term outcome of patients with three-vessel disease undergoing coronary artery bypass surgery. A report from the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1992;86:446–457. doi: 10.1161/01.cir.86.2.446. [DOI] [PubMed] [Google Scholar]
- 66.Pepine CJ, Sharaf B, Andrews TC, et al. Relation between clinical, angiographic and ischemic findings at baseline and ischemia-related adverse outcomes at 1 year in the Asymptomatic Cardiac Ischemia Pilot study. ACIP Study Group. J Am Coll Cardiol. 1997;29:1483–1489. doi: 10.1016/s0735-1097(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 67.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 68.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with angina like chest pain and normal coronary angiographic findings. J Am Coll Cardiol. 1995;25:1013–1018. doi: 10.1016/0735-1097(94)00519-v. [DOI] [PubMed] [Google Scholar]
- 69.Gaemperli O, Valenta I, Schepis T, et al. Coronary 64-slice CT angiography predicts outcome in patients with known or suspected coronary artery disease. Eur Radiol. 2008;18:1162–1173. doi: 10.1007/s00330-008-0871-7. [DOI] [PubMed] [Google Scholar]
- 70.Carrigan TP, Nair D, Schoenhagen P, et al. Prognostic utility of 64-slice computed tomography in patients with suspected but no documented coronary artery disease. Eur Heart J. 2009;30:362–371. doi: 10.1093/eurheartj/ehn605. [DOI] [PubMed] [Google Scholar]
- 71.Abdulla J, Asferg C, Kofoed KF. Prognostic value of absence or presence of coronary artery disease determined by 64-slice computed tomography coronary angiography: a systematic review and meta-analysis. Int J Cardiovasc Imaging. 2011;27(3):413–420. doi: 10.1007/s10554-010-9652-x. [DOI] [PubMed] [Google Scholar]
- 72.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 73.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009;55:1–16. doi: 10.1016/j.jacc.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 74.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun Z, Choo GH, Ng KH. Coronary CT angiography: current status and continuing challenges. Br J Radiol. doi: 10.1259/bjr/15296170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun Z, Ng KH. Prospective versus retrospective ECG-gated multislice CT coronary angiography: A systematic review of radiation dose and diagnostic accuracy. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.01.070. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 77.Paul JF, Abada HT. Strategies for reduction of radiation dose in cardiac multislice CT. Eur Radiol. 2007;17:2028–2037. doi: 10.1007/s00330-007-0584-3. [DOI] [PubMed] [Google Scholar]
- 78.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 79.Sun Z, Ng KH. Multislice CT angiogreaphy in cardiac imaging. Part III: radiation risk and dose reduction. Singapore Med J. 2010;51:374–380. [PubMed] [Google Scholar]


