Abstract
Postural orthostatic tachycardia syndrome (POTS) has been recognized since at least 1940. A review of the literature identifies differences in the definition for this condition and wide variations in treatment and outcomes. This syndrome appears to describe a group of conditions with differing pathophysiology, which requires treatment tailored to the true underlying disorder. Patients need to be fully evaluated to guide treatment. Further research is required to effectively classify the range of underlying pathophysiology that can produce this syndrome and to guide optimal management.
Keywords: Postural orthostatic tachycardia syndrome, Syncope, tachycardia, Diagnosis, Treatment
1. Introduction
Postural orthostatic tachycardia syndrome (POTS) has been recognized since at least 1940,[1],[2] annually affecting around 500,000. Americans between the ages of 15∼50 years[3] with a female to male ratio around 5:1. Until recently, there has been no clear definition of the syndrome, although a consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome has recently been published.[4] POTS is a disorder in which the autonomic nervous system fails to compensate for upright body posture, but there is disagreement surrounding the precise definition.[1] Most authors agree POTS is characterized by an excessive increase in heart rate (tachycardia), either by 30 beats per minute or to a rate of more than 120 beats per minutes, after standing (5∼30 min). However, opinions diverge on the inclusion, or exclusion, of blood pressure changes (orthostatic hypo- or hyper-tension) as a delineating feature of POTS.[2],[4]–[6] Some authors note that high levels of plasma noradrenaline when upright, and low blood volume, are also observed in some POTS patients. This raises two questions: Is POTS a meaningful diagnosis, or is it a symptoms-labelled disease? Are all disorders labelled as POTS truly the same condition? This review compiles the literature on symptoms, diagnosis, treatment and management of POTS to demonstrate further research is required to fully characterize this syndrome and guide optimal management.
2. Symptoms
Patients with POTS experience a variety of symptoms ranging from mild to severe. The most common underlying condition is cerebral hypo-perfusion, which might be due to excessive tachycardia, neurological dysfunction or other idiopathic causes.[5] These symptoms include: light-headedness, fatigue, diaphoresis, tremor, palpitations, exercise intolerance, near syncope and recurrent syncope on upright posture.[5],[7] Patients with POTS may complain of exacerbation of symptoms after simple activities, such as eating, showering or low-intensity exercise, associated with a high degree of functional disability.[8] POTS patients may also suffer from mental clouding (“brain fog”), blurred vision, shortness of breath, early satiety, nausea, headache and chest discomfort;[8] Other symptoms include anxiety, flushing,[9] postprandial hypotension,[9] lower back pain,[10] aching neck and shoulders,[10] cold hands (and often feet & nose),[11] and hypovolemia.[11]
3. Diagnosis
The first step in the diagnosis of POTS is to rule out or exclude other causes of tachycardia, such as specific heart conditions and diseases with symptoms, which overlap POTS.[12] According to some physicians, the patient should meet the following specific criteria.
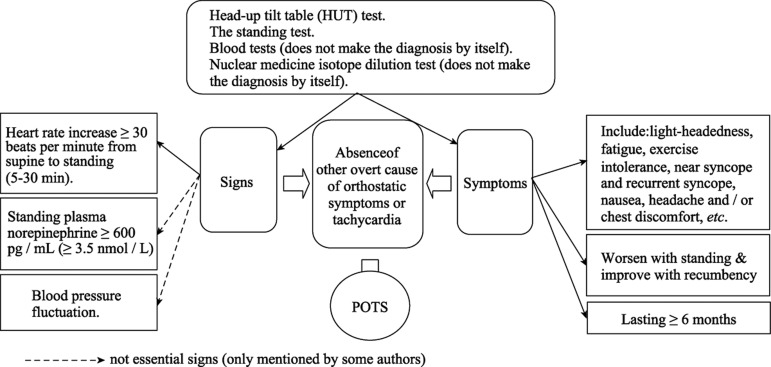
3.1. Diagnostic criteria for POTS (See also Figure 1)
Figure 1. Diagnosis and evaluation of postural orthostatic tachycardia syndrome (POTS).
Heart rate increase ≥ 30 beats per minute from supine to standing (5∼30 min), symptoms that worsen with standing and improve with recumbency. The recently published consensus statement notes that an increase of > 40 beats per minute should be used for patients in the age range 12∼19 years.[4] symptoms should last ≥ 6 months, and the absence of other overt causes of orthostatic symptoms or tachycardia (e.g., active bleeding, acute dehydration, medications).
Head-up tilt table (HUT) testing is the standard method to assess a patient's reaction to postural change. This involves placing the patient on the tilt table, and measuring blood pressure and heart rate. Then the table is tilted upright to a 60∼80 degree vertical angle for approximately 45 min and blood pressure and heart rate are again measured, either continuously, or at least every 2∼3 min.[12]
The standing test, considered to mimic real life, is another test for POTS.[13] The patient is asked to stand upright without any assistance, so the patient supports his own weight and maintains balance.[13] One study suggested that, though both the standing test and HUT have the same criteria to diagnose POTS, the standing test had a specificity of 79% compared to only 23% for the HUT.[13]
A high plasma level of noradrenaline level is also considered useful to identify POTS patients, and is sometimes referred to as a hyper-adrenergic form of POTS. Noradrenaline is measured after drawing a blood sample from the patient in both a supine and a standing position,[8] with the patient in each position for at least 15 min prior to blood sampling.[8] The increase in noradrenaline in the standing position indicates the patient belongs to this sub-group.[8]
The nuclear medicine isotope dilution test is another potential test to assist in POTS diagnosis. This method is not diagnostic by itself, [8] since some POTS patients suffer from low blood volume, but the test identifies the presence of hypovolaemia.[8]
4. Pathophysiology
A number of mechanisms may be contributing to the symptoms of POTS. Some may contribute to reduced oxygen to the brain upon standing.[14] Translation from supine to upright posture requires rapid and effective neurologic and circulatory compensation to ensure blood pressure and consciousness is maintained.[15] When upright, the human body pools a certain amount of blood in the veins of the ankles and legs resulting in a transient reduction in venous return, thus reducing blood pressure. Normal compensation is swift and asymptomatic, primarily through sympathetic stimulation. The heart rate is increased, but rarely exceeds 100 beats per minute. This also causes peripheral vasoconstriction to maintain the circulating volume in the upper body. It appears that blood pooling in the veins of the lower body is a major factor in most POTS patients. Many authors speculate on the possible causes for this, including impaired venous innervation and/or reduced venous response to sympathetic stimulation.[3] This theory supports a neuropathy that mainly affects the lower extremities.
Another explanation suggests alpha-1-adrenergic receptor denervation and/or hypo-sensitivity,[16] as the cause, but there is a controversy relating to skeletal muscle alpha-1 receptor involvement, because during orthostasis, alpha-1 is said to be important only in splanchnic and skin circulation.[16]
Others attribute the venous pooling to beta-adrenergic receptor super-sensitivity. This may result in indirect alteration in venous filling due to arterial vasoactivity.[17] Altered vasoconstriction and increased capillary permeability are also mentioned as components of POTS pathophysiology.[18] Diehl suggests there is growing evidence the increased sympathetic cardiac activation is a compensatory mechanism to keep blood pressure constant in the presence of disturbed vasoconstriction.[18] Finally, POTS patients may exhibit abnormal vascular structure and/or muscle tone which results in impairment in the venous capacitance properties and hence venous pooling.[18]
An abnormality in the baroreflex mechanism of POTS patients has also been considered an etiological factor. The baroreflex plays an essential role in neuro-circulatory control.[15] The arterial baroreflex maintains blood circulation to the brain and other body organs via blood pressure regulation.[15] Baroreceptors sense systemic blood pressure indirectly, by the extent of stretching of receptors in the walls of the carotid arteries and of the aorta. Heart rate, cardiac contractility, vascular resistance, and venous return are all adjusted by baroreflex feedback upon changes in blood pressure. Muenter swift et al.[19] showed POTS patients have exaggerated muscle sympathetic nerve activity (MSNA) responses to baroreflex challenges compared to healthy control subjects, although resting supine MSNA values did not differ between the groups.
Endogenous nitric oxide is also a mechanism involved in blood pressure control, but only for short-term control through a feedback mechanism. Nitric oxide also modulates release of noradrenaline. One study shows that genotype frequencies for particular polymorphs of nitric oxide synthase isoform 3 differ significantly between patients with POTS and healthy control subjects.[20] In addition, these genotypes correlated significantly with the orthostatic rise in HR and plasma noradrenaline in POTS patients.[20] Moreover, the same study reported higher levels of eNOS endogenous nitric oxide synthase (eNOS) in POTS patients.[20]
A 2010 study conducted by Fu et al.[21] suggested the tachycardia associated with POTS patients, in the absence of blood pressure changes, is due to small stroke volume, cardiac output, left ventricular mass and blood volume.
Raj et al.[8] suggested the chest pains experienced in POTS are almost never thought to be due to coronary artery obstruction, but may be associated with electrocardiographic (EKG) changes in the inferior leads, particularly when upright. They suggest the left sided heart pain common among POTS sufferers is due to differences in heart chamber pressures, abnormal heart wall motions, and/or nerve damage. However, based on the Qi Fu's study[21] the chest pain may be associated with reduced blood supply to the heart, hence ischemia, which will result in the angina like chest pain.
Other theories include sudomotor (relating to the nerves that stimulate the sweat glands) abnormalities,[22] and the exclusion of anxiety as primary causes of the excessive orthostatic tachycardia.[23]
5. Treatment
5.1. Diet
Changing diet and eating habits and increasing fluid intake help people with POTS. Eating small meals has been said to reduce the severity of postprandial hypotension, because the amount of blood required for digestion is reduced.[24] Increasing electrolyte and water intake was shown to decrease tachycardia in POTS patients with idiopathic forms of the disease, as a result of increased blood pressure through increasing blood volume.[24] This is also thought to be helpful in POTS patients with pooling blood and/or hypovolemia.[8] The study recommends two to three litres per day, however, drinking excessive amounts of water has the potential to disturb electrolyte concentrations which may affect the heart rhythm.[8] Increasing salt in the diet is another option for POTS patients found to have impaired urinary sodium retention, which precipitates hypovolaemia episodes. The levels of renin and aldosterone in some POTS sufferers are found to be low. These hormones increase plasma volume by promoting sodium retention.[25] Thus, increasing sodium intake by taking salt tablets or an electrolyte solution helps expand blood volume, which will alleviate the hypotension some POTS patients suffer. Some physicians suggest patients take ten to fifteen grams of sodium[26] daily, which is equivalent to 5.85 g (approximate. 250 mmol, 250 mEq) of sodium.
5.2. Exercise
In a number of studies, exercise has been reported to be beneficial both in alleviating POTS symptoms, as well as playing a role in curing the condition.[21] In one study, POTS patients without blood pressure fluctuations,[21] were trained gradually to move from lying to sitting to a standing position during different activities, such as swimming, rowing, and cycling. The same study combined the exercise training with increased water and salt intake to 3∼4 L/d and 6∼8 g/d, respectively and also included elevating the head of the bed while sleeping at night.[21] Grubb reported aerobic exercise three times a week for 20 min is also beneficial for patients who can tolerate it.[27]
5.3. Sodium chloride 0.9% (Normal saline)
Sodium chloride 0.9% infusion has been reported very beneficial in decreasing symptoms in POTS patients and improving quality of life.[28] The infusion loads the POTS patient with sodium,[28] enhances blood and blood cell volume, and results in a slight elevation in blood pressure. Freitas et al.[28] reported sodium chloride 0.9% infusion was the most effective among a range of treatments, producing a reduction in heart rate and reducing systolic blood pressure fluctuation in treated POTS patients. This appears to be a good, low-cost treatment option with few side effects for POTS patients who suffer hypovolemia caused by sympathetic neuropathy, which leads to venous pooling during upright posture, or other idiopathic causes.[28] However, the problem in this study is that intravenously infusion is time consuming as the patient must normally go to the hospital or to the physician's office for IV cannulation and infusion.[28] Indwelling, peripherally inserted central catheters (PICC) have been used by some POTS patients to administer their infusion at home, but there are possible risks associated with a PICC line, such as infection, occlusion or displacement.[28] We speculate that techniques, such as proctoclysis (rectal infusion of hydration) as employed in emergency resuscitation in rural and remote areas, may offer an alternative for home-administered therapy,[29] but no published studies had evaluated this option.
5.4. Beta-blockers
Beta blockers have been reported to be a useful treatment for POTS patients with beta-receptor super-sensitivity, high noradrenaline levels and/or hyper-adrenergic states. However, β-blockers may exacerbate hypotension and reduce renin levels. Thus, β-blockers must be used with caution since some POTS patients have low blood pressure and/or plasma renin.[30] Consequently, beta blocker usage in hypovolemic patients may be counterproductive. Additionally, as beta- blockers have the potential to activate mast cells, they should be used with caution in patients with mast cell activation diseases. A study reports combining the beta-blocker, bisoprolol, with fludrocortisone, a mineralocorticoid that increases salt retention, is a beneficial treatment for POTS patients with autonomic and hemodynamic disturbances.[28],[31] Conversely, a randomized, crossover, controlled study showed low-dose oral propranolol significantly benefited POTS patients by attenuating tachycardia and improving their symptoms. However, the same study showed no improvement in the symptoms of POTS patients with higher-dose propranolol and may even worsen them.[32] In a small survey of patients treated with midorine (n = 13) and beta blockers (n = 14), 100% of patients on the beta blocker claimed improvement, although only 63% of the patients attributed their improvement to medication.[33]
5.5. Fludrocortisone
Fludrocortisone increases plasma volume in patients with POTS[31],[34] due to salt and water retention and also sensitizes the blood vessels to constriction.[35] Some physicians combine salt tablets with fludrocortisone in order to ensure its effectiveness, although this must depend on salt intake.[36] Patients on fludrocortisone should be given magnesium and potassium supplements due to their concurrent depletion. Also, fludrocortisone has the potential to raise intracranial pressure, therefore it cannot be used in some cases, which involve the brain. Like beta-blockers, fludrocortisone reduces renin levels and may be counterproductive for POTS patients with low renin levels.[30] Some patients suffer severe side effects, particularly severe headache.[36]
5.6. Ivabradine
Due to the presence of sinus tachycardia in some POTS patients, usage of a sinus node blocker, in particular, Ivabradine, was reported to improve their symptoms. Ivabradine may be preferable to beta-blockers as it reduces the heart rate without sexual disturbance, negative ionotropic effects and vasodilation, which are commonly associated with beta-blocker.[37]
5.7. Erythropoietin
Erythropoietin is considered a treatment option[25] in some POTS patients noted to have a low red blood cell volume, and impairment of erythropoietin function and/or production. This is due to its ability to increase cell mass and hence blood pressure.[27] In addition, erythropoietin is a potent vasoconstrictor, which further assists in raising blood pressure.[27] One study of erythropoietin in POTS patients with hypotension showed benefit.[38] However, a later study of only 8 patients with orthostatic tachycardia reported erythropoietin did not help the tachycardia,[39] since the underlying pathophysiology of these particular patients is not related to the red blood cells and blood volume.[39] Procrit (epoetin alpha) has been used instead of erythropoietin, which has to be injected and is expensive.[39] Moreover, erythropoietin may result in a rise in heamatocrit, so patients may require iron supplementation.[27] Erythropoetin has been associated with increased mortality in certain populations, this was described in detail by Fishbane and Besarab.[40]
5.8. Pyridostigmine bromide
Occasionally pyridostigmine bromide is used to treat POTS.[41],[42] Pyridostigmine bromide enhances the effect of acetylcholine by inhibiting its breakdown,[43] but some studies suggest that the pathophysiology in some POTS atients is related to the production of antibodies that block the acetylcholine receptor. Pyridostigmine bromide has the potential to increase agonist activity and overcome the blockage of these receptors. Thus, it may be useful for POTS patients of postviral, paraneoplastic or autoimmune forms. The dose is initially 30 mg two times a day increased to 60 mg two times a day when required.[27] A randomised, placebo controlled, crossover study showed that acute intake of pyridostigmine bromide resulted in symptom improvement in POTS patients.[44]
Another study shows that pyridostigmine is safe for use by children with POTS (absence of significant orthostatic hypotension) in three divided doses.[43] A further study concluded a single dose (30 mg orally) in POTS patients produced moderate but statistically significant hemodynamic improvement, but concluded the long-term usage of pyridostigmine bromide was insufficient to treat patients with orthostatic intolerance.[45]
5.9. Vasoconstrictors
In patients with diseases related to venous pooling, a vasoconstrictor may be an option. Available medications include: ergotamine, midodrine, octreotide, ephedrine, pseudoephedrine, yohimbine, theophylline and methylphenidate. These medications reduce pooling of blood by improving the venous tone. Midodrine is a vasoconstrictor found to be very helpful for POTS patients, including children, with peripheral denervation.[26],[46] Patients usually start midodrine at 5 mg every eight hours; and the dose can be increased to up to 15∼20 mg orally every six hours.[27] Long term use of midodrine is not recommended due to chronic activation of the sympathetic nervous system which can reduce blood volume.[3] Octreotide is another vasocon strictor which has been used to decrease splanchnic pooling by preventing vasodilation in the gut and thus will prevent hypotension after meals.[10] Octreotide inhibits a range of peptides released in the gastrointestinal tract and may reduce hypotension induced by exercise.[10] Thus, octreotide is useful for POTS patients where hypotension is one of their symptoms. One study reports octreotide has the potential to “enhance supine nocturnal hypertension”.[38] Theophylline also has a vasoconstriction action, and has been reported to treat dysautonomia.[47] As methylphenidate stimulates alpha receptor, and hence increases vascular resistance in the periphery, some physicians prescribe it for POTS patients with hypotension, but there is a risk of addiction.[48] Clonidine, a direct-acting α2 adrenergic agonist is somewhat controversial as it has been reported to worsen the orthostatic symptoms in one study,[28] but a recent study in children reported it to be an effective treatment for hyperadrenergic POTS patients.[49]
5.10. NSAIDs
POTS patients who suffer postprandial hypotension might benefit from ibuprofen or indomethacin[50] since these medications reduce prostaglandin effects, hence blocking its effect in decreasing blood pressure.[51]
5.11. Others
Other medications which have been reported to help POTS patients include: methyldopa and antidepressants.[27]
6. Conclusion
The pathophysiology of POTS is complex and the result of a number of separate mechanisms producing a common pattern of symptoms. The large number of clinical manifesttations that characterize this disorder and the wide range of medications available, plus the clear evidence that certain medications and treatment strategies work in some, but not all POTS patients, demonstrates that POTS is a range of disorders requiring comprehensive investigation and characterisation to guide selection of the most appropriate treatment. The recent consensus statement will help to direct further research into the underlying conditions that lead to POTS.
References
- 1.Carew S, Cooke J, O'Connor M, et al. What is the optimal duration of tilt testing for the assessment of patients with suspected postural tachycardia syndrome? Europace. 2009;11:635–637. doi: 10.1093/europace/eup044. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478–480. doi: 10.1136/pgmj.2006.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci. 1999;317:88–101. doi: 10.1097/00000441-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Masuki S, Eisenach JH, Schrage WG, et al. Arterial baroreflex control of heart rate during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103:1136–1142. doi: 10.1152/japplphysiol.00176.2007. [DOI] [PubMed] [Google Scholar]
- 6.Grubb BP. The postural tachycardia syndrome: A brief review of etiology, diagnosis and treatment. Hellenic J Cardiol. 2002;43:47–52. [Google Scholar]
- 7.Ojha A, McNeeley K, Heller E, et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245–249. doi: 10.1016/j.amjmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 9.Grubb BP, Kanjwal MY, Kosinski DJ. Review: The postural orthostatic tachycardia syndrome: Current concepts in pathophysiology diagnosis and management. J Interv Card Electrophysiol. 2001;5:9–16. doi: 10.1023/a:1009845521949. [DOI] [PubMed] [Google Scholar]
- 10.Mathias CJ. Autonomic diseases: Management. J Neurol Neurosurg Psychiatry. 2003;74(Suppl 3):S42–S47. doi: 10.1136/jnnp.74.suppl_3.iii42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS) Neurol. 1995;45(4 Suppl 5):S19–S25. [PubMed] [Google Scholar]
- 12.Braune S, Wrocklage C, Schulte-Monting J, et al. Diagnosis of tachycardia syndromes associated with orthostatic symptoms. Clin Auton Res. 1999;9:97–101. doi: 10.1007/BF02311766. [DOI] [PubMed] [Google Scholar]
- 13.Raj SR, Dzurik MV, Biaggioni I, et al. Diagnosing postural tachycardia syndrome: comparison of tilt versus standing. Circulation. 2006;6:84–99. [Google Scholar]
- 14.Stewart JM, Medow MS, Cherniack NS, et al. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol. 2006;291:H904–H913. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: Interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283:R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute alpha-1 adrenergic agonist and a beta-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation. 2002;106:2946–2954. doi: 10.1161/01.cir.0000040999.00692.f3. [DOI] [PubMed] [Google Scholar]
- 17.Bush VE, Wight VL, Brown CM, et al. Vascular responses to orthostatic stress in patients with postural tachycardia syndrome (POTS), in patients with low orthostatic tolerance, and in asymptomatic controls. Clin Autonom Res. 2000;10:279–284. doi: 10.1007/BF02281110. [DOI] [PubMed] [Google Scholar]
- 18.Diehl RR. Continuous progression of orthostatic tachycardia as a further feature of the postural tachycardia syndrome. Pacing Clin Electrophysiol. 2005;28:975–979. doi: 10.1111/j.1540-8159.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- 19.Muenter Swift N, Charkoudian N, Dotson RM, et al. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2005;289:H1226–H1233. doi: 10.1152/ajpheart.01243.2004. [DOI] [PubMed] [Google Scholar]
- 20.Garland EM, Winker R, Williams SM, et al. Endothelial NO synthase polymorphisms and postural tachycardia syndrome. Hypertension. 2005;46:1103–1110. doi: 10.1161/01.HYP.0000185462.08685.da. [DOI] [PubMed] [Google Scholar]
- 21.Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peltier AC, Garland E, Raj SR, et al. Distal sudomotor findings in postural tachycardia syndrome. Clin Auton Res. 2010;20:93–99. doi: 10.1007/s10286-009-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocon AJ, Medow MS, Taneja I, et al. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H664–H673. doi: 10.1152/ajpheart.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478–480. doi: 10.1136/pgmj.2006.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 26.Low PA, editor. Orthostatic intolerance. National Dysautonomia Research Foundation Patient Conference; Minneapolis, Minnesota, USA. 2000. [Google Scholar]
- 27.Grubb BP, Kanjwal Y, Kosinski DJ. The postural tachycardia syndrome: A concise guide to diagnosis and management. J Cardiovasc Electrophysiol. 2006;17:108–112. doi: 10.1111/j.1540-8167.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 28.Freitas J, Santos R, Azevedo E, et al. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res. 2000;10:293–299. doi: 10.1007/BF02281112. [DOI] [PubMed] [Google Scholar]
- 29.Tremayne V. Proctoclysis: Emergency rectal fluid infusion. Nurs Stand. 2009;24:46–48. doi: 10.7748/ns2009.09.24.3.46.c7271. [DOI] [PubMed] [Google Scholar]
- 30.Jacob G, Robertson D, Mosqueda-Garcia R, et al. Hypovolemia in syncope and orthostatic intolerance: Role of the renin-angiotensin system. Am J Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 31.Freitas J, Santos R, Azevedo E, et al. Reversible sympathetic vasomotor dysfunction in POTS patients. Rev Port Cardiol. 2000;19:1163–1170. [PubMed] [Google Scholar]
- 32.Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: Less is more. Circulation. 2009;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol. 2009;32:234–238. doi: 10.1111/j.1540-8159.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Hermosillo JA. Orthostatic intolerance syndromes. Arch Cardiol Mex. 2001;71(Suppl 1):S58–S62. [PubMed] [Google Scholar]
- 35.Haran C. Pressure drop: Treating orthostatic hypotension (Interview with Dr. Blair P. Grubb) 2004. http://www.healthology.com/focus_article.asp (accessed on July 30, 2010).
- 36.Schondorf R, Freeman R. The importance of orthostatic intolerance in the chronic fatigue syndrome. Am J Med Sci. 1999;317:117–123. doi: 10.1097/00000441-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Khan S, Hamid S, Rinaldi C. Treatment of inappropriate sinus tachycardia with ivabradine in a patient with postural orthostatic tachycardia syndrome and a dual chamber pacemaker. Pacing Clin Electrophysiol. 2009;32:131–133. doi: 10.1111/j.1540-8159.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- 38.Hoeldtke RD, Bryner KD, Hoeldtke ME, et al. Treatment of autonomic neuropathy, postural tachycardia and orthostatic syncope with octreotide LAR. Clin Auton Res. 2007;17:334–340. doi: 10.1007/s10286-007-0436-x. [DOI] [PubMed] [Google Scholar]
- 39.Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med. 1995;99:525–529. doi: 10.1016/s0002-9343(99)80230-7. [DOI] [PubMed] [Google Scholar]
- 40.Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2:1274–1282. doi: 10.2215/CJN.02380607. [DOI] [PubMed] [Google Scholar]
- 41.Grubb BP. The heterogeneity of symptoms related to dysautonomia. 2002. Symposium conducted at the meeting of the National Dysautonomia Research Foundation Northwest Ohio Support Group, Toledo, Ohio, USA.
- 42.Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing Clin Electrophysiol. 2011;34:750–755. doi: 10.1111/j.1540-8159.2011.03047.x. [DOI] [PubMed] [Google Scholar]
- 43.Filler G, Gow RM, Nadarajaha R, et al. Pharmacokinetics of pyridostigmine in a child with postural tachycardia syndrome. Pediatrics. 2006;118:E1563–E1568. doi: 10.1542/peds.2006-0904. [DOI] [PubMed] [Google Scholar]
- 44.Raj SR, Black BK, Biaggioni I, et al. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 45.Gales BJ, Gales MA. Pyridostigmine in the treatment of orthostatic intolerance. Ann Pharmacother. 2007;41:314–318. doi: 10.1345/aph.1H458. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Wang L, Sun J, et al. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ J. 2011;75:927–931. doi: 10.1253/circj.cj-10-0514. [DOI] [PubMed] [Google Scholar]
- 47.Grubb BP, McMann MC. The fainting phenomenon: Understanding why people faint and what can be done about it. Futura Publishing Company; New York, USA: 2001. [Google Scholar]
- 48.Grubb BP, Kosinski D, Mouhaffel A, et al. The use of methylphenidate in the treatment of refractory neurocardiogenic syncope. Pacing Clin Electrophysiol. 1996;19:836–840. doi: 10.1111/j.1540-8159.1996.tb03367.x. [DOI] [PubMed] [Google Scholar]
- 49.Kanjwal K, Saeed B, Karabin B, et al. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single centre experience. Cardiology. 2011;18:527–531. doi: 10.5603/cj.2011.0008. [DOI] [PubMed] [Google Scholar]
- 50.Hilz MJ, Marthol H, Neundorfer B. Syncope-a systematic overview of classification, pathogenesis, diagnosis and management. Fortschr Neurol Psychiatr. 2002;70:95–107. doi: 10.1055/s-2002-19923. [DOI] [PubMed] [Google Scholar]
- 51.Hain TC. Orthostatic hypotension. Information about dizziness, ataxia and hearing disorders 2001. http://www.tchain.com/otoneurology/disorders/ (accessed on August 21, 2010).