Abstract
Background
Chronic congestive heart failure is a complex condition that leads to dysfunction in the peripheral microcirculation. We have previously shown that vascular reactivity is reduced with increasing age. In this study, we examined a group of very old patients with severe chronic heart failure to test the hypothesis that vascular function is further compromised by a combination of heart failure and aging.
Methods
Cutaneous forearm blood flow was measured by laser Doppler flowmetry and compared among three groups: Group 1 (n = 20, mean ± SE: 85.5 ± 4 years), heart failure patients with New York Heart Association class IV (NYHA IV) and with a NT-proBNP level ≥ 5000 ng/L; Group 2 (n = 15, mean ± SE: 76.5 ± 2 years), heart failure patients with NYHA II and NT-proBNP ≤ 2000 ng/L, and Group 3 (n = 10, mean ± SE: 67.6 ± 3.0 years), healthy controls with no clinical signs of heart failure. The vasodilator response to the iontophoretic administration of acetylcholine (ACh), acting via an endothelial mechanism, and sodium nitroprusside (SNP), acting via a smooth muscle cell mechanism, were studied.
Results
All patients with heart failure had significantly reduced vascular reactivity independent of the mode of stimulation (ACh, SNP or heat) when compared to healthy controls. However, the responses did not differ between the two groups of heart failure patients.
Conclusions
Cutaneous vascular reactivity is reduced in heart failure patients and does not correlate with the severity of the condition or age of patients.
Keywords: heart failure, cutaneous microcirculation, endothelial responses, acetylcholine, smooth muscle responses
1. Introduction
Congestive heart failure is a multi symptomatic disease that is more abundant in the elderly population.[1] Patients with chronic heart failure are characterized by multi organ dysfunction and frequently seek hospital care because of shortness of breath and peripheral oedema. One of the features of heart failure is a low grade inflammation of unknown origin that is associated with reduced vascular responses to vasodilator stimuli.[2] It has been proposed that this vascular dysfunction could contribute to symptoms like fatigue and intolerance to heat.[3]
The vast majority of clinical research on heart failure has focused on middle aged (40-65 years) and the younger elderly (65–75 years) however. In previous studies, we have shown that cutaneous microvascular function declines in older patients and also that the endothelium-dependent vascular reactivity appears to negatively correlate to the severity of heart failure.[2]
In this study, we aimed to investigate these phenomena by studying if age and severity of heart failure could have a synergistic effect on the microvasculature; or if the vascular dysfunction mainly is an early response in the heart failure syndrome.
2. Methods
2.1. Patients
The study population consisted of three groups. Group 1 consisted of 20 patients, 12 men and 8 women, mean age of 85.5 years. The patients were diagnosed earlier with chronic congestive heart failure. The heart failure patients arrived due to worsening of the condition to Lund University Hospital (Lund University, Sweden) with New York Heart Association class IV (NYHA IV) symptoms and NT pro-BNP levels ≥ 5000 ng/L. Group 2 consisted of 15 heart failure patients, 9 men and 6 women, who were obtained from the out patients clinic in the same geographic region, with mean age of 76.5 years. They were considered clinically stable with NYHA II symptoms and NT pro-BNP levels of about 2000 ng/L. Group 3 consisted of 10 healthy elderly age- and gender-matched subjects recruited from the community registry. These subjects had a mean age of 67.5 years. Their NT pro-BNP levels were in the normal range, 50–300 ng/L. They did not take any medication for cardiovascular disease.
The two groups of chronic congestive heart failure patients had reduced left ventricular function upon echocardiography and were all non-current smokers when entering the clinical study to avoid any effects on flow measurements.[4] Healthy controls have ejection fraction > 50%.[5] All patients were kept on their prescribed medication but refrained from long lasting nitrates six hours before the laser Doppler blood flow measurement. No other co-morbidity resulted in exclusion of participation in the study, only tremor was considered not suitable for the laser Doppler blood flow method. For demographic details on the subjects, see Table 1.
Table 1. The demographics of severe and moderate congestive heart failure patients vs. healthy subjects. Data are given as mean ± SE, and/or range in parenthesis.
| NYHA IV n = 20 | NYHA II n = 15 | Healthy n = 10 | |
| Age | 85.5 ± 1.2 (78–96)* | 76.5 ± 1.9 (68–84) | 67.6 ± 3.0 (56–81) |
| Sex, F/M | 8/12 | 5/10 | 6/4 |
| BMI, kg/m2 | 24.6 (19–34) | 26.4 (20–34) | 28.4 (24–35) |
| MABP | 94 ± 3.6 | 101 ± 3.2 | 90 ± 3.0 |
| Pulse/min | 74 (60–90) | 74 (52–110) | 70 (56–86) |
NYHA: New York Heart Association classification; BMI: Body mass index; MABP: Mean arterial blood pressure. *P < 0.05 NYHA IV vs. NYHA II.
The investigation conformed to the principles outlined in the Declaration of Helsinki (Seoul 2008). The Ethics Committee of Lund University approved of the protocol (LU 465-03). Written informed consent was obtained from all patients by the investigator before they were entered into the study.
2.2. Clinical parameters
Hemodynamic measurements consisted of arterial blood pressure and heart rate. Blood pressure was measured non-invasively in the supine position from the upper left arm with the cuff inflated at heart level. Blood pressure was taken after the blood flow measurement when the patients had been resting for about one hour. The diastolic value was accepted as Korotkoff's phase V. All blood pressure measurements were taken by the same investigator. Heart rate was counted for one minute.
2.3. Blood analysis
Plasma levels of inflammatory markers, C-reactive protein (P-CRP), interleukin 6 (IL-6) and soluble IL 2 receptor (s-IL2r) were measured as well as pro-brain natriuretic peptide (NT-proBNP), P-LDL (low density lipoprotein) cholesterol, P-HLD (high density lipoprotein) cholesterol, and blood glucose levels. In addition, haemoglobin (Hb), P-sodium, P-potassium, P-creatinine and P-uric acid were analyzed at the Department of Clinical Chemistry and Pharmacology. Interleukins were measured at Clinical Immunology laboratory at Lund University Hospital. All blood samples were obtained from a peripheral venous access in heart failure patients and measured by validated techniques.
2.4. Blood flow measurements
Cutaneous blood flow was measured using the PeriFlux system 5000 (Perimed, Järfälla, Sweden). This method is non-invasive and gives minimal discomfort to the patients which makes it suitable for severely ill patients at bedside.[6] Laser-generated light at a wavelength of 780 nm is directed to the skin using a fibre optic probe. The light reflected from moving blood cells in the superficial skin microvessels undergoes a shift in frequency (Doppler effect) that is proportional to the number and velocity of moving blood cells. The laser-Doppler output is semi-quantitative, and we have presented all data as the percentage change compared with the baseline perfusion value. Temperature of the skin was recorded continuously.
2.5. Iontophoresis
Constant current iontophoresis was used to enhance the perfusion of charged molecules into the skin of the dorsal side of the lower arm. Endothelium–dependent vasodilatation was provoked by iontophoresis of the acetylcholine (Ach, 2% dissolved in MilliQ water, Sigma) using anodal current to deliver the positively charged molecule.
Endothelium-independent vasodilatation was provoked by iontophoresis of nitric oxide (NO) donor, sodium nitroprusside (SNP, 1% dissolved in MilliQ water, Sigma) using the cathode current for this negatively charged molecule.[2] The PeriIont System (Perimed) used in this study contains of an applicator with a small recess in the centre and of circular temperature probe surrounding the application site. The recess in the centre allows the insertion of a fibre optic probe to measure the blood flow in the stimulated area. An additional temperature probe containing a fibre optic probe was placed at a distance suitable to avoid large veins. This was used as a reference during the iontophoresis and was subsequently used to determine the response to local warming.
All studies were performed at room temperature (22°C–24°C). For the severely ill congestive heart failure (CHF) patients, the measurements were obtained at the hospital emergency ward. For both the CHF patient group from the out patient clinic and the healthy subjects, blood flow measurements were carried out at the Clinical Trial Centre, Lund University Hospital, Lund, Sweden. All subjects were resting in a supine position. Blood pressure and heart rate were measured before and after stimulation and the lowest value is given. The skin of the dorsal lower arm was gently cleansed and the iontophoretic applicators/fibre optic probes were applied to the forearm resting on a pillow to give comfort and provide stabilization. The basal blood flow was studied for 2 min after which ACh was transferred by iontophoresis (anodal current, 0.2 mA for 20 s). The current alone did not affect the blood flow (results not shown). The protocol was based on our previous study[2] when we determined that successive iontophoretic stimuli at 60 s intervals, produces a cumulative stimuli-response curve. We measured the maximum response after five stimuli. Endothelium-independent vasodilatation was studied by iontophoresis of SNP as above (cathode current, 0.1 mA for 60 s). The stimulation was repeated four times at 60 s intervals. Finally, the response to heat was measured following local warming to +44°C for 10 min. This responses were considered as maximum vasodilatation in the micro vessels of the skin using this technique.
2.6. Laser Doppler calculation
Light is transmitted to the tissue via a fibre-optic probe. When the light hits moving blood cells, it undergoes a change in wavelength (Doppler shift). The magnitude and frequency distribution of these changes are directly related to the number and velocity of blood cells, i.e., the blood perfusion. Measurements are expressed in arbitrary Perfusion Units (PU). Full linear correlation to absolute perfusion value is achieved using perimed's analysis technology (including a linearization function to avoid underestimation in highly perfused tissues) and calibration using automatic instrument zeroing and Perimed's Motility Standard.
The responses are expressed as the maximum percent change in PU from baseline flow (set as 100% in each subject) to the iontophoretic administration of ACh and SNP. The perfusion change after local heating (e.g., +44°C) is a measure of the tissue reserve capacity.
2.7. Statistical analysis
Statistical analysis was performed by Mann-Whiney U test. Statistical differences with a P value < 0.05 were considered significant. Calculations were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
3. Results
3.1. Basic characteristics
There was no significant difference between the two groups of heart failure patients regarding gender, body mass index (BMI) and blood pressure; however, the NYHA IV patients were older (Table 1). The concomitant diseases and the pharmacological treatment in the heart failure groups are given in Table 2. The difference in ejection fraction (EF) between the groups was expected due to the difference in severity of the heart failure. EF was 43% ± 3% in NYHA II and 34% ± 2 % in NYHA IV (P < 0.05 between the two heart failure groups). Blood samples also showed much higher levels of proBNP in NYHA IV (16859 ± 1966 ng/L) as compared to NYHA II (1959 ± 569 ng/L; P < 0.05). The healthy individuals had proBNP levels that were lower than 500 ng/L.
Table 2. Medical history and treatment of the chronic congestive heart failure patients.
| CHF patients in hospital (n = 20) | CHF from out patient clinic (n = 15) | |
| NYHA II | 0 | 15 |
| NYHA IV | 20 | 0 |
| Co-existing disease | ||
| Hypertension | 2/20 | 2/15 |
| Diabetes | 4/20 | 3/15 |
| Coronary artery disease | ||
| Prior myocardial infarction | 8/20 | 4/15 |
| Electrocardiogram | ||
| Arterial fibrillation | 9/20 | 7/15 |
| Bundle branch block | 6/20 | 0/15 |
| Pacemaker | 4/20 | 1/15 |
| Chest X-ray | ||
| Pulmonary oedema | 12/20 | 0/15 |
| Cardiomegaly | 16/20 | 0/15 |
| Pharmacological treatment | ||
| Beta-adrenoceptor antagonists | 13/20 | 8/15 |
| ACE-inhibitors | 12/20 | 12/15 |
| Digoxin | 1/20 | 2/15 |
| ARB | 1/20 | 0/15 |
| Diuretics | 20/20 | 6/15 |
| ASA | 12/20 | 6/15 |
| Warfarin | 4/20 | 6/15 |
| Spironolactone | 4/20 | 2/15 |
CHF: congestive heart failure; n: number of patients; NYHA: New York Heart Association classification; ACE: angiotensin converting enzyme; ASA: acetylsalicylic acid; ARB: angiotensin receptor blockers.
Other clinical parameters that differed between the groups were significantly lower Hb and HDL and higher CRP, creatinine, uric acid, IL-6 and s-IL2r in NYHA IV as compared to NYHA II. The calculated absolute glomerular filtration rate[7] was 64 ± 4 mL/min in NYHA II and 35 ± 5 mL/min in NYHA IV (P < 0.0001).
The above differences are consistent with a more severe disease state in NYHA IV patients as compared to the NYHA II group and provide clear indication of ongoing inflammation in the former group. Six months after participation in this study, 12 out of 20 subjects had died in the NYHA IV group while only one patient had died in the NYHA II group. These findings agree with a previous mortality study of severe heart failure.[8]
3.2. Microvascular responses
ACh stimulates the release of NO and elicits a subsequent dilatation of cutaneous blood vessels. The control subjects showed a mean dilatation of 975 ± 120%, relative to baseline resting flow (which was set as 100%), the NYHA II patients showed a mean dilatation to ACh of 659 ± 124% (P < 0.05 vs. healthy controls) while the severe heart failure subjects (NYHA IV) had a mean dilatation of 588 ± 127% (P < 0.005 vs. healthy controls).
SNP mediates its effect directly on the smooth muscle cells independent of the endothelium.
The responses to SNP in the controls were more variable than responses to ACh (Figure 1). The mean value was 869 ± 153% in the healthy controls. There was a markedly lower response to SNP in the NYHA II group (389 ± 63%; P < 0.05) as compared to controls. A reduction in responsiveness were seen also in the severe heart failure group (544 ± 79%; P < 0.005 as compared to controls).
Figure 1. Percent increase in blood flow (perfusion units) compared to base line (set as 100%) in healthy individuals (n = 10), patients with congestive heart failure of NYHA II (n = 15) and NYHA IV (n = 20). (A) endothelium-dependent responses to acetylcholine; (B) endothelium–independent relaxation response to sodium nitroprusside; (C) general vasodilator response to local heating to +44 °C. Values represent mean maximum relaxation ± SE. NYHA: New York Heart Association classification; ACh: Acetylcholine; SNP: sodium nitroprusside.
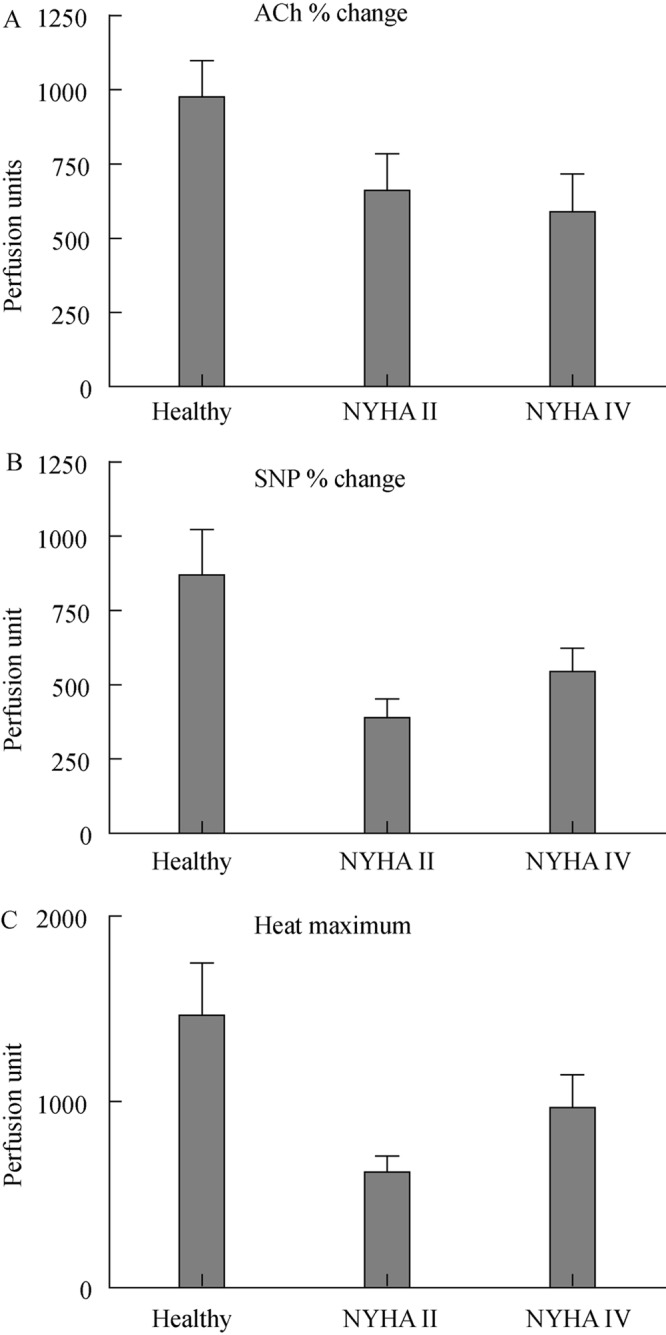
Heat causes a non-endothelium dependent dilatation of the vascular bed and is used as a way to cause near maximum dilation of the local cutaneous vascular bed. We found that the response to heat was 1467 ± 283% in the healthy controls, and this was markedly reduced in both mild (621 ± 87%; P < 0.01) as compared to healthy controls and severe heart failure (969 ± 177%; P < 0.05) as compared to healthy controls.
4. Discussion
The present study has shown that patients with CHF have reduced microvascular reactivity to acetylcholine, nitroprusside and local heat. In a previous study, we found that the microvascular relaxant capacity in skin vessels was reduced in healthy elderly people and attenuated further by presence of heart failure.[2] However, contrary to our expectations, there was no significant correlation between the severity of heart failure and cutaneous microvascular dysfunction. Even though they were elderly, the patients with more severe symptoms (NYHA IV) showed similar vasodilatory responses as less severely ill patients (NYHA II).
In the present study, we explored the relation between these parameters (age, heart failure) by measuring the vascular responses in a group of elder elderly patients with heart failure at the terminal stage. Unexpectedly, the vascular responses did not differ markedly between the two heart failure groups. Our previous studies had shown that vasomotor reactivity is diminished during transition from youth to middle-aged due to normal aging processes.[2] Heart failure further decreases vasodilatory responses in elderly patients of 65–70 years. However, in our data from very old heart failure patients (78–96 years), there was no further age related decline and, the blood vessels ability to relax appeared stable.
It is remarkable that our group of very old patients who were seriously ill and had all indications of ongoing elevated inflammation had similar vasodilatory responses as the group of milder heart failure patients. This suggests that microvascular insufficiency is not worsening with the progression of severe chronic congestive heart failure.
This study has shown that subjects with CHF have reduced vascular capacity as assessed using local application of heat (+44°C) as compared to matched elderly control subjects. Similarly the iontophoretic administration of ACh and SNP resulted in significantly lower dilator responses as compared to the healthy group; this difference was somewhat larger for the dilatation to SNP. It was obvious that the two heart failure patients groups had markedly lower cutaneous vasoreactivity as compared to the healthy controls. There was no stepwise reduction in cutaneous responses that depended on the severity of heart failure.
The reason behind this is not clear, but we can exclude some factors based on our choice of the design: (1) Age and gender were not confounders because the subjects were matched to a similar degree as we have analysed before.[2] (2) Smoking may severely affect the cutaneous responses, however we excluded such subjects in this study.[4] (3) Degree of inflammation in the vasculature may be a confounder and we have earlier published that homocysteine, CRP and cytokines could be a marker of heart failure.[9] The present subjects had several markers of inflammation that differed between the two groups of heart failure (Table 3). Thus, higher levels of CRP, IL6 and IL2r were seen in NYHA IV patients as compared to NYHA II. Interestingly, both heart failure groups had attenuated responses to ACh, SNP and heat as compared to that seen in the healthy controls. If anything, the reductions were more pronounced in the mild NYHA II group as compared to the severe heart failure group which may argue against a purely causative role of inflammation in diminished vasorelaxant reactivity.[9] and (4) Other clinical parameters such as plasma sodium and potassium were within acceptable values and can thus not be confounders. The levels of NT-proBNP were typical for the two stages of heart failure: > 2000 ng/L vs. > 16000 ng/L, and are somewhat in accordance with the ejection fraction measurements obtained in conjunction with echocardiography (43% and 36%, respectively). We suggest that proBNP is a better predictor of severity of heart failure as compared to echocardiography in the standard clinical setting.[8] Since the microvascular response was not different for the moderate and severe heart failure patients groups, it may indicate that the cutaneous vascular reactivity to vasodilators is an early sign in CHF and reveals alteration of the circulatory system to congestion.
Table 3. Laboratory blood analysis, mean ± SE.
| CHF NYHA II out patients n = 15 | CHF NYHA IV hospitalized n = 20 | |
| NT pro BNP (ng/L) | 1959 ± 569 | 16859 ± 1966* |
| Hemoglobin (g/L) | 143 ± 3.7 | 116.5 ± 4.8* |
| Sodium (mmol/L) | 141 ± 0.9 | 141 ± 1.1 |
| Potassium (mmol/L) | 4.2 ± 0.1 | 3.9 ± 0.1 |
| Creatinine (µmol/L) | 96.8 ± 5.4 | 152.7 ± 16.0* |
| Uric acid (µmol/L) | 450 ± 21 | 559 ± 36* |
| LDL (mmol/L) | 3.1 ± 0.2 | 2.1 ± 0.2 |
| HDL (mmol/L) | 1.8 ± 0.2 | 1.1 ± 0.1* |
| CRP (mg/L) | 4.7 ± 0.7 | 13.3 ± 4.6* |
| HbA1c (%) | 4.8 ± 0.1 | 7.0 ± 0.3* |
| IL-6 (ng/L) | 5.8 ± 2.1 | 7.0 ± 0.3* |
| IL-2r (kU/L) | 666 ± 88 | 1127 ± 123* |
CHF: Congestive heart failure; NYHA: New York Heart Association classification; NT pro BNP: Nerve terminal-pro-brain natriuretic peptide; LDL: Low density lipoprotein; HDL: High density lipoprotein; CRP: Sensitive C Reactive Protein; IL: Interleukin; IL-2r: Soluble IL 2 receptor. *P < 0.05 compared between the two groups.
One consideration is that the age of the NYHA IV patients was beyond the average life span. Thus, patients in this group may reflect a selected group who, due to genetics, lifestyle, diet and other factors, may have been better able to preserve endothelial function as compared to the general population of heart failure patients.
In conclusion, patients with heart failure have reduced microvascular responses to endothelial and smooth muscle cell stimulants. However, there was no relation to the degree of chronic heart failure which may suggest that the vascular reduction in responses may be an early sign, but not a progression indicator of chronic heart failure.
Acknowledgments
This study was supported by the Lisa & Johan Grönbergs Stiftelse, SEB Enskilda Banken, Stockholm, Sweden.
References
- 1.Krum H, Abraham WT. Heart failure. Lancet. 2009;373:941–955. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 2.Andersson SE, Edvinsson ML, Edvinsson L. Cutaneous vascular reactivity is reduced in aging and in heart failure: association with inflammation. Clin Sci (Lond) 2003;105:699–707. doi: 10.1042/CS20030037. [DOI] [PubMed] [Google Scholar]
- 3.Bank AJ, Lee PC, Kubo SH. Endothelial dysfunction in patients with heart failure: relationship to disease severity. J Card Fail. 2000;6:29–36. doi: 10.1016/s1071-9164(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 4.Edvinsson ML, Andersson SE, Xu CB, et al. Cigarette smoking leads to reduced relaxant responses of the cutaneous microcirculation. Vasc Health Risk Manag. 2008;4:699–704. doi: 10.2147/vhrm.s2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 6.Edvinsson L, Andersson SE. Commentary on viewpoint: the human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:381. doi: 10.1152/japplphysiol.90301.2008. author reply 389. [DOI] [PubMed] [Google Scholar]
- 7.Grubb A, Nyman U, Björk J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 8.Andersson SE, Edvinsson ML, Bjork J, et al. High NT-proBNP is a strong predictor of outcome in elderly heart failure patients. Am J Geriatr Cardiol. 2008;17:13–20. doi: 10.1111/j.1076-7460.2007.06674.x. [DOI] [PubMed] [Google Scholar]
- 9.Andersson SE, Edvinsson ML, Edvinsson L. Reduction of homocysteine in elderly with heart failure improved vascular function and blood pressure control but did not affect inflammatory activity. Basic Clin Pharmacol Toxicol. 2005;97:306–310. doi: 10.1111/j.1742-7843.2005.pto_146.x. [DOI] [PubMed] [Google Scholar]


