Abstract
Depression is a common medical problem and is more prevalent among patients with coronary artery disease. Whether early detection and treatment of depression will enhance cardiovascular outcome is uncertain. Obviously, the safety and efficacy of the anti-depression drugs is an important link. This article reviews the patho-physiologic and behavioural links between depression and cardiovascular disease progression, the treatment of depression, and the potential benefits of anti-depressants in patients with coronary disease.
Keywords: depression, coronary disease, pharmacologic treatment
1. Introduction
Depression and cardiovascular disease (CVD) are the leading causes of mortality and morbidity that pose a significant global health burden and the two are associated. Among 129 499 US non-institutionalized adults (data obtained from 38 states which administered an Anxiety and Depression Module as part of the 2006 Behavioral Risk Factor Surveillance System), CVD was assessed with three questions on coronary heart disease and stroke.
The prevalence of a CVD history was 15.3%. Persons with a CVD history were more likely than those without to experience current depression (15.8% vs. 7.1%, demographics adjusted prevalence ratio (APR) [95% CI] = 1.69 [1.54–1.85]), to have a lifetime diagnosis of depressive disorders (22.3% vs. 15.1%, APR [95% CI] = 1.56 [1.45–1.67]).[1]
Other psychological factors such as hopelessness, anger and anxiety are also risk factors for CVD.[1]–[4] Recently, in patients with atrial fibrillation and heart failure, Frasure-Smith et al.[5] have reported that depression is associated with increased cardiovascular mortality with relative risk of 1.57 (95% CI = 1.20–2.07, P < 0.001). This observation is consistent with a large body of previous literatures on patients with heart attacks[6]–[8] and heart failure.[9]
Numerous studies have proposed and investigated plausible mechanisms of interaction between depression and cardiovascular disease. Depressed subjects may have increased biomarkers I-CAM, C-reactive protein, β-thromboglobulin and interleukin-6, changes indicative of heightened platelet and inflammation responses.[10] Additionally, depression is associated with altered endothelial function. The endothelium plays an important role in the vascular homeostasis via the nitric oxide mediated vasodilation and the modulation of the platelet and leukocyte function. In a number of studies, patients with depressive symptoms have reduced flow mediated dilation (a measure of endothelial function).[11],[12] These mechanisms promote atherogenesis and increase cardiovascular risks.
Another possible mechanism is autonomic nervous system dysfunction. Under normal physiology, the heart is under sympathetic and parasympathetic control, which can be partly measured by heart rate variability (beat to beat variation in the heart rate). Evidences link depression and reduced heart rate variability, suggesting increased sympathetic input and/or reduced parasympathetic input.[13]–[15] In addition to physiological interaction, several studies propose behavioural interaction in that depressed patients have poor adherence to the treatment regime.[16]–[18] Figure 1 summarizes some pathophysiological mechanisms that may link depression to CVD.
Figure 1. The pathophysiological link between depression, an appropriate example of chronic stress and affective disorder, and cardiovascular disease. HPA: Hypothalamo-pituitary-adrenal axis; SNS: sympathetic nervous system. Reproduced with permission from Rozanski et al.[49].
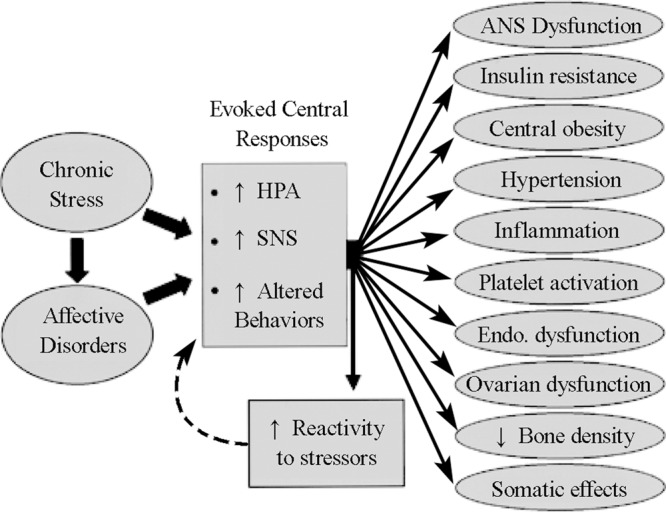
The pathophysiology of depression is not completely understood. The monoamine theory associates depression with reduced norepinephrine and/or serotonin. This is extrapolated from the observations decades ago that depression can be caused by drugs that depletes monoamines such as reserpin.[19],[20] The monoamine theory remains controversial[21]–[23] and consequently the current therapies for depression are based largely on clinical evidence of efficacy and safety. However, publication bias may have exaggerated the true efficacy of the antidepressants.[24],[25] Meanwhile, antidepressant drugs are commonly prescribed, and up to 10.12% of the population was under antidepressant treatment in the United States in 2005.[26]
2. Tricyclic anti-depressant
The tricyclic antidepressants (TCAs) are one of the older classes of anti-depressant drugs available for half of a century. These compounds are characterized by dibenzazepine and dibenzocycloheptadine ring structures. The tricyclics block the reuptake of monamines and raise the extracellular concentration of serotonin and norepinephrine, but many agents also exhibit potent antihistamine H1 antagonism causing sedation and weight gain.[27] The newer agents blocking serotonin and nor-epinephrine reuptake (SNRIs) are classified separately to TCAs. Since 1970s studies have reported the tricyclic antidepressants as cardiotoxic due to its anti-cholinergic properties and increased extracellular norepinephrine, particularly in situations of an overdose. Its use in the patients with heart disease has been considered contraindicated due to the cardiac side-effects including sinus tachycardia, postural hypotension and electrocardioghic changes (prolongation of the PR, QRS and QT intervals) even at the therapeutic dosage.[27]–[30]
3. Serotonin reuptake inhibitors
The serotonin reuptake inhibitors (SSRIs) are the newer class of antidepressants designed to treat depressive disorders. Following the development of the TCAs and the monoamine theory that the antidepressant effect of TCAs was attributed to increased extracellular monoamines, SSRIs was modelled to inhibit the neuronal uptake pumps.[31] In addition to more favourable side effect profile compared to TCAs, SSRIs has long been associated with altered platelets with reduced activity. Studies have observed that the SSRIs family of drugs such as sertraline hydrochloride and paroxetine have a dose-dependent negative impact on the serotonin mediated platelet activation.[32]–[34] The anti-platelet attribute of SSRIs may provide a new treatment modality in patients with depression and cardiovascular disease to prevent further thrombotic cardiovascular events.
4. Mechanistic studies of vascular benefit from SSRIs
Morel-Kopp et al.[35] recently reported a cohort study of 108 outpatients with depression and 45 controls without depression, evaluating the effects of psychotherapy and antidepressant therapy (predominantly citalopram or other SSRIs) on platelet function. Platelet function was assessed at four weeks and six months mark to determine the short term and long term effect of the treatments. The cell count, CD62p surface expression, platelet-leukocyte aggregates and platelet microparticles were measured as the biomarkers of platelet function. Initially, the patients had higher platelet cell count (254 × 109 L−1 vs. 233 × 109 L−1, P = 0.013) and higher platelet activity (due to the elevated CD62p positive platelets, 0.76 × 109 L−1 vs. 0.46 × 109 L−1, P = 0.019) and elevated platelet-leukocyte count, compared to the controls. This observation contributes to the hypothesis that depression is linked with cardiovascular disease through platelet dysregulation. During the trial, it was found that in the short term treatment had no effect on the platelet parameters, but at 6 mo there were notable changes. The platelet count, platelet-leukocyte aggregate remained unchanged but there was a significant reduction in the CD62p platelet and the platelet microparticles. While both psychotherapy and antidepressant succeeded in improving the mood of the patients, the measured changes of platelet activation correlated with the changes of depressive symptoms. Thus, treatment of depression may reverse the platelet dysregulation and the anti-platelet properties of the SSRIs may be clinically beneficial.
The SSRIs may also affect the inflammation and the endothelial function. Pizzi et al.[36] investigated the effect of sertraline on inflammation and endothelial function in 100 patients with CVD and symptoms of depression, in a randomized double-blind placebo-controlled fashion. After 20 weeks, setraline treatment significantly reduced the Becks Depresion Inventroy (BDI) score, C-Reactive Protein and IL-6 levels, and flow dependent endothelial dilatation, whereas no change was seen in the placebo group. Similar results have been reported in other small scale studies.[37],[38]
Collectively, these studies suggest that the use of psycho- or behavioural therapy and SSRIs in treating depressed patients is linked with the improvements of several cardiovascular biomarkers and physiological parameters. However, the therapeutic values of antidepressants in cardiovascular disease setting have been difficult to establish.
5. Studies with clinical endpoints
Setraline antidepressant heart attack randomized trial was an early attempt to assess the safety and efficacy of the antidepressant treatment in CVD patients. This randomized 369 patients with major depressive disorder and a recent history of acute myocardial infarction or angina[39] to setraline or placebo for 24 weeks. The study was successful in documenting the safety of the sertaline treatment in CVD patients. There was no difference in the mean left ventricular ejection fraction, the electrocardiogram parameters, blood pressure and heart rate between the placebo and the sertraline group. The efficacy of sertraline in treating depression was mixed, as assessed by the Clinical Global Impression (CGI) scale and the Hamilton Rating Scale for Depression (HAM-D). While sertraline was superior to placebo in the CGI-I, there was no difference in the HAM-D. However sertraline was superior to the placebo in both scales in those with recurrent depression and those with severe depression. There was a trend to a lower adverse cardiovascular event rates (death, myocardial infarction, congestive heart failure, stroke and recurrent angina) with sertraline (14.5% vs. 22.4%) but this was not statistically significant.
The Canadian cardiac randomized evaluation of Antidepressant and Psychotherapy Efficacy trial randomized 284 patients with the four weeks history of depression and established coronary artery disease to citalopram/interpersonal psychotherapy versus placebo/control clinical management. During 12 weeks follow up, citalopram was much more effective in treating depression compared to the placebo while there was no difference between interpersonal psychotherapy and clinical management. However, the cardiovascular event rate was low in all groups.[40]
The small sample sizes and short duration of follow-up rendered these trials under-powered in assessing the efficacy of anti-depressant in improving cardiovascular outcome. The recent meta-analyses by Pizzi et al.[41] and Mazza et al.[42] help address these issues. Pizzi's review (up to April 2010) focussed on the use of SSRIs and endpoints of readmission of coronary heart disease (CHD) and mortality, including six randomized controlled studies with 2461 participants. However, only three randomized control trials with 734 patients were deemed properly randomized trials and contributed to their main analysis, and the use of SSRIs was not associated with significant differences in CHD readmission (Risk ratio [RR] = 0.74, 95% CI = 0.44–1.23) and mortality (RR = 0.3, 95% CI = 0.08–2.01). However, any meta-analysis cannot compensate for the lack of high quality evidences. Two of the three trials showed the trend that the SSRIs use was associated with decreased risk of CHD readmission, the third trial was completely neutral; and only one study reported mortality data. In a secondary analysis including the two other observational studies, there was a significant reduction in CHD readmission in the SSRIs group (RR = 0.63, 95% CI = 0.46–0.86) and an improved mortality rate in the SSRIs group (RR = 0.57, 95% CI = 0.36–0.92). Pizzi et al.[41] also demonstrated the efficacy of SSRIs in treating depression in the cardiovascular disease setting. The study also reported that the use of SSRIs was associated with improvements in depressive symptoms.
The review by Mazza et al.[42] is another attempt to evaluate the efficacy of antidepressant therapy. Five studies have been selected in this analysis, including 353 receiving antidepressant therapy and 448 controls. While having overlap with the analysis by Pizzi et al.[41], there are some disparities in both the included studies and the results. Some notable differences are as follows. Mazza et al.[42] noted that the SSRIs therapy was favourable over the control in improving the depressive symptoms but this association was not statistically significant (P = 0.21). Mazza et al.[42] found that the antidepressant therapy was associated with a significantly reduced rate of rehospitalization (Risk difference = 14%, P = 0.001).
In summary, two meta-analyses show a trend based on the published evidences that with modest duration (about six months) of antidepressant therapy, there are at least positive trends towards an improvement of both depression symptoms and cardiovascular outcome. However, this does not detract the need of having larger scale trials with stronger statistical power to investigate the efficacy of antidepressant therapy in CVD patients.
6. Insights from “primary prevention” trials
From the US Department of Veterans Affairs patient records,[43] a cohort aged 25–80 years and free of cardiovascular disease was identified in the years 1999 – 2000, who had an ICD-9CM code indicating an episode of depression (n = 93653). These depressed patients, as compared to non-depressed Veterans Administration patients, were at increased risk for incident myocardial infarction (Hazard ratio [HR] = 1.39; 95% CI = 1.34–1.45).[44]
Incident myocardial infarction and all-cause mortality were modeled in patients who received 12 weeks or more of antidepressant pharmacotherapy as compared with 0-11 weeks during follow-up.[43] Receipt of 12 or more weeks of continuous antidepressant therapy was associated with significantly reduced rates of incident myocardial infarction across classes of antidepressants: SSRIs (HR = 0.48, 95%CI = 0.44 – 0.52), SNRIs (HR = 0.35, 95% CI = 0.32 – 0.40), TCAs (HR = 0.39, 95% CI = 0.34 – 0.44), and “Other” (HR = 0.41; 95% CI = 0.37 – 0.45). Risk of all-cause mortality was also decreased with 12 weeks of pharmacotherapy with all classes of antidepressants (SSRIs, SNRIs, TCAs, Other), with HRs ranging from 0.50 to 0.66.[43]
Of note, the mechanism for this association remains uncertain. Compliance with pharmacotherapy for depression may reflect compliance with cardiovascular medications, or a direct vaso-protective effect from the medications or that improved depressed mood attenuates the risk of myocardial infarction in depressed patients.
7. Current clinical perspectives
The link between depression and CVD is becoming clear (Figure 1). In a recent study[45], 1019 patients with stable CHD in the Heart and Soul Study were assessed using the Patient Health Questionnaire to determine the presence of the nine depressive symptoms included in the DSM-4, comparing cognitive vs. somatic symptoms. After adjustment for demographic data and cardiac risk factors, each somatic symptom was associated with 14% greater risk for events (HR = 1.14, 95% CI = 1.05 – 1.24, P = 0.002). The somatic symptoms of fatigue, appetite problems, and sleeping difficulties were most strongly predictive of cardiovascular events. In contrast, cognitive symptoms (HR = 1.08, 95% CI = 0.99 – 1.17, P = 0.09) were not significantly associated with cardiovascular events.
These findings highlight the biological effect from depressive disorder, but behavioural interactions may also play a significant role. Apart from being potentially non-compliant to medications, depressed patients often present later with heart attacks and miss the golden hour of reperfusion therapy.[46]
Despite the increasing awareness of the problem at hand, it is challenging to recommend an appropriate intervention to manage depression in cardiovascular disease setting. In the primary care setting in subjects without known CVD, the current US Preventive Task Force (USPTF) 2009 recommendation is to screen adults for depression only when staff assisted supportive care are in place for accurate diagnosis, effective treatment and follow-up.[47] The clinical diagnosis of depression in patients with CVD is summarized in Figure 2.
Figure 2. The diagnosis of depression in patients with heart disease. Adapted from Whooley et al.[50].
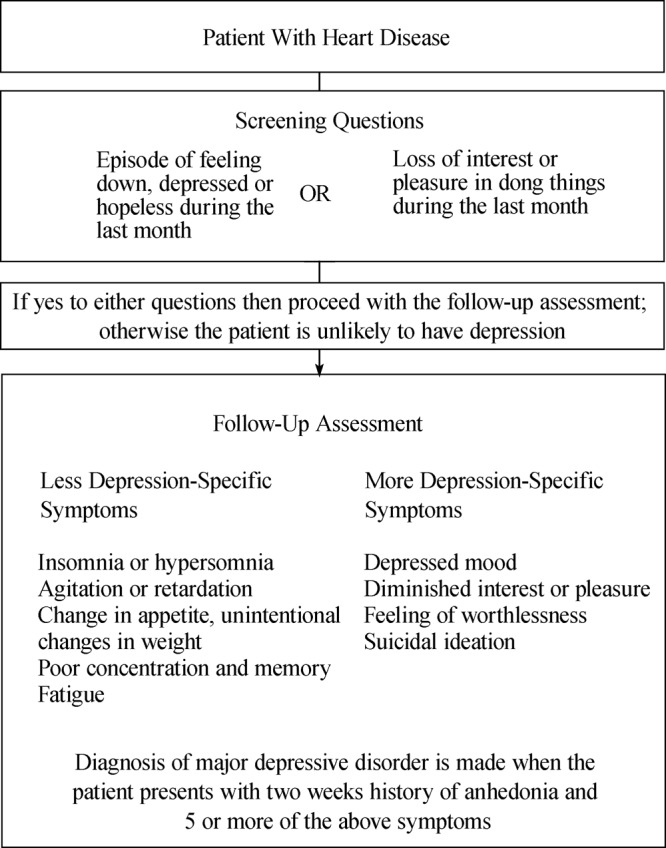
Numerous studies have demonstrated the value of SSRIs antidepressants in alleviating the inflammatory, endothelial and platelet function changes associated with depression but the handful of clinical studies investigating the effect of SSRIs in improving depressive symptoms and cardiovascular outcome have failed to show a statistically significant results in patient with documented CHD. Nonetheless, the safety of these anti-depressive drugs is clear and is in stark contrast to the risks of anti-psychotic agents which may cause sudden death through ventricular arrhythmias and QT prolongation in a dose-dependent manner.[48]
Published data have displayed at least trends towards a benefit between the use of SSRIs and management of depression and CVD. Further research is much needed to generate recommendations on the use of a regular psychological care and anti-depressive medications in the current treatment strategy of CHD.
References
- 1.Fan AZ, Strine TW, Jiles R, et al. Depression and anxiety associated with cardiovascular disease among persons aged 45 years and older in 38 states of the United States, 2006. Prev Med. 2008;46(5):445–450. doi: 10.1016/j.ypmed.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Everson SA, Kaplan GA, Goldberg DE, et al. Hypertension incidence is predicted by high levels of hopelessness in Finnish men. Hypertension. 2000;35(2):561–567. doi: 10.1161/01.hyp.35.2.561. [DOI] [PubMed] [Google Scholar]
- 3.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131(2):260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 4.Denollet J, Maas K, Knottnerus A, et al. Anxiety predicted premature all-cause and cardiovascular death in a 10-year follow-up of middle-aged women. J Clin Epidemiol. 2009;62(4):452–456. doi: 10.1016/j.jclinepi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Frasure-Smith N, Lespérance F, Habra M, et al. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120(2):134–140. doi: 10.1161/CIRCULATIONAHA.109.851675. [DOI] [PubMed] [Google Scholar]
- 6.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: A meta-analysis. Psychosom Med. 2004;66(6):814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 7.Dickens C, McGowan L, Percival C, et al. New onset depression following myocardial infarction predicts cardiac mortality. Psychosom Med. 2008;70(4):450–455. doi: 10.1097/PSY.0b013e31816a74de. [DOI] [PubMed] [Google Scholar]
- 8.Glassman AH, Bigger JT, Jr, Gaffney M, et al. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: Seven-years follow-up of SADHART participants. Arch Gen Psychiatry. 2009;66(9):1022–1029. doi: 10.1001/archgenpsychiatry.2009.121. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor CM, Jiang W, Kuchibhatla M, et al. Antidepressant use, depression, and survival in patients with heart failure. Arch Intern Med. 2008;168(20):2232–2237. doi: 10.1001/archinte.168.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizzi C, Manzoli L, Mancini S, et al. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29(9):1110–1117. doi: 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Brook R, Rubenfire M, et al. Abnormal brachial artery flow mediated vasodilatioan in young adults with major depression. Am J Cardiol. 2001:196–198. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 12.Sherwood A, Hinderliter AL, Watkins LL, et al. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46(4):656–659. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 13.Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005;165(13):1486–1491. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 14.Krittayaphong R, Cascio WE, Light KC, et al. Heart rate variability in patients with coronary artery disease: differences in patients with higher and lower depression scores. Psychosom Med. 1997;59(3):231–235. doi: 10.1097/00006842-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 16.Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol. 2006;48:2218–2222. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 17.Gehi A, Hass D, Pipkin S, et al. Depression and medication adherence in outpatients with coronary heart disease: findings from the heart and soul study. Arch Intern Med. 2005;165(21):2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carney RM, Freedland KE, Eisen SA, et al. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychology. 1995;14(1):88–90. doi: 10.1037//0278-6133.14.1.88. [DOI] [PubMed] [Google Scholar]
- 19.Brodie BB, Shore PA. A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Ann N Y Acad Sci. 1957;66(3):631–642. doi: 10.1111/j.1749-6632.1957.tb40753.x. [DOI] [PubMed] [Google Scholar]
- 20.Checkley SA. Neuroendocrine tests of monoamine function in man: a review of basic theory and its application to the study of depressive illness. Psychol Med. 1980;10(1):35–53. doi: 10.1017/s0033291700039593. [DOI] [PubMed] [Google Scholar]
- 21.Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29(1):2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- 22.Hindmarch I. Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur Psychiatry. 2002;17(Suppl 3):294–299. doi: 10.1016/s0924-9338(02)00653-3. [DOI] [PubMed] [Google Scholar]
- 23.Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61(Suppl 6):4–6. [PubMed] [Google Scholar]
- 24.Kirsch I. Initial Severity and Antidepressant Benefits: A meta-analysis of data submitted to the food and drug administration. PLoS Med. 2008;5(2):e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner EH, Mathews AM, Linardato E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 26.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 27.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151(6):737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jefferson JW. A review of the cardiovascular effects and toxicity of tricyclic antidepressants. Psychosom Med. 1975;37(2):160–179. doi: 10.1097/00006842-197503000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Biggs JT, Spiker DG, Petit JM, et al. Tricyclic antidepressant overdose. JAMA. 1977;238(2):135–138. [PubMed] [Google Scholar]
- 30.Callaham M, Kassel D. Epidemiology of fatal tricyclic antidepressant ingestion: implications for management. Ann Emerg Med. 1985;14(1):1–9. doi: 10.1016/s0196-0644(85)80725-3. [DOI] [PubMed] [Google Scholar]
- 31.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders–I. Basic pharmacology. J Psychopharmacol. 1998;12(3) Suppl B:S5–20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- 32.Markovitz JH, Shuster JL, Chitwood WS, et al. Platelet activation in depression and effects of sertraline treatment: An open-label study. Am J Psychiatry. 2000;157(6):1006–1008. doi: 10.1176/appi.ajp.157.6.1006. [DOI] [PubMed] [Google Scholar]
- 33.Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/Endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the sertraline antidepressant heart attack randomized trial (SADHART) platelet substudy. Circulation. 2003;108(8):939–944. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- 34.Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol. 2000;20(2):137–140. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Morel-Kopp MC, McLean L, Chen Q, et al. The association of depression with platelet activation: evidence for a treatment effect. J Thromb Haemost. 2009;7(4):573–581. doi: 10.1111/j.1538-7836.2009.03278.x. [DOI] [PubMed] [Google Scholar]
- 36.Pizzi C, Mancini S, Angeloni L, et al. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86(5):527–532. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 37.O'brien SM, Scott LV, Dinan TG. Antidepressant therapy and C-reactive protein levels. Br J Psychiatry. 2006;188(5):449–452. doi: 10.1192/bjp.bp.105.011015. [DOI] [PubMed] [Google Scholar]
- 38.Lekakis J, Ikonomidis I, Papoutsi Z, et al. Selective serotonin re-uptake inhibitors decrease the cytokine-induced endothelial adhesion molecule expression, the endothelial adhesiveness to monocytes and the circulating levels of vascular adhesion molecules. Int J Cardiol. 2010;139(2):150–158. doi: 10.1016/j.ijcard.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 40.Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297(4):367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 41.Pizzi C, Rutjes AW, Costa GM, et al. Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol. 2011;107(7):972–979. doi: 10.1016/j.amjcard.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Mazza M, Lotrionte M, Biondi-Zoccai G, et al. Selective serotonin reuptake inhibitors provide significant lower re-hospitalization rates in patients recovering from acute coronary syndromes: evidence from a meta-analysis. J Psychopharmacol. 2010;24(12):1785–1792. doi: 10.1177/0269881109348176. [DOI] [PubMed] [Google Scholar]
- 43.Scherrer JF, Garfield LD, Lustman PJ, et al. Antidepressant drug compliance: reduced risk of MI and mortality in depressed patients. Am J Med. 2011;124(4):318–324. doi: 10.1016/j.amjmed.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Scherrer JF, Chrusciel T, Zeringue A, et al. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed veterans administration patients. Am Heart J. 2010;159(5):772–779. doi: 10.1016/j.ahj.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 45.Hoen PW, Whooley MA, Martens EJ, et al. Differential associations between specific depressive symptoms and cardiovascular prognosis in patients with stable coronary heart disease. J Am Coll Cardiol. 2010;56(11):838–844. doi: 10.1016/j.jacc.2010.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong CK, Tang EW, Herbison P, et al. Pre-existent depression in the 2 weeks before an acute coronary syndrome can be associated with delayed presentation of the heart attack. QJM. 2008;101:137–144. doi: 10.1093/qjmed/hcm153. [DOI] [PubMed] [Google Scholar]
- 47.US Preventive Task Force Screening for depression in adults: US preventive task force recommendation statement. Ann Intern Med. 2009;151:784–792. doi: 10.7326/0003-4819-151-11-200912010-00006. [DOI] [PubMed] [Google Scholar]
- 48.Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozanski A, Blumenthal JA, Davidson KW, et al. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am CollCardiol. 2005;45(5):637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Whooley MA. Depression and cardiovascular disease: healing the broken-hearted. JAMA. 2006;295(24):2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]


