Abstract
Abdominal aortic aneurysm is a common vascular disease that affects elderly population. Open surgical repair is regarded as the gold standard technique for treatment of abdominal aortic aneurysm, however, endovascular aneurysm repair has rapidly expanded since its first introduction in 1990s. As a less invasive technique, endovascular aneurysm repair has been confirmed to be an effective alternative to open surgical repair, especially in patients with co-morbid conditions. Computed tomography (CT) angiography is currently the preferred imaging modality for both preoperative planning and post-operative follow-up. 2D CT images are complemented by a number of 3D reconstructions which enhance the diagnostic applications of CT angiography in both planning and follow-up of endovascular repair. CT has the disadvantage of high cummulative radiation dose, of particular concern in younger patients, since patients require regular imaging follow-ups after endovascular repair, thus, exposing patients to repeated radiation exposure for life. There is a trend to change from CT to ultrasound surveillance of endovascular aneurysm repair. Medical image visualizations demonstrate excellent morphological assessment of aneurysm and stent-grafts, but fail to provide hemodynamic changes caused by the complex stent-graft device that is implanted into the aorta. This article reviews the treatment options of abdominal aortic aneurysm, various image visualization tools, and follow-up procedures with use of different modalities including both imaging and computational fluid dynamics methods. Future directions to improve treatment outcomes in the follow-up of endovascular aneurysm repair are outlined.
Keywords: Abdominal aortic aneurysm, Computed tomography, Follow-up, Stent graft, Treatment, Visualization.
1. Introduction
An abdominal aortic aneurysm (AAA) occurs when the abdominal aortic wall becomes weakened, resulting in focal enlargement of the blood vessel. An AAA is defined as an enlargement of the aorta of at least 1.5 times its normal aortic diameter or greater than 3 cm diameter in the maximum transverse dimension. Most of the AAAs (> 80%) are located in an infrarenal position, while only a small percentage of them belong to juxtarenal or suprarenal AAAs. Common risk factors attributable to the development of AAA include increased age, smoking, atherosclerosis and hypertension.[1] AAAs are about three to four times more common in men than in women.
Most people with AAAs do not have aneurysm-related symptoms and therefore the diagnosis depends mainly on incidental clinical investigations (e.g., physical examination or ultrasound or X-ray examination). Because most AAAs are asymptomatic, it is difficult to estimate their prevalence, but screening studies in the UK reported an occurrence of 1.3%∼12.7% depending on the age group studied.[2] The incidence of symptomatic AAAs in men is approximately 25 per 100,000 at age 50, increasing to 78 per 100,000 in those older than 70 years.[2] The implementation of a national screening program for AAA with the aim of reducing aneurysm associated mortality is recommended.[3]
Once an aneurysm has been detected by routine physical exam and radiographic studies, the risk of rupture is weighed against the risk of surgical repair for each individual patient. The major determinant for risk of rupture is aneurysm diameter. In the absence of historical data on patients with aortic aneurysms, the risk of rupture is estimated from the respective diameters of the abdominal aorta. The risk of operative complications is determined not only by age, cardiac and pulmonary function, but also by the extent of aorta involved.
Without surgery, studies indicate the 5-year survival rate for patients with aneurysms larger than five centimerter is about 20%.[2] Elective surgery is recommended in current clinical practice for patients with aneurysms larger than 5.5 cm in diameter and with aneurysms larger than 4.5 cm in diameter that have increased by more than 0.5 cm in the past six months.[3] Although the progression of AAA is that of a continuing growth, the rupture risk at small aneurysm size may be low, thus, surveillance is regarded as a safe approach, while avoiding exposure of the patients to the unnecessary risk of early or late procedure-related complications.[4]–[6] Current guidelines of the Vascular Society and the National Screening Committee recommend that patients with asymptomatic aneurysms of less than 4.5 cm in diameter should be followed up with ultrasound every 6 months, and aneurysms of 4.5∼ 5.5 cm in diameter should be followed up every three or six months.[3]
2. Treatment options
Definitive therapy for aortic aneurysms is to prevent aneurysm rupture, for example, by placement of the dilated segment of aorta with a prosthetic graft. To determine whether an individual patient is a candidate for graft replacement, many factors need to be considered, including the risk of aneurysm rupture, life expectancy, anticipated quality of life after the operation, and the risk of surgical treatment.[7]
2.1. Open surgical repair
For more than half a century, open surgical repair has been regarded as the gold standard to treat AAAs with a high degree of success, and it is still widely performed in many clinical centers. The basic goal of surgical repair is the exclusion of the aortic aneurysm from the systemic circulation with preservation of blood flow to the pelvis and legs via an implanted new vascular conduit (usually a synthetic fabric or expanded polytetrafluoroethylene). This is usually achieved by incision of the aneurysm sac; removal of mural thrombus; ligation of patent branch vessels (such as superior mesenteric or inferior mesenteric artery) arising from the aneurysm sac; selection of a graft of appropriate size and shape; suturing anastomosis of the graft to the artery at proximal and distal segments to the aneurysm; and, finally, closure of the decompressed aneurysm sac over the synthetic graft material.
This is a major and invasive surgery and the overall operative mortality for elective surgical repair is 4% or less, but can be as high as 8.4%, depending on the experience of operating centers and the patient's cardiovascular condition.[8],[9] The major causes of peri-operative morbidity are cardiovascular, hemorrhagic and septic complications.[10],[11]
2.2. Endovascular aneurysm repair-minimally invasive technique
In an attempt to reduce the surgical risk in patients with associated medical conditions, less invasive techniques of AAA repair have been considered. About two decades ago, minimally invasive techniques emerged limited only to the repair of infrarenal aneurysms but recent technical developments allowed them to be extended to the suprarenal aorta.[12]–[14] Instead of graft replacement via an extensive procedure through abdominal or flank incision under general anesthesia, a thin-walled prosthesis is compressed into a catheter, introduced into the femoral artery via a limited groin incision under local anesthesia, and advanced into the abdominal aorta to exclude the aneurysm from the systemic circulation.
Endovascular repair of AAA, as evaluated by the endovascular aneurysm repair (EVAR) and the Dutch Randomized Endovascular Aneurysm Management (DREAM) trials, is reported to have lower 30-day mortality than conventional open repair.[15],[16] The EVAR trial 1 is one of the largest of planned trials of endovascular versus open surgery repair of AAA with recruitment of 1082 patients from various clinical centers distributed in European countries.[15] In the trial, a clear short-term benefit of EVAR has been reported with 1.7% of patients dying within 30 days compared with 4.7% of those treated with open surgery. In addition, EVAR had at least two-thirds lower 30-day and in-hospital mortality compared with open repair. Similarly, the DREAM trial, comparing open and endovascular repair of AAA, concluded that endovascular repair is preferable to open repair over the first 30 days after the procedure.[16] These randomized, controlled trials indicated that in patients who are candidates for both open surgery and EVAR, endovascular repair leads to lower rates of operative mortality and complications and the significant reduction in the rate of systemic complications, thus, it is a preferable approach in these patients.
The EUROSTAR (The European Collaborators Registry on Stent-Graft Techniques For AAA repair) evaluated the quality-adjusted life expectancy post-operatively for both open surgical repair and endovascular repair of AAA.[17] The report showed that open surgery is preferred in younger patients, but EVAR is better in older patients as it prolonged a 70-year-old male's life in poor health by three months. EVAR is still considered the only choice of elective intervention for very unfit AAA patients, (patients with cardiac disease, chronic obstructive pulmonary disease, diabetes, renal disease, cerebrovascular disease or peripheral artery disease). However, the UK EVAR Trial 2 has demonstrated that immediate endovascular repair does not offer any benefit in terms of mortality, quality of life or costs within the first four years after treatment.[18] Since unfit patients (patients with cardiac disease, chronic obstructive pulmonary disease, diabetes, renal disease, cerebrovascular disease or peripheral artery disease) are most likely vulnerable to a number of co-morbidities, in particular, at high risk of developing cardiovascular events (such as myocardial infarction, stroke and atrial fibrillation), endovascular intervention should be justified in these patients. Brown et al.[19] in their recent study reported that there is only weak evidence to suggest a higher rate of cardiovascular events after EVAR than surveillance alone, and the impact of EVAR in relation to no intervention is not being influenced strongly by patient fitness. Their results suggested that optimization of co-morbidities and improvement of patient fitness should remain the goal in these unfit patients before aneurysm repair is considered.
Although many dramatically successful early and midterm results have been achieved with EVAR, and many advantages have been demonstrated compared to open surgery,[20]–[24] the repeated qualifying statement concluding so many articles on the topic of endovascular repair of AAA is that the long-term results are yet to be determined. Medical imaging techniques play an important role in the follow-up of EVAR with regard to monitoring the aneurysm changes and detecting complications associated with the procedure.
3. Image visualizations
Unlike conventional graft procedures, the success of endovascular stent-graft repair of AAA cannot be determined by direct examination and therefore, relies on imaging assessment. While conventional angiography has been losing its dominant role for arterial imaging, spiral computed tomography (CT) angiography has been confirmed as the best single imaging technique for both preoperative patient assessment and aortic stent-graft surveillance.[25],[26]
3.1. Pre-operative EVAR-planning of stent-grafts
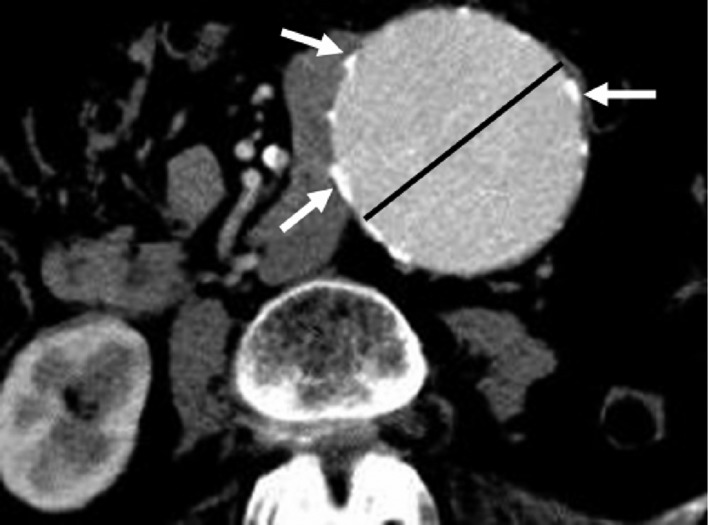
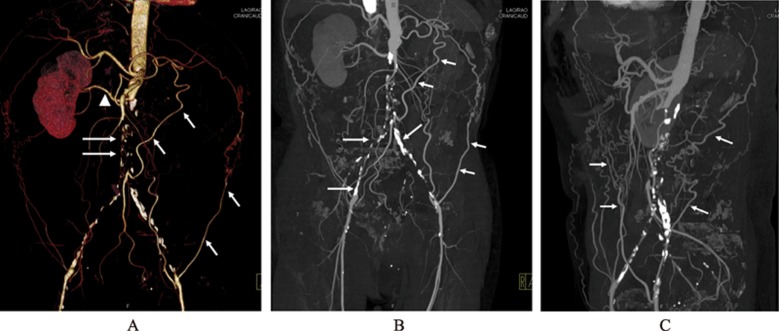
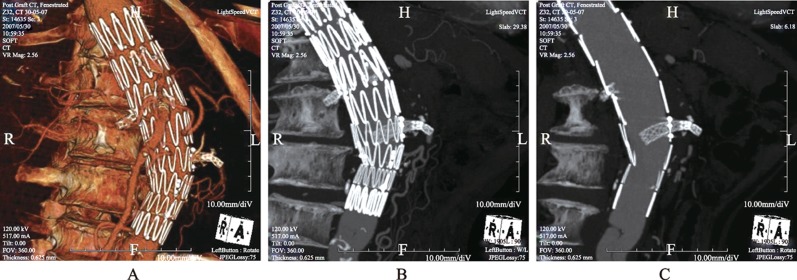
Successful endovascular AAA repair requires secure placement of the stent's proximal and distal segment in non-dilated portions of the aorta and the iliac arteries. Diagnostic imaging performed before AAA stent-graft placement determines the anatomical suitability for the type of stent grafting and provides measurements to size the stent-graft. Axial CT images are most commonly used to determine the maximal aneurysm diameter, including both patient lumen and thrombus (Figure 1). However, 2D and 3D reconstructions, such as multi-planar reformation, maximum- intensity projection and volume rendering visualizations are also routinely generated to enhance the role of CT angiography in planning EVAR, and provide additional information for assessment of extent of the aneurysm, as well as the relationship between aneurysms and arterial branches (Figure 2).
Figure 1. An axial CT image shows a large aortic aneurysm with extensive artery wall calcification. The black line refers to measurement of the aneurysm diameter at transverse dimension, while arrows indicate the calcification in the aortic wall.
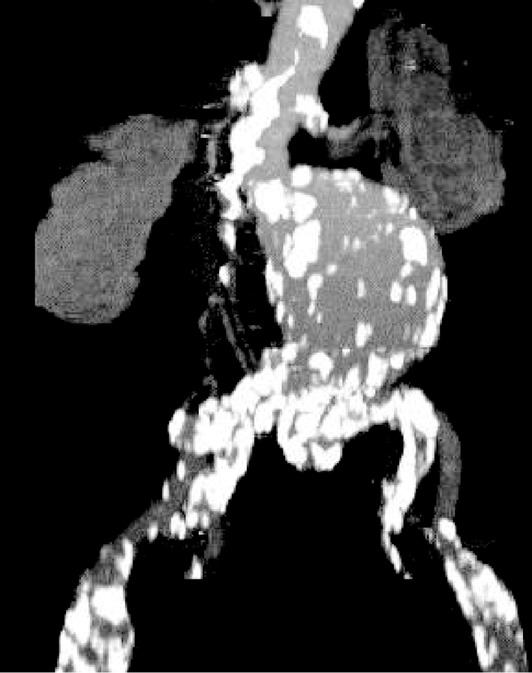
Figure 2. 3D volume rendering shows an infrarenal aortic aneurysm with near total occlusion of the abdominal aorta extending to the common iliac arteries (long arrows in A). Left renal artery is also occluded with no enhancement of the left kidney. Coronal and sagittal maximum-intensity projection images (B and C) demonstrate similar findings with extensive calcifications in the common iliac arteries (long arrows in B). Short arrows refer to the collateral arteries, while arrowhead indicates the patent right renal artery.
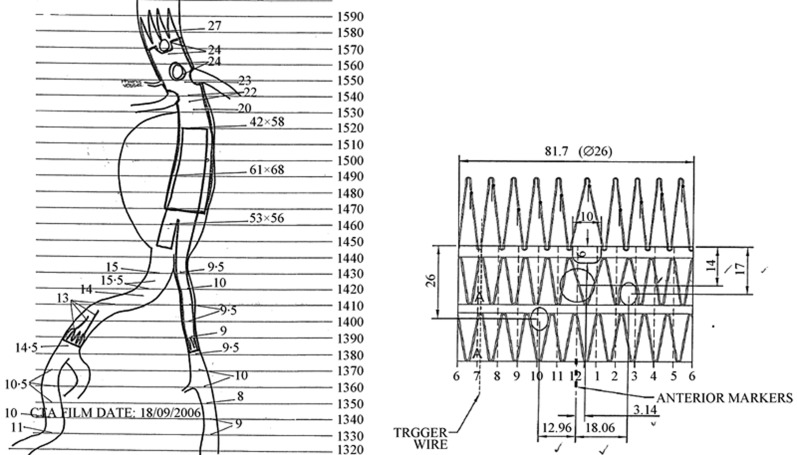
Pre-operative measurements include: the diameter and length of the aneurysm, the centre line of the aneurysm with respect to any curvature of the aorta, the diameter of the aneurysm neck, the neck length (distance from the lowest level of the renal arteries to the proximal segment of the aneurysm), and the diameter and length of the common iliac arteries (Figure 3).[27],[28]
Figure 3. Diagram shows preoperative planning of endovascular aneurysm repair with detailed measurements of the relevant parameters and design of the stent-graft to be implanted in a patient with an infrarenal aortic aneurysm. (A). Viewing from the top to the bottom, scallop fenestration, large fenestration and small fenestrations are recommended for the celiac axis, superior mesenteric artery and bilateral renal arteries, respectively (B).
The dimensions and quality of the aneurysm neck are of critical importance both for deployment of the stent-graft and for satisfactory long-term results. An aneurysm neck less than 1.5 cm in length, excessive tortuosity or angulation of the neck (greater than 60° relative to the axis of the aneurysm) and barrel or conical shaped necks are adverse features for infrarenal endovascular repair (Figure 4).[26] These situations suggest the need to choose a modified stent-grafts with a suprarenal component for firm fixation (with uncovered stent struts crossing the renal orifices) or fenestrated stent-grafts with branched stents inserted into the renal and other visceral arteries (Figure 3).[23],[24]
Figure 4. Extensive calcifications are noticed in a large infrarenal aortic aneurysm with angulation in the proximal and distal aneurysm necks.
3.2. Post-operative EVAR-routine imaging follow-up
Stent-graft integrity is of paramount to post-procedural imaging. Because aneurysm shrinkage is the sure sign that the stent-graft is working, sac size is closely monitored. Thus, aneurysm sac change is a significant indicator for determining the success or failure of EVAR. Diameter measurement is the most commonly used method for determining aneurysm size change, although various methods of diameter measurements have been reported.[29]–[31] Traditionally, the preferred imaging method for surveillance of EVAR is CT angiography, which involves a series of maximal diameter measurements of the aneurysm at regular intervals to determine whether the aneurysm is shrinking, enlarging or unchanged. Some studies, however, have demonstrated the inaccuracy of diameter measurements, noting discrepancies between volume changes and maximal diameter measurements.[32],[33]
It is believed that volume measurement is superior to diameter measurement, since it reflects all dimensional changes of the aneurysm, but one has to admit that volume monitoring of sac size is too time-consuming for routine use. Attempts have been made to produce faster segmentation methods for volume measurements,[34] and both manual and semi-automatic segmentation techniques have shown satisfactory results, with good intra- and inter-observer variability.[35],[36] van Prehn et al.[36] demonstrated that fast and repeatable CT angiography volume measurements could be achieved with a semi-automatic method, indicating the possible clinical application of volumetry to routine surveillance of AAA after EVAR.
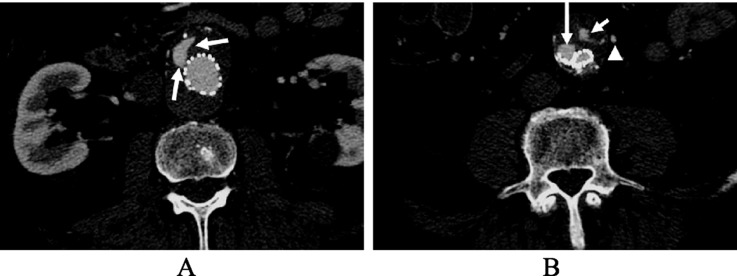
One of the principal reasons for EVAR failure is the occurrence of endoleaks, defined as persistent blood flow outside the graft and within the aneurysm sac. Endoleaks have been categorized in detail,[37],[38] with their reported incidence varying widely from 15% to 52%.[39],[40] Type 1 endoleaks are due to proximal or distal attachment site leaks (Figure 5), type 2 endoleaks are due to retrograde flow from aortic branches (due to back-filling of the aneurysm sac via branch vessels, such as lumbar arteries and inferior mesenteric artery) (Figure 6) and type 3 endoleaks are due to component separation of stent-graft or fabric tears. Type 4 endoleaks can only occur within 30 days of stent-graft insertion and are due to an increase in graft wall (fabric) porosity. A further category, known as type 5, or endotension, has been identified and described as an increased intra-sac pressure after EVAR without a visualised endoleak on contrast-enhanced CT scans.[41] Types 1 and 3 endoleaks are often considered high-pressure leaks, requiring immediate attention to prevent rupture.[42] In contrast, type 2 endoleaks, the most common type, are regarded as low-pressure leaks and thus, are frequently dealt with by conservative approaches.
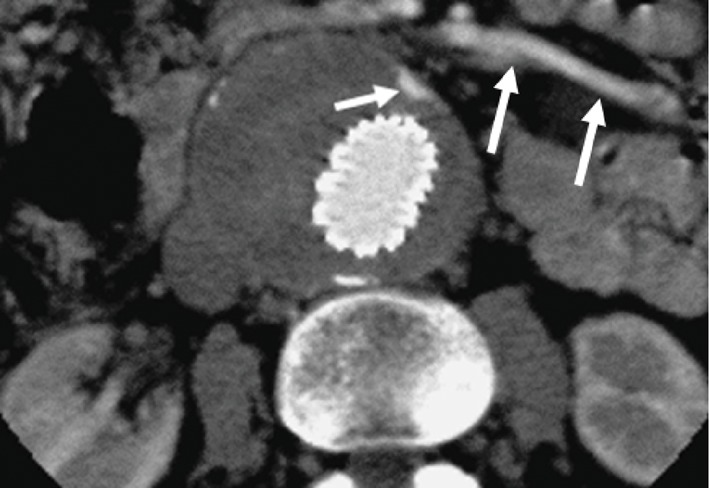
Figure 5. A type 1 endoleak is present in the proximal and distal segments of aortic stent graft, as demonstrated on the axial CT images (long arrows in A and B). A type II endoleak (short arrow in B) is also noticed within the aneurysm sac at the level of common iliac artery due to patent inferior mesenteric artery (arrowhead in B).
Figure 6. A type 2 endoleak (short arrows) is present in the anterior aspect of an aortic aneurysm following endovascular repair due to backfilling from the patent inferior mesenteric artery (long arrows).
There is much debate concerning the management of type 2 endoleaks, since it is not clear about the effect of type 2 endoleaks on long-term outcome of endovascular repair. The management of type 2 endoleaks has consequently evolved with time. Initially, many of these endoleaks were treated by radiological or surgical intervention due to the fear of aneurysm rupture. Recently, a more conservative approach has been recommended because many of them seem to be relatively benign. Rayt et al.[42] showed that regular surveillance for type 2 endoleaks was not associated with any adverse events. The wide variation in the incidence of endoleaks is largely related to the identification of as a type 2 endoleak, which is a reflection of acquisition techniques and imaging quality, especially at the end of the procedure when lumbar vessels are likely to be still open. Thus, accurate detection and identification of type 2 endoleaks by imaging modalities is essential to determine the success of EVAR. The preferred opinion among experts in the field is to take a non-interventional approach unless the aneurysm is enlarging.
It is generally accepted that contrast-enhanced spiral CT angiography is the standard imaging method in the follow-up of EVAR patients due to its high sensitivity and specificity in detecting endoleaks.[43],[44] However, CT angiography has the disadvantages of being used as a routine modality to follow- up endovascular repair of AAA. Although some authors feel that CT angiography is a useful modality for identifying endoleaks, they believe it is less effective in helping to classify endoleaks compared to conventional angiography because it is difficult to determine direction of blood flow from a routine CT angiography.
Stavropoulos et al.[45] showed that classifying endoleaks using conventional angiography resulted in a change in management in 11% of patients compared with CT angiography results. Others recommended the use of multi-phase CT scans to improve detection of endoleaks and differentiate progressive aneurysm expansion as a result of low-flow endoleaks from endotension.[46],[47] This leads to another disadvantage of CT angiography for routine follow-up, which is radiation exposure. Repeated CT scans for EVAR follow-up put patients at risk of receiving cumulative radiation dose, which could contribute to the radiation- induced malignancy.[48] A recent study investigating the radiation exposure during EVAR reported that the mean effective dose of invasive angiography was 27 mSv during EVAR, and the entrance skin dose exceeded the threshold value (2 Gy is generally accepted as the safe threshold dose for avoiding skin damage) in 29% of patients,[49] which explains the trend to move from CT to ultrasound surveillance.
Colour duplex ultrasound (CDU) scanning is less expensive and does not involve ionizing radiation or potentially nephrotoxic contrast. In addition, contrast-enhanced ultrasound displays promising results in the detection of endoleaks.[50],[51] Several studies have reported excellent results with CDU and contrast- enhanced ultrasound compared to CT angiography for better identification and characterization of endoleaks.[50]–[52] In current clinical practice, CT and ultrasound are both used collectively as surveillance imaging tools, especially in the early follow-up. When the aneurysm sac begins to shrink, it is reasonable to move from CT to ultrasound, reserving CT for patients with suspected aneurysm sac re-enlargement or endoleaks.[53]
Magnetic resonance imaging (MRI) is less commonly used than CT angiography and ultrasound in the follow-up of EVAR, mainly because the stent-grafts must be MR compatible. Patients with stainless steel devices are ineligible for MRI surveillance because of the significant metallic artifacts that affect image quality. Although promising results have been reported in some studies showing the superiority of MRI over CT in detecting or classifying endoleaks,[54],[55] MRI is unsuitable for routine follow-up of EVAR due to its high cost and limited availability. In addition, further studies are required to define the exact role of MRI/MR angiography for follow-up of patients after EVAR, especially since the design of MR-compatible stent-grafts would be essential.
3.3. Post-operative EVAR-3D imaging follow-up
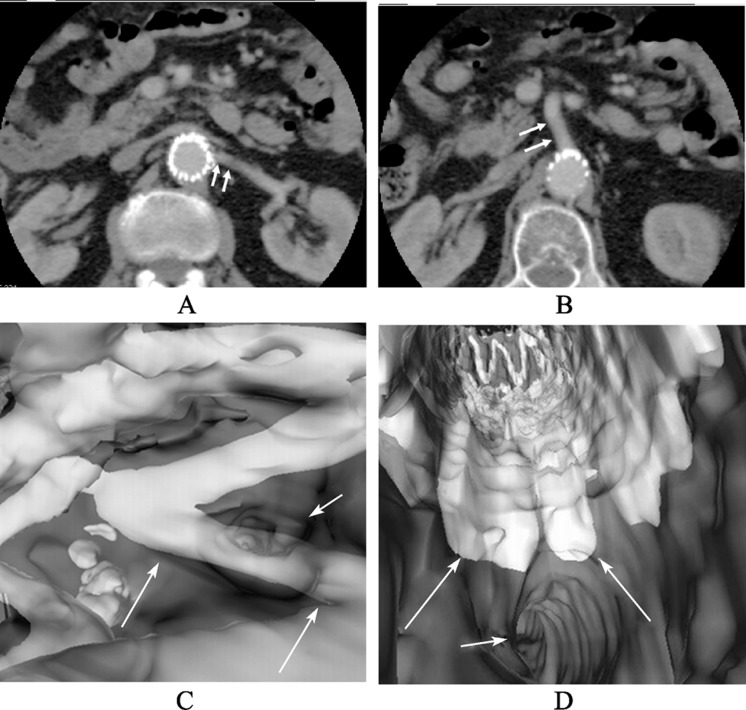
Similar to the role in preoperative planning, CT angiography has been complemented by a number of post-processing methods to produce a 3D representation of anatomical structures in the EVAR follow-up (Figure 7). Among these 3D reconstructions, virtual intravascular endoscopy (VIE) is a unique visualization tool as it provides intraluminal views of the abdominal aorta, its arterial branches and stent- grafts (Figure 8). Studies using VIE in the evaluation of patients treated with stent-grafts have demonstrated that VIE provides advantages over traditional 2D visualization methods.[56]–[59]
Figure 7. 3D volume rendering (A) in a patient treated with fenestrated stent-graft shows different colours, such as red and white, are coded to blood vessels and bones and stent wires, respectively. Coronal maximum-intensity projection (MIP) (B) shows that fenestrated renal stents are placed inside the renal arteries with successful exclusion of the aneurysm, while thin-slab MIP image (C) clearly demonstrates the intra-aortic fenestrated stents.
Figure 8. Virtual intravascular endoscopy demonstrates intraluminal appearances of the celiac axis and superior mesenteric artery (SMA) ostia (A), left renal ostium (arrows in B) and right renal ostium (arrows in C).
The primary application of VIE in aortic stent grafting is to demonstrate the stent wire-renal ostia relationships in patients treated with suprarenal stent-grafts, since there are concerns about the potential effect of the suprarenal component on subsequent renal blood flow and renal function.[56]–[58] VIE is regarded as a valuable technique since it is superior to other visualization tools and offers a clear 3D intraluminal view of the stent wires and their position relative to the renal ostia (Figure 9). The long-term effects of suprarenal stent grafting are not well understood, thus, the ability of VIE to characterize the stent wire–ostia relationship will prove a useful research and diagnostic tool that will enable identification of any detrimental effects that suprarenal stent struts may have on the renal artery ostium and hence on renal function.
Figure 9. 2D axial images show that suprarenal stent graft is placed above the left renal artery (arrows in A) and superior mesenteric artery (SMA) (arrows in B) in a patient treated with aortic stent-graft. Corresponding virtual intravascular endoscopy confirms that the left renal and SMA ostia are crossed by a single and multiple stent wires, respectively. Short arrows in C and D indicate the renal and SMA ostia, while long arrows refer to the suprarenal stent wires.
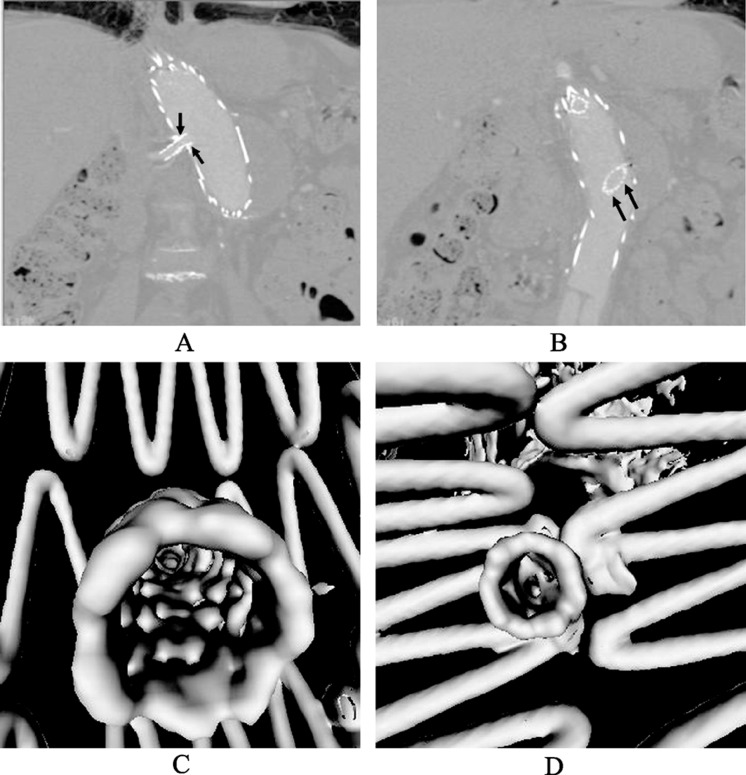
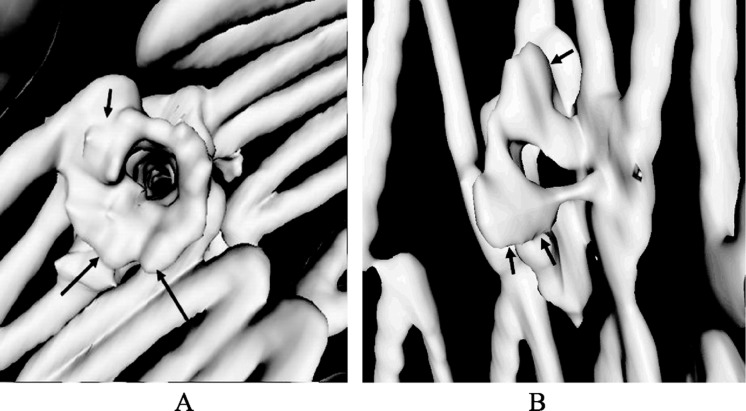
Another useful application of VIE in aortic stent grafting with the aim of treating patients with short aneurysm necks is to evaluate a recently developed endovascular technique, fenestrated stent grafting which represents technical developments over conventional infrarenal and suprarenal stent-grafts with the aim of treating patients with short aneurysm necks. The fenestration involves creating an opening in the graft fabric to accommodate the orifice of the vessel targeted for preservation. Fixation of the fenestration to the renal and other visceral arteries can be achieved by implantation of bare or covered stents across the graft-artery ostia interfaces so that a portion of the stents protrudes into the aortic lumen. Hence, there are concerns about the loss of target vessels, such as renal arteries, due to the fenestrated technique. Branched fenestrated grafts may offer an alternative solution. A tapered graft with internal or external branches provides greater flexibility in graft morphology. This allows for an increased margin of error in the stent-graft deployment without risking target vessel coverage.[59],[60] VIE visualization has proved to provide valuable information about the intraluminal appearances of fenestrated renal stents (Figure 10), and any procedure-related complications such as deformity of the stents (Figure 11). These studies suggested that VIE could be used as a complementary tool to conventional visualizations for the accurate assessment of treatment outcomes of endovascular repair of AAA.[56]–[58],[61]
Figure 10. Coronal reformatted images (A,B) reveal the fenestrated renal stents that were implanted in a patient treated with fenestrated stent-graft. Corresponding virtual intravascular endoscopy images show the fenestrated renal stents (C and D) with normal circular appearance. Arrows indicate the intra-aortic protrusion of bilateral renal stents (arrows in A and B) with a large intra-aortic extension from the left renal stents (arrows in B).
Figure 11. Virtual intravascular endoscopy shows the flaring effect at the inferior component of right renal stent (A) due to balloon inflation during fenestrated stent grafting procedure, and deformed right renal stent (B). Arrows indicate the intraluminal appearances of fenestrated renal stents.
3.4. Post-operative EVAR-computer modeling
CT angiography provides excellent anatomical details of abdominal aorta and stent-grafts, thus enabling assessment of the diameter of aneurysms and stent-grafts relative to the aortic branches. Despite these advantages, CT angiography is limited to image visualization and does not provide information about hemodynamic changes to the abdominal aorta and renal arteries following implantation of stent-grafts. Although the mechanisms are unknown, stent placement may alter local hemodynamcis, which might lead to the dispersion of late multiple emboli when coupled with wall movement.[62] Thus, studies based on computer modeling of AAA pre-and post-stent grafting will assist analysis of hemodynamic changes of the blood vessel, even before the morphological changes such as stenosis or occlusion to the renal or other visceral arteries are actually formed.
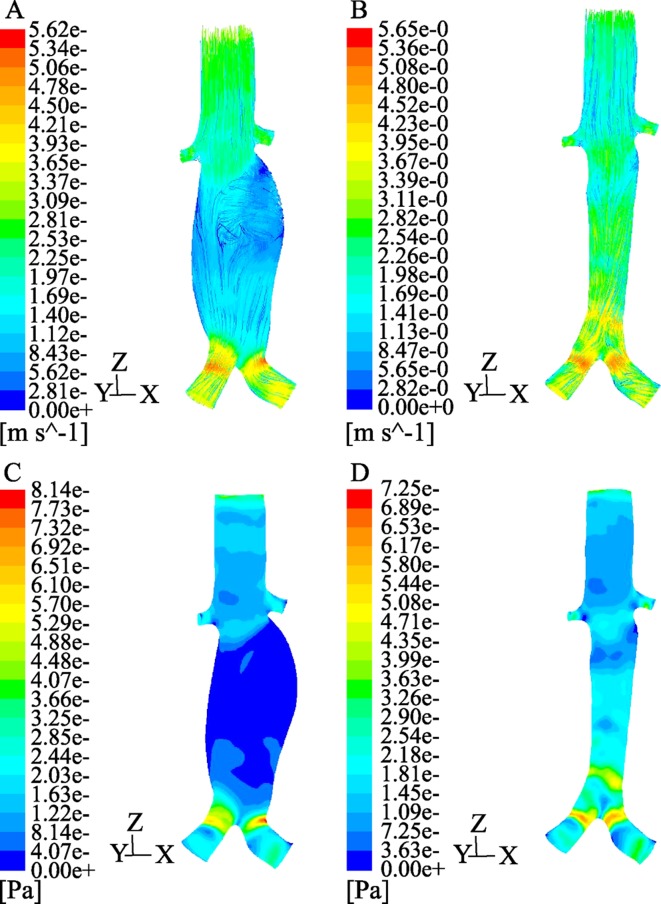
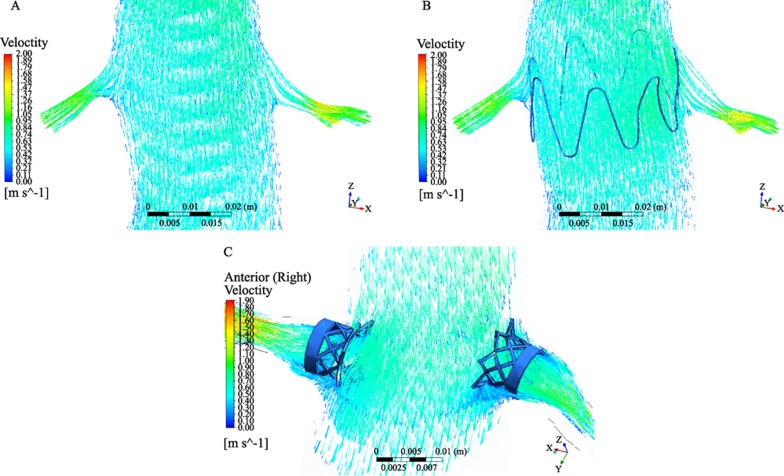
Computer simulation of conventional stent-grafts and fenestrated stents in anatomically and physiologically- accurate patient models allows improvement of stent-graft design and investigation of stent-grafts interaction with the arterial wall in a 3D patient-specific basis. Computational fluid dynamics (CFD) is a numerical method which can provide valuable information that is extremely difficult to be obtained experimentally. Rigorous CFD analysis is increasingly performed to study the fluid phenomenon inside the human vascular system. Different experimental and numerical studies have focused on the hemodynamic changes in AAA with and without a stent-graft.[62]–[69] These studies either computed wall stresses[63] or simulated the interaction between blood flow and aneurysm wall[64] in order to assess prognostic factors for aneurysm rupture risk,[64],[65] or measured the flow pattern in stented AAA (Figure 12),[54] or investigated the forces on bifurcated stent-grafts.[66],[67] Only a few studies focused on determining the changes of blood flow after stent-graft implantation by application of coupled fluid structure interaction dynamics.[67]–[69]
Figure 12. A computer modelling of aortic aneurysm pre-and post-stent grafting is based on a realistic patient CT data. Turbulent flow pattern with low flow velocity is noticed inside the aneurysm (A) prior to stent grafting, but the flow becomes laminar and flow rate increases after stent graft placement (B). Low wall shear is observed inside the aneurysm (C), with the shear stress increasing following stent graft placement (D).
CFD analysis of the flow patterns before and after EVAR and calculation of the resulting flow velocity, wall pressure and shear stress improves understanding of the stent-graft performance (Figure 13A and B). Technical developments such as patient-specific modeling and 3D finite element methods for simulated blood flow allow for realistic computational modeling of blood flow in the abdominal aorta,[69]–[71] thus increasing our understanding of the effect of stent-grafts or stents on renal function (Figure 13C).
Figure 13. Computer modelling of abdominal aorta with a focus on blood flow to the renal artery prior to stent graft placement (A). After simulation of suprarenal stent wires crossing the renal arteries, flow pattern and flow velocity are not affected (B). With simulation of fenestrated renal stents with a 5 mm intra-aortic protrusion (C), flow recirculation is seen in the proximal parts of renal arteries due to stent protrusion.
Given the availability of these computational methodologies, future endovascular devices can be tested in 3D computational models that accurately reflect the in vivo flow conditions, thus, long-term durability will be tested in simulated models prior to implantation of the devices in patients. This allows for improved endovascular device designs with improved long-term safety and effectiveness of the devices.
4. Future directions
While open surgery still remains the gold standard for treatment of patients with AAA, there is no doubt that EVAR has been confirmed as an effective alternative to open surgery. EVAR continues to benefit more patients and it will become more applicable and durable with technical improvements. New stent-graft technology, such as fenestrated and branched grafts, makes this technique available to more patients, especially in those with unsuitable aneurysm necks,[23],[24],[72],[73] although long-term follow-up is needed to prove the stability and patency of fenestrated vessels. Lifelong surveillance is necessary after EVAR, and there is increasing evidence of a trend from using conventional CT follow-up to ultrasound monitoring. Recent data suggest that EVAR is most beneficial in the fittest patients, who may survive longer.[49] Hence, the long-term risk of radiation- induced malignancy needs to be considered when choosing CT as the method of choice for routine follow-up.
Traditional post-EVAR imaging-based surveillance restricted to the monitoring of changes in AAA morphology and the detection of endoleaks has proven unreliable in preventing aneurysm rupture.[53] The expansion of the aneurysm sac depends on sac pressure. Pressure measurements of the aneurysm sac are increasingly being recognized as the most accurate indication of AAA exclusion.[53],[74]–[76] Quantitative hemodynamic changes of flow rate and flow pattern caused by stent-graft implantation can be analysed with CFD, thus allowing more accurate assessment of treatment outcomes. CFD is a highly promising technique and improves our understanding of the local structural and fluid dynamic conditions in patients with AAA after stent-graft placement. The future development of more realistic, patient-specific models will demonstrate the potential to assist stent-graft design and improve the success rate of endovascular aneurysm repair.
References
- 1.Alcorn HG, Wolfson SK, Jr, Sutton-Tyrell K. Risk factors for abdominal aortic aneurysms in older adults enrolled in Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. doi: 10.1161/01.atv.16.8.963. [DOI] [PubMed] [Google Scholar]
- 2.Endovascular stent–grafts for the treatment of abdominal aortic aneurysms. NICE technology appraisal guidance 167. www.nice.org.uk (accessed on October 26, 2011) [DOI] [PubMed]
- 3.Desai M, Eaton-Evans J, Hillery C, et al. AAA stent-grafts: past problems and future prospects. Ann Biomed Eng. 2010;38:1259–1274. doi: 10.1007/s10439-010-9953-1. [DOI] [PubMed] [Google Scholar]
- 4.The United Kingdom EVAR trial investigators Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863–1871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 5.The United Kingdom EVAR trial investigators Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med. 2010;362:1872–1880. doi: 10.1056/NEJMoa0911056. [DOI] [PubMed] [Google Scholar]
- 6.Cao P, De Rango P, Verzino F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13–25. doi: 10.1016/j.ejvs.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Norrgard O, Angqvist KA, Johnson O. Familial aortic aneurysms: serum concentrations of triglyceride, cholesterol, HDL-cholesterol and (VLDL + LDL)-cholesterol. Br J Surg. 1985;72:113–116. doi: 10.1002/bjs.1800720215. [DOI] [PubMed] [Google Scholar]
- 8.Ernst C. Abdominal aortic aneurysm. N Engl J Med. 1993;328:1167–1172. doi: 10.1056/NEJM199304223281607. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence PF, Gazak C, Bhirangi L, et al. The epidemiology of surgically repaired aneurysms in the United States. J Vasc Surg. 1999;30:632–640. doi: 10.1016/s0741-5214(99)70102-3. [DOI] [PubMed] [Google Scholar]
- 10.Hallett JW, Jr, Naesens JM, Ballard DJ. Early and late outcomes of surgical repair for small abdominal aortic aneurysms: a population-based analysis. J Vasc Surg. 1993;18:684–691. [PubMed] [Google Scholar]
- 11.Galland RB, Simmons MJ, Torrie EPH. Prevalence of abdominal aortic aneurysm in patients with occlusive peripheral vascular disease. Br J Surg. 1991;78:1259–1260. doi: 10.1002/bjs.1800781036. [DOI] [PubMed] [Google Scholar]
- 12.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 13.Woodburn KR, May J, White GH. Endoluminal abdominal aortic aneurysm surgery. Br J Surg. 1998;85:435–443. doi: 10.1046/j.1365-2168.1998.00775.x. [DOI] [PubMed] [Google Scholar]
- 14.May J, White G, Waugh R, et al. Treatment of complex abdominal aortic aneurysms by a combination of endoluminal and extraluminal aortofemoral grafts. J Vasc Surg. 1994;19:924–933. doi: 10.1016/s0741-5214(94)70020-6. [DOI] [PubMed] [Google Scholar]
- 15.Greenhalgh RM, Brown LC, Kwong GP, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843–848. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 16.Prinssen M, Verhoeven EL, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;14:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 17.Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors or late rupture, conversion and death after endovascular repair of infrarenal aortic aneurysm: the EUROSTAR experience. European Collaboration on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32:739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 18.EVAR Trial Participants Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2187–2192. doi: 10.1016/S0140-6736(05)66628-7. [DOI] [PubMed] [Google Scholar]
- 19.Brown LC, Greenhalgh RM, Thompson SG, et al. Does EVAR alter the rate of cardiovascular events in patients with abdominal aortic aneurysm considered unfit for open repair: Results from the randomized EVAR Trial 2. Eur J Vasc Endovasc Surg. 2010;39:396–402. doi: 10.1016/j.ejvs.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell M, Sun Z, Winder J, et al. Suprarenal fixation of endovascular aortic stent grafts: Assessment of medium-term to long-term renal function by analysis of juxtarenal stent morphology. J Vasc Surg. 2007;45:694–670. doi: 10.1016/j.jvs.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Lau LL, Hakaim AG, Oldenburg WA, et al. Effect of suprarenal versus infrarenal aortic endograft fixation on renal function and renal artery patency: a comparative study with intermediate follow-up. J Vasc Surg. 2003;37:1162–1168. doi: 10.1016/s0741-5214(03)00083-1. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg RK, Chuter TA, Lawrence-Brown M, et al. Analysis of renal function after aneurysm repair with a device using suprarenal fixation (Zenith AAA Endovascular Graft) in contrast to open surgical repair. J Vasc Surg. 2004;39:1219–1228. doi: 10.1016/j.jvs.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Muhs BE, Verhoeven EL, Zeebregts C, et al. Mid-term results of endovascular aneurysm repair with branched and fenestrated endografts. J Vasc Surg. 2006;44:9–15. doi: 10.1016/j.jvs.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 24.Verhoeven ELG, Prins TR, Tielliu IFJ, et al. Treatment of short-necked infrarenal aortic aneurysms with fenestrated stent-grafts: short-term results. Eur J Vasc Endovasc Surg. 2004;27:477–483. doi: 10.1016/j.ejvs.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Rydberg J, Kopecky KK, Johnson MS, et al. Endovascular repair of abdominal aortic aneurysms: Assessment with multislice CT. Am J Roentgenol. 2001;177:607–614. doi: 10.2214/ajr.177.3.1770607. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z. Helical CT angiography of abdominal aortic aneurysms treated with suprarenal stent grafting. Cardiovasc Intervent Radiol. 2003;26:290–295. doi: 10.1007/s00270-003-0034-9. [DOI] [PubMed] [Google Scholar]
- 27.Rydberg J, Kopecky KK, Lalka SG, et al. Stent grafting of abdominal aortic aneurysms: Pre- and postoperative evaluation with multislice helical CT. J Comput Assit Tomgr. 2001;25:580–586. doi: 10.1097/00004728-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Whitaker SC. Imaging of abdominal aortic aneurysm before and after endoluminal stent graft repair. Eur J Radiol. 2001;39:3–15. doi: 10.1016/s0720-048x(01)00337-0. [DOI] [PubMed] [Google Scholar]
- 29.Parry DJ, Kessel DO, Robertson I, et al. Type II endoleaks: predictable, preventable, and sometimes treatable? J Vasc Surg. 2002;36:105–110. doi: 10.1067/mva.2002.125023. [DOI] [PubMed] [Google Scholar]
- 30.Wolf YG, Hill BR, Rubin GD, et al. Rate of change in abdominal aortic aneurysm diameter after endovascular repair. J Vasc Surg. 2000;32:108–115. doi: 10.1067/mva.2000.107754. [DOI] [PubMed] [Google Scholar]
- 31.White RA, Donayre CE, Walot I, et al. Computed tomography assessment of abdominal aortic aneurysm morphology after endograft exclusion. J Vasc Surg. 2001;33:S1–S10. doi: 10.1067/mva.2001.111680. [DOI] [PubMed] [Google Scholar]
- 32.Kritpracha B, Beebe HG, Comerota AJ. Aortic diameter is an insensitive measurement of early aneurysm expansion after endografting. J Endovasc Ther. 2004;11:184–190. doi: 10.1583/03-976.1. [DOI] [PubMed] [Google Scholar]
- 33.Bargellini I, Cioni R, Petruzzi P, et al. Endovascular repair of abdominal aortic aneurysms: analysis of aneurysm volumetric changes at mid-term follow up. Cardiovasc Intervent Radiol. 2005;28:426–433. doi: 10.1007/s00270-004-0171-9. [DOI] [PubMed] [Google Scholar]
- 34.Prinssen M, Verhoeven EL, Verhagen HJ, et al. Decision making in follow-up after endovascular aneurysm repair based on diameter and volume measurements: a blinded comparison. Eur J Vasc Endovasc Surg. 2003;26:184–187. doi: 10.1053/ejvs.2002.1892. [DOI] [PubMed] [Google Scholar]
- 35.Wever JJ, Blankensteijn JD, van Rijn JJ, et al. Inter- and intraobserver variability of CT measurements obtained after endovascular repair abdominal aortic aneurysms. Am J Roentgenol. 2000;175:1279–1282. doi: 10.2214/ajr.175.5.1751279. [DOI] [PubMed] [Google Scholar]
- 36.van Prehn J, van der Wal MB, Vincken K, et al. Intra- and interobserver variability of aortic aneurysm volume measurement with fast CTA postprocessing software. J Endovasc Ther. 2008;15:504–510. doi: 10.1583/08-2478.1. [DOI] [PubMed] [Google Scholar]
- 37.May J, White G, Waugh R, et al. Life-table analysis of primary and secondary success following endoluminal repair of abdominal aortic aneurysms: role of supplementary endovascular intervention in improving outcome. Eur J Vasc Endovasc Surg. 2000;19:648–655. doi: 10.1053/ejvs.1999.1060. [DOI] [PubMed] [Google Scholar]
- 38.Schurink GW, Aarts NJ, van Bockel JH. Endoleak after stent-graft treatment of abdominal aortic aneurysm: a meta analysis of clinical studies. Br J Surg. 1999;86:581–587. doi: 10.1046/j.1365-2168.1999.01119.x. [DOI] [PubMed] [Google Scholar]
- 39.Chuter TA, Faruqi RM, Sawhney R, et al. Endoleak after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2001;34:98–105. doi: 10.1067/mva.2001.111487. [DOI] [PubMed] [Google Scholar]
- 40.Zarins CK, White RA, Hodgson KJ, et al. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg. 2000;32:90–107. doi: 10.1067/mva.2000.108278. [DOI] [PubMed] [Google Scholar]
- 41.Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029–1035. doi: 10.1067/mva.2002.123095. [DOI] [PubMed] [Google Scholar]
- 42.Rayt HS, Sandford RM, Salem M, et al. Conservative management of type 2 endoleaks is not associated with increased risk of aneurysm rupture. Eur J Vasc Endovasc Surg. 2009;38:718–723. doi: 10.1016/j.ejvs.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Rozenblit A, Patlas M, Rosenbaum AT, et al. Detection of endoleaks after endovascular repair of abdominal aortic aneurysm repair: value of unenhanced and delayed helical CT acquisitions. Radiology. 2003;227:426–433. doi: 10.1148/radiol.2272020555. [DOI] [PubMed] [Google Scholar]
- 44.Armerding MD, Rubin GD, Beaulieu CF, et al. Aortic aneurysmal disease: assessment of stent-graft treatment-CT versus conventional angiography. Radiology. 2000;215:138–146. doi: 10.1148/radiology.215.1.r00ap28138. [DOI] [PubMed] [Google Scholar]
- 45.Stavropoulos SW, Clark TW, Carpenter JP, et al. Use of CT angiography to classify endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2005;16:663–667. doi: 10.1097/01.RVI.0000152386.97448.F1. [DOI] [PubMed] [Google Scholar]
- 46.Roberto I, Raffaele CA, Antonella F, et al. Multidetector-row computed tomography angiography in abdominal aortic aneurysm treated with endovascular repair: evaluation of optimal timing of delayed phase imaging for the detection of low-flow endoleaks. J Comput Assist Tomogr. 2008;32:609–615. doi: 10.1097/RCT.0b013e31814b271d. [DOI] [PubMed] [Google Scholar]
- 47.McWilliams RG, Martin J, White D, et al. Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. J Endovasc Ther. 2002;9:170–179. doi: 10.1177/152660280200900206. [DOI] [PubMed] [Google Scholar]
- 48.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 49.Weerakkody RA, Walsh SR, Cousin C, et al. Radiation exposure during endovascular aneurysm repair. Br J Surg. 2008;95:699–702. doi: 10.1002/bjs.6229. [DOI] [PubMed] [Google Scholar]
- 50.Napoli V, Bargellini I, Sardella SG, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology. 2004;233:217–225. doi: 10.1148/radiol.2331031767. [DOI] [PubMed] [Google Scholar]
- 51.Giannoni MF, Palombo G, Sbarigia E, et al. Contrast- enhanced ultrasound imaging for aortic stent-graft surveillance. J Endovasc Ther. 2003;10:208–217. doi: 10.1177/152660280301000208. [DOI] [PubMed] [Google Scholar]
- 52.Sun Z. Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol. 2006;17:759–764. doi: 10.1097/01.RVI.0000217944.36738.02. [DOI] [PubMed] [Google Scholar]
- 53.Lawrence-Brown MMD, Sun Z, Semmens JB, et al. Type II endoleaks: when is intervention indicated and what is the index of suspicion for types I or III? J Endovasc Ther. 2009;16(Suppl 1):S106–S118. doi: 10.1583/08-2585.1. [DOI] [PubMed] [Google Scholar]
- 54.van der Laan MJ, Bartels LW, Viergever MA, et al. Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. Eur J Vasc Endovasc Surg. 2006;32:361–365. doi: 10.1016/j.ejvs.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 55.Lookstein RA, Goldman J, Pukin L, et al. Time resolved magnetic resonance angiography as a noninvasive method to characterize endoleaks: initial results compared with conventional angiography. J Vasc Surg. 2004;39:27–33. doi: 10.1016/j.jvs.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 56.Sun Z, Winder J, Kelly B, et al. Diagnostic value of CT virtual intravascular endoscopy in aortic stent grafting. J Endovasc Ther. 2004;11:13–25. doi: 10.1177/152660280401100102. [DOI] [PubMed] [Google Scholar]
- 57.Sun Z, Winder J, Kelly B, et al. CT virtual intravascular endoscopy of abdominal aortic aneurysms treated with suprarenal endovascular stent grafting. Abdom Imaging. 2003;28:580–587. doi: 10.1007/s00261-002-0069-4. [DOI] [PubMed] [Google Scholar]
- 58.Sun Z, O'Donnell M, Winder R, et al. Effect of suprarenal fixation of aortic stent grafts on renal ostium: Assessment of morphological changes by virtual intravascular endoscopy. J Endovasc Ther. 2007;14:650–660. doi: 10.1177/152660280701400508. [DOI] [PubMed] [Google Scholar]
- 59.Bicknell CD, Cheshire NJ, Riga CV, et al. Treatment of complex aneurysmal disease with fenestrated and branched stent grafts. Eur J Vasc Endovasc Surg. 2009;37:175–181. doi: 10.1016/j.ejvs.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Nordon IM, Hinchliffe RJ, Manning B, et al. Toward an “off-the-shelf” fenestrated endograft for management of short-necked abdominal aortic aneurysms: an analysis of current graft morphological diversity. J Endovasc Ther. 2010;17:78–85. doi: 10.1583/09-2895R.1. [DOI] [PubMed] [Google Scholar]
- 61.Sun Z, Allen Y, Nadkarni S, et al. CT virtual intravascular endoscopy in the visualization of fenestrated endovascular grafts. J Endovasc Ther. 2008;15:42–51. doi: 10.1583/07-2234.1. [DOI] [PubMed] [Google Scholar]
- 62.Richter GM, Palmaz JC, Noeldge G, et al. Relationship between blood flow, thrombus, and neointima in stents. J Vasc Interv Radiol. 1999;10:598–604. doi: 10.1016/s1051-0443(99)70090-4. [DOI] [PubMed] [Google Scholar]
- 63.Chong CK, How TV. Flow patterns in an endovascular stent-graft for abdominal aortic aneurysm repair. J Biomech. 2004;37:89–97. doi: 10.1016/s0021-9290(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 64.Di Martino ES, Guadagni G, Fumero A, et al. Fluid–structure interaction within realistic three-dimensional models of the aneurysmatic aorta as a guidance to assess the risk of rupture of the aneurysm. Med Eng Phys. 2001;23:647–655. doi: 10.1016/s1350-4533(01)00093-5. [DOI] [PubMed] [Google Scholar]
- 65.Wang DH, Makaroun MS, Webster MW, et al. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J Vasc Surg. 2002;36:598–604. doi: 10.1067/mva.2002.126087. [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Kleinstreuer C. Fluid–structure interaction effects on sac blood pressure and wall stress in a stented aneurysm. J Biomech Eng. 2005;127:662–671. doi: 10.1115/1.1934040. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Kleinstreuer C. Blood flow and structure interactions in a stented abdominal aortic aneurysm model. Med Eng Phys. 2005;27:369–382. doi: 10.1016/j.medengphy.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Frauenfelder T, Lotfey M, Boehm T, et al. Computational fluid dynamics: Hemodynamic changes in abdominal aortic aneurysm after stent-graft implantation. Cardiovasc Intervent Radiol. 2006;29:613–623. doi: 10.1007/s00270-005-0227-5. [DOI] [PubMed] [Google Scholar]
- 69.Sun Z, Chaichana T. Investigation of hemodynamic effect of stent wires on renal arteries in patients with abdominal aortic aneurysms treated with suprarenal stent grafts. Cardiovasc Intervent Radiol. 2009;32:647–657. doi: 10.1007/s00270-009-9539-1. [DOI] [PubMed] [Google Scholar]
- 70.Liffman K, Lawrence-Brown MD, Semmens JB, et al. Suprarenal fixation: effect on blood flow of an endoluminal stent wire across an arterial orifice. J Endovasc Ther. 2003;10:260–274. doi: 10.1177/152660280301000216. [DOI] [PubMed] [Google Scholar]
- 71.Sun Z, Chaichana T. Fenestrated stent graft repair of abdominal aortic aneurysm: Hemodynamic analysis of effect of fenestrated stents on renal arteries. Korean J Radiol. 2010;11:95–106. doi: 10.3348/kjr.2010.11.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Z, Mwipatayi BP, Semmens JB, et al. Short to midterm outcomes of fenestrated endovascular grafts in the treatment of abdominal aortic aneurysms: a systematic review. J Endovasc Ther. 2006;13:747–753. doi: 10.1583/06-1919.1. [DOI] [PubMed] [Google Scholar]
- 73.Stanley BM, Semmens JB, Lawrence-Brown MM, et al. Fenestration in endovascular grafts for aortic aneurysm repair: new horizons for preserving blood flow in branch vessels. J Endovasc Ther. 2001;8:16–24. doi: 10.1177/152660280100800103. [DOI] [PubMed] [Google Scholar]
- 74.Baum RA, Carpenter JP, Cope C, et al. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2001;33:32–41. doi: 10.1067/mva.2001.111807. [DOI] [PubMed] [Google Scholar]
- 75.Dias NV, Ivancev K, Malina M, et al. Intra-aneurysm sac pressure measurements after endovascular aneurysm repair: differences between shrinking, unchanged, and expanding aneurysms with and without endoleaks. J Vasc Surg. 2004;39:1229–1235. doi: 10.1016/j.jvs.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 76.Sonesson B, Dias N, Malina M, et al. Intra-aneurysm pressure measurements in successfully excluded abdominal aortic aneurysm after endovascular repair. J Vasc Surg. 2003;37:733–738. doi: 10.1067/mva.2003.138. [DOI] [PubMed] [Google Scholar]