Abstract
Objective
Several studies have indicated that miR-15a, miR-15b and miR-16 may be the important regulators of apoptosis. Since attenuate apoptosis could protect myocardium and reduce infarction size, the present study was aimed to find out whether these miRNAs participate in regulating myocardial ischemia reperfusion (I/R) injury.
Methods
Apoptosis in mice hearts subjected to I/R was detected by TUNEL assay in vivo, while flow cytometry analysis followed by Annexin V/PI double stain in vitro was used to detect apoptosis in cultured cardiomyocytes which were subjected to hypoxia/reoxygenation (H/R). Taqman real-time quantitative PCR was used to confirm whether miR-15a/15b/16 were involved in the regulation of cardiac I/R and H/R.
Results
Compared to those of the controls, I/R or H/R induced apoptosis of cardiomyocytes was significantly increased both in vivo (24.4% ± 9.4% vs. 2.2% ± 1.9%, P < 0.01, n = 5) and in vitro (14.12% ± 0.92% vs. 2.22% ± 0.08%). The expression of miR-15a and miR-15b, but not miR-16, was increased in the mice I/R model, and the results were consistent in the H/R model.
Conclusions
Our data indicate miR-15 and miR-15b are up-regulated in response to cardiac I/R injury, therefore, down-regulation of miR-15a/b may be a promising strategy to reduce myocardial apoptosis induced by cardiac I/R injury.
Keywords: miR-15a/b, Apoptosis, Myocardial reperfusion injury, Ischemia/Reperfusion injury
1. Introduction
miRNAs, a subset of small non-coding single-strand RNAs approximately 22 nucleotides (nt) long, have emerged as a group of important regulators of gene expression at the post-transcriptional level.[1]–[5] The action of mature miRNAs rely on the miRNA-induced silencing complex (miRISC), in which miRNA recognize target mRNA by binding to the complementary sequence of 3′ untranslated region of the target.[2]–[4] MiRISC promote target mRNA encoded protein down-regulation by either degrade or repress the translation of mRNA.[5]
Recent studies have suggested miRNAs are involved in the regulation of myocardial ischemia reperfusion (I/R) injury.[6],[7] Cardiac stress including myocardial reperfusion injury could induce altered expression of miRNAs, and studies using miRNAs arrays or real-time quantitative PCR have consistently found that the miR-15 family are up-regulated in myocardial ischemia and heart failure.[8] The miR-15 family members include miR-15a, miR-15b, miR-16, miR-195, miR-424 and miR-497.[9],[10] One study showed that miR-195 was up-regulated during cardiac hypertrophy, an overexpression of which could result in pathological cardiac growth and heart failure in transgenic mice.[11] But the exact function of other members of the family is still unclear.
Cardiomyocytes are conventionally thought to be terminally differentiated with limited regenerate capacity; the amount of acute and chronic cell loss after myocardial infarction is the key factor that affects cardiac function and patient prognosis. Reperfusion may induce more cell apoptosis, which is detrimental for cardiac function recovery.[12]–[15] Research involving tumor cells suggest miR-15a, miR-15b, and miR-16 regulate apoptosis by post-transcriptionally down- regulating Bcl-2 protein.[16]–[18] In the present study, we tested the hypotheses that miR-15a, miR-15b, miR-16 may play a role in myocardial reperfusion injury by regulating apoptosis.
2. Methods
2.1. I/R injury model in mice
Thirty male mice (Kunming, eight to ten weeks) were randomly divided into control group and I/R group, then anesthetized intramuscularly with 2% sodium pentobarbital. Ischemia was achieved by using a 7.0 prolene suture around the left anterior descending coronary artery (LAD) and tied with a slipknot for 30 min, after which the knot was relaxed and the heart was allowed reperfusion for 24 hours. After reperfusion, mice were sacrificed and hearts were harvested for RNA isolation and TUNEL assay (Roche, Germany). The same procedure was performed on control hearts without tying the suture.
2.2. TUNEL assay
The determination of cell apoptosis was performed using TUNEL assay according to manufacturer's instruction. Briefly, the cardiac tissue from infarct border zones was sectioned at 50 µm thickness and stained. The ratio of apoptotic (green) nuclei vs. total nuclei (blue) was calculated and compared with the control group.
2.3. Isolation and culture of neonatal rat cardiomyocytes
The hearts from Sprague-Dawley rats born within 24 hours were minced and digested with trypsin. The primary neonatal rat cardiomyocytes were cultured in DMEM containing 10% neonatal bovine serum (NBS) and gentamicin. After synchronization, the medium was replaced with DMEM free of NBS before the cells were exposed to hypoxia, which was induced by placing the culture dishes into a hypoxic incubator filled with 95% N2 and 5% CO2 at 37°C. After 6 hours hypoxia, the dishes were transferred to a normoxic incubator for 24 hours reoxygenation. All the cell experiments were repeated for three times.[19]
2.4. Cell viability assessment
Trypan blue staining was used to determine cell death with the number of Trypan blue-positive and Trypan blue- negative cells counted by hemocytometer under microscope.
2.5. Flow cytometry analysis of cell apoptosis by labeled Annexin V/PI
After hypoxia/reoxygenation (H/R), (1∼5) × 105 primary neonatal cardiomyocytes were collected and washed with phosphate buffer solution (PBS), the cells were resuspended in binding buffer followed by Annexin V-FITC and propiduim iodide (PI) double-stain according to the manufacturer's instruction, and then the samples were examined by a flow cytometer (BD, USA).
2.6. Isolation of total RNA and real-time PCR
Total RNA was isolated from mice heart tissues or cultured cells using Trizol (Invitrogen, USA) according to the manufacturer's instructions. The concentration of extracted RNA was quantified by UV spectrophotometer, the A260/A280 needs to be about 1.8∼2.0. Then real-time PCR was used to quantify the expression of miR-15a, miR-15b, miR-16 with specific Taqman assays (Applied Biosystems, USA) and Taqman universal master mix (Applied Biosystems, USA). Briefly, we constructed 15 µL reverse transcript system with 10 ng total RNA, and real-time PCR system of 10 µL, containing eight µL Taqman Universal PCR master mix, one microlitre Taqman assay and one microlitre RT product. The 2−ΔΔCT relative quantification method was applied, and U6 served as internal reference. The 2−ΔΔC indicates the fold change in gene expression relative to the untreated control, the equation displayed as ΔΔCT= (CT,Target – CT,U6)I/R or H/R – (CT,Target – CT,U6)control.[20]
2.7. Animal care
All animal experiments were approved by the animal research ethics committee of the Chinese PLA General Hospital, Beijing, China.
2.8. Statistical analysis
Quantitative data are presented as mean ± SE. Statistical significance was determined using student t test for two group comparisons. P < 0.05 was considered as statistically significant.
3. Results
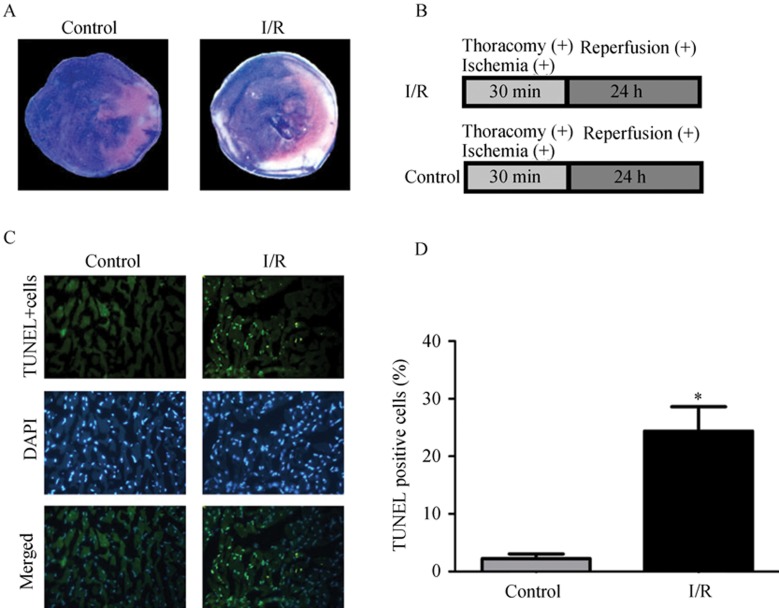
The mice I/R model was successfully made by temporary occlusion of the LAD coronary artery for 30 min while monitoring with ECG; the ST segment elevation could be observed in lead II. After 24-h reperfusion, triphenyltetrazolium chloride (TTC) and Evans blue double stain was performed to evaluate the extent of infarction area, the I/R group exhibited significant infarction area (I/R infarction size, 21.38 ± 2.22%, n = 5) whereas control group showed no infarction. Representative photos of both control group and I/R group are shown in Figure 1A.
Figure 1. Myocardial infarction induced by I/R through occlusion of LAD. Mice I/R models were established by occluding the LAD coronary artery for 30-min and followed by 24-h reperfusion. (A): middle transverse section of the heart stained with TTC and Evans blue (white–gray area represents area of necrosis, blue area represents normal perfusion zone, brick-red area represents ischemic zone); (B): the surgery protocols for I/R group and control group; (C): TUNEL assay was performed in infarct border zone (blue-positive indicates all the nuclei, green-positive indicate apoptotic nuclei, × 400); (D), the proportion of apoptotic cells (24.4% ± 9.4% vs. 2.2% ± 1.9%, compared with sham group, P < 0.01, n = 5). I/R: ischemia reperfusion; LAD: left anterior descending coronary artery.
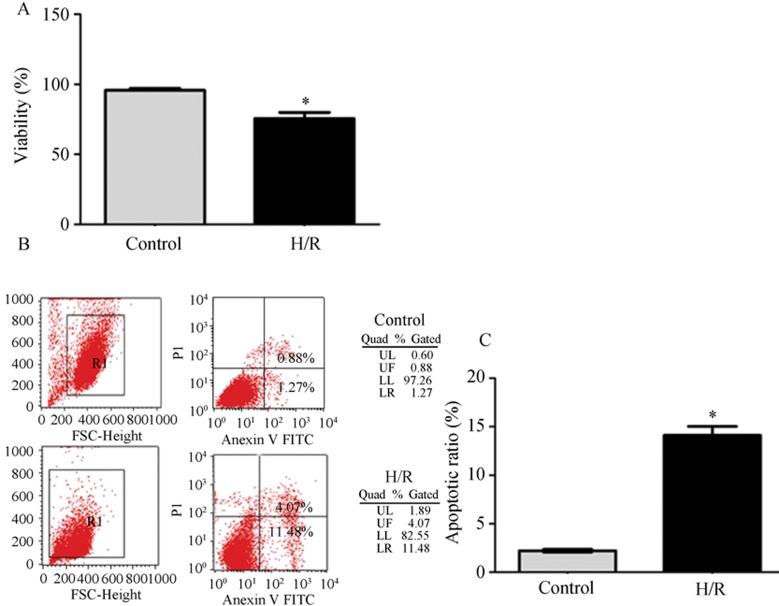
To further investigate the injury induced by I/R in vivo, TUNEL assay was performed to detect the apoptotic cells. As displayed in Figure 1B, TUNEL assay showed the proportion of apoptotic cells were significantly increased in I/R group compared with control group (24.4% ± 9.4% vs. 2.2% ± 1.9%, P < 0.01, n = 5). Figure 2 shows that the neonatal cardiomyocytes subjected to H/R presented significant cell death, and flow cytometry analysis confirmed that the cell death is mainly contributed by apoptosis (14.12% ± 0.92% vs. 2.22% ± 0.08%, P < 0.05 compared with control, n = 3), (Figure 2B).
Figure 2. H/R induced cell apoptosis and death. (A): cell viability detected by trypan blue (the percentage of trypan blue-negative cells, n = 3); (B): cells were labeled by Annexin V-FITC and PI (upper right represent late phrase apoptotic cells, lower right represent early phrase apoptotic cells, upper left represent necrotic cells, lower left represent normal cells); (C): the percentage of lower right region cells plus upper right region cells, H/R induced significant increase of cell apoptosis compared with control (P < 0.01, n = 3). H/R: hypoxia/reoxygenation; PI: propiduim iodide.
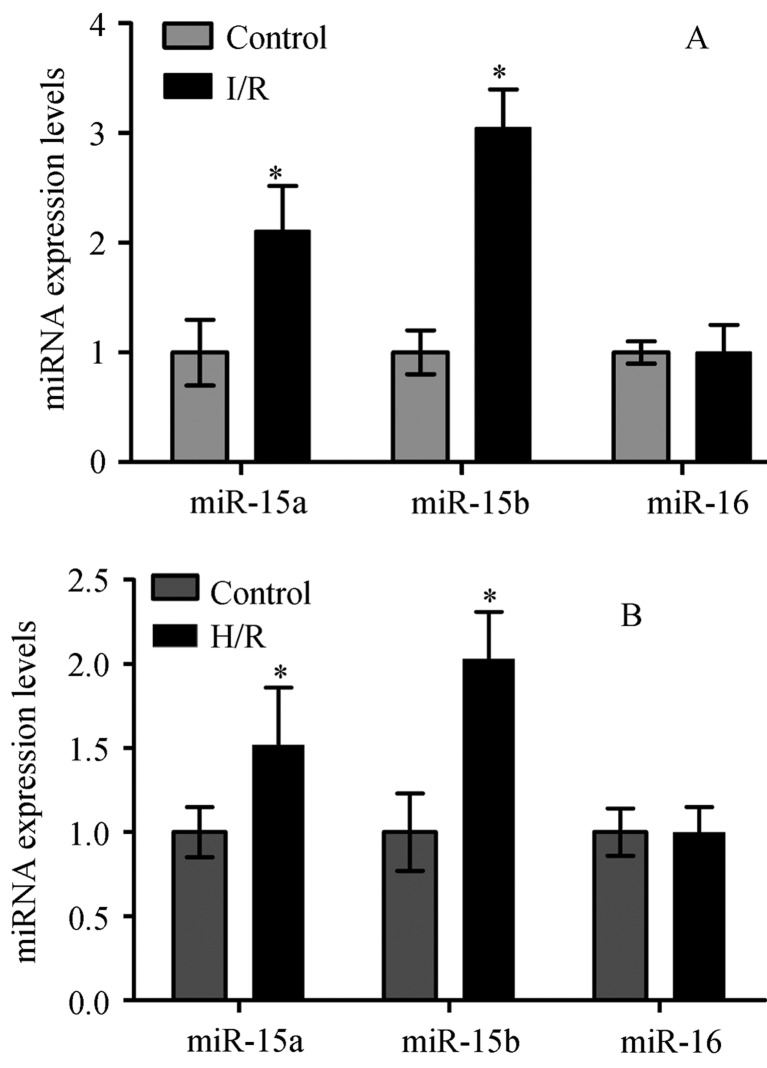
To further explore the molecular mechanism of myocardial I/R injury, we performed real-time quantitative PCR analyses to investigate whether miR-15a, miR-15b and miR-16 were involved in I/R or H/R. As shown in Figure 3A and B, we found the miR-15a and miR-15b expression levels were increased in the mice I/R model and cardiomyocytes exposed to H/R in vitro (P < 0.05). The MiR-16 expression had no significant difference between I/R or H/R in the control group, both in vivo and in vitro.
Figure 3. Quantitative, real-time PCR (Taqman) displaying miR-15a, miR-15b, miR-16 fold change in mice I/R model group (A), and in cardiomyocytes H/R model group (B) compared with control (normalized to U6), repectively, *P < 0.05.
4. Discussion
Cardiomyocytes are cells with limited regenerative capacity, and their viability is very important for the maintenance of the heart function. In myocardial I/R injury, cardiomyocytes undergo apoptosis on a large scale. These apoptotic cell deaths account for the acute cell loss in the infarct area and the chronic cell loss in the infarct border zone. TUNEL data from this study revealed apoptosis was dramatically increased in hearts subjected to I/R. The data of Annexin-V/PI labeling flow cytometry also revealed apoptosis was increased in the cultured cardiomyocytes subjected to H/R. Our findings are consistent with previous experimental and clinical studies.[12],[19]
Over the past decade, there were many studies investigating the functions of miRNAs, showing some of these RNAs participate in regulating cardiovascular disease. Some miRNAs are up-regulated in response to I/R, as displayed by our results, while I/R could induce dynamic expression changes of miR-15a and miR-15b. Also, our results indicated that cardiomyocytes exposed to hypoxia lead to similar change. These data suggest that miR-15a and miR-15b may play an important role during I/R injury. Accumulating evidence strongly suggests the miR-15 family has a relationship with apoptosis. In rat activated hepatic stellate cells, miR-15b and miR-16 were decreased and were found to be related to apoptosis by targeting Bcl-2.[17].
Previous studies have found that the mitochondrial permeability transition (mPTP) opening would lead to the collapse of the membrane potential and the efflux of pro- apoptosis factors is particularly important if myocardial I/R injury happened on the moment of blood flow restore.[21],[22]. Whereas mPTP is regulated mainly by Bcl-2 family, and especially Bcl-2/Bax is a crucial factor for mitochondrial apoptotic pathway by controlling mitochondrial membrane integrity.[23].
Since both miR-15a and miR-15b are the upstream regulators of Bcl-2, miR-15a and miR-15b are up-regulated in myocardial I/R injury, miR-15a and miR-15b may regulate mitochondrial apoptotic pathway by targeting Bcl-2. It is suggested that the knockdown of miR-15a and miR-15b could be a therapeutic strategy for myocardial I/R injury.
4.1. Study limitation
The limitation of the current study is that we haven't directly investigated the effect of miR-15a and miR-15b on apoptosis of cardiomyocytes and the downstream signaling transduction through over expression or down expression, although the studies in tumor cells have provided evidence that miR-15a and miR-15b have an intimate relationship with apoptosis. In the future, we will attempt to verify the role of miR-15a and miR-15b in myocardial I/R induced apoptosis and the signaling pathway.
4.2. Conclusion
Both miR-15a and miR-15b are related to myocardial I/R injury, and these RNAs may play an essential role in apoptosis by targeting Bcl-2 and caspase signaling pathway. The down-regulation of miR-15a/b may be a promising strategy to reduce myocardial apoptosis induced by cardiac I/R injury.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Yi R, Qin Y, Macara IG, et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutvagner G, Mclachlan J, Pasquinelli AE, et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valencia-Sanchez MA, Liu J, Hannon GJ, et al. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 6.Small E M, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condorelli G, Latronico MV, Dorn GN. microRNAs in heart disease: putative novel therapeutic targets? Eur Heart J. 2010;31:649–658. doi: 10.1093/eurheartj/ehp573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latronico MV, Catalucci D, Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circ Res. 2007;101:1225–1236. doi: 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- 9.van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy S, Khanna S, Hussain S R, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rooij E, Sutherland L B, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prech M, Marszalek A, Schroder J, et al. Apoptosis as a mechanism for the elimination of cardiomyocytes after acute myocardial infarction. Am J Cardiol. 2010;105:1240–1245. doi: 10.1016/j.amjcard.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez B, Fuster V, Jimenez-Borreguero J, et al. Lethal myocardial reperfusion injury: A necessary evil? Int J Cardiol. 2011;151:3–11. doi: 10.1016/j.ijcard.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb RA, Burleson KO, Kloner RA, et al. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner C, Kroemer G. Apoptosis. Mitochondria-the death signal integrators. Science. 2000;289:1150–1151. doi: 10.1126/science.289.5482.1150. [DOI] [PubMed] [Google Scholar]
- 16.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo CJ, Pan Q, Li DG, et al. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50:766–778. doi: 10.1016/j.jhep.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Xia L, Zhang D, Du R, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Ge X, Liu X. The cardioprotective effect of postconditioning is mediated by ARC through inhibiting mitochondrial apoptotic pathway. Apoptosis. 2009;14:164–172. doi: 10.1007/s10495-008-0296-4. [DOI] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Ola M S, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]