Abstract
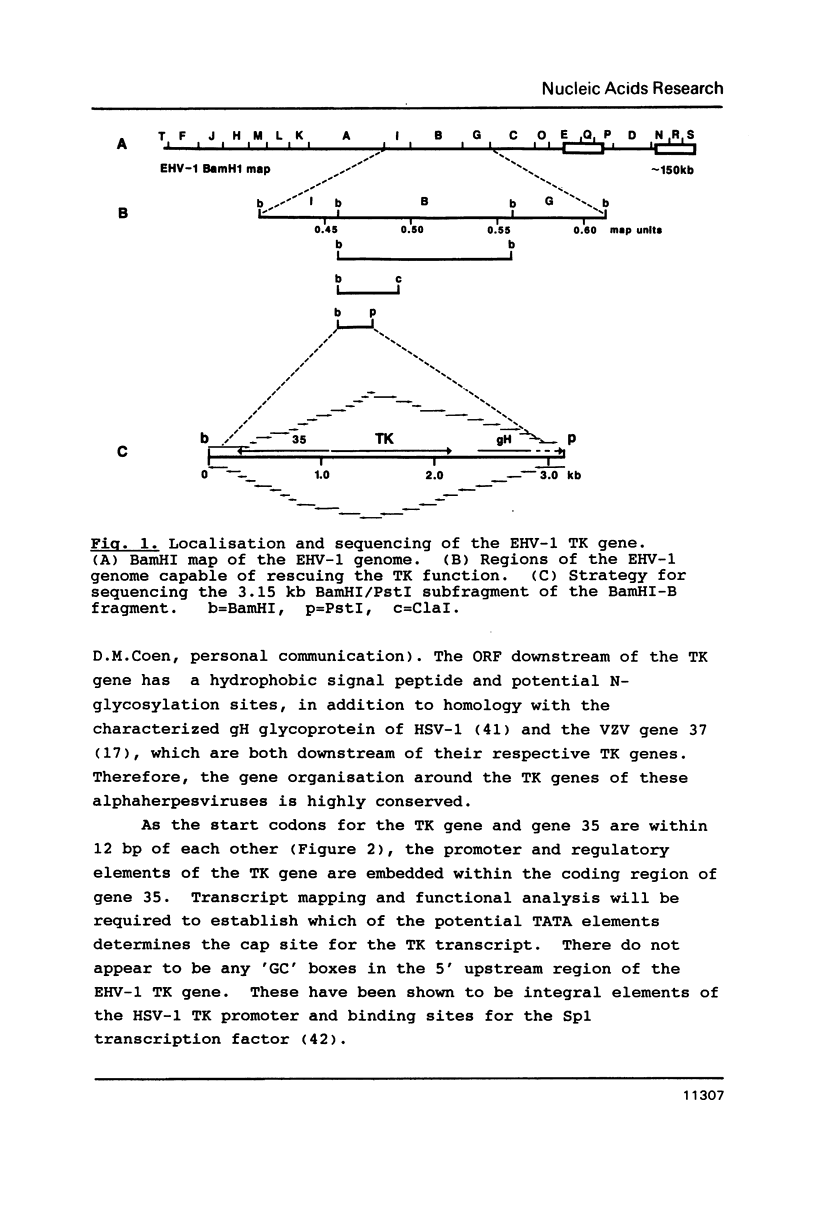
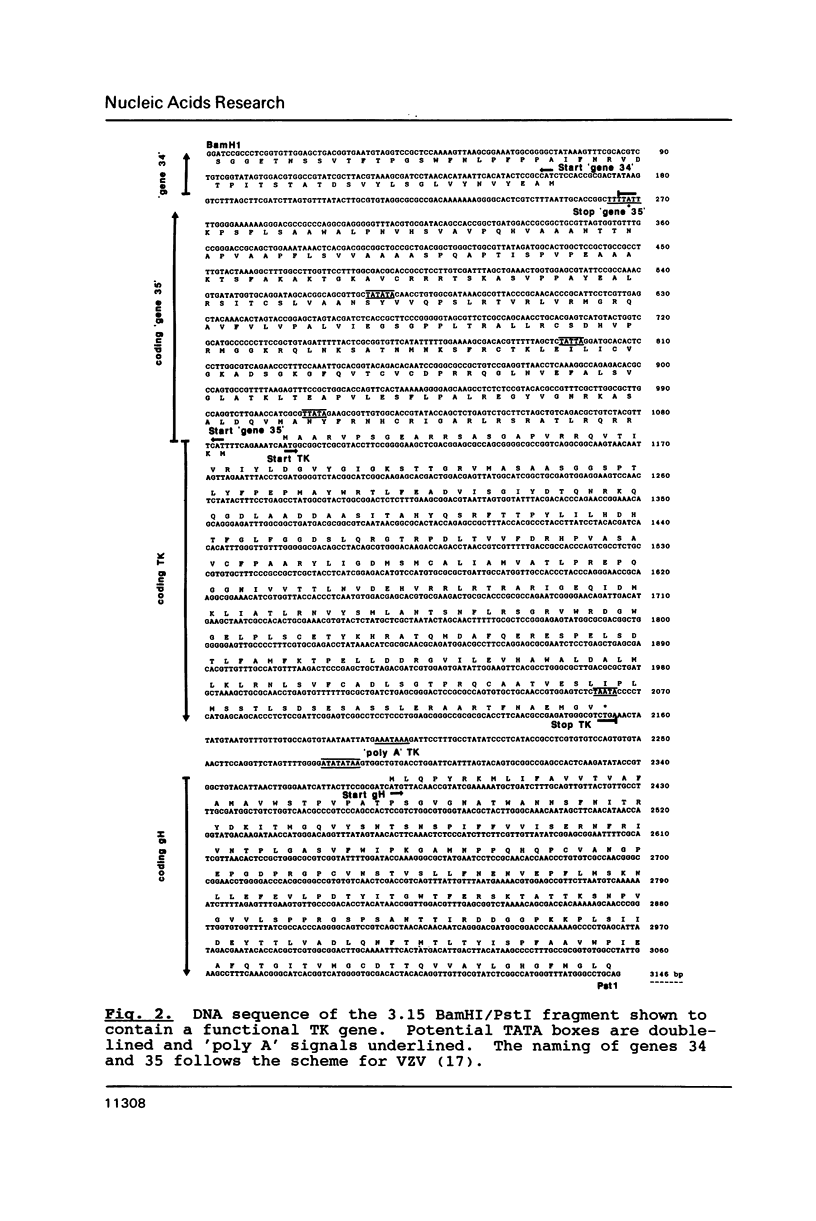
We have identified the equine herpesvirus 1 (EHV-1) thymidine kinase gene (TK) by DNA-mediated transformation and by DNA sequencing. Alignment of the amino acid sequence of the EHV-1 TK with the TKs from 3 other herpesviruses revealed regions of homology, some of which correspond to the previously identified substrate binding sites, while others have as yet, no assigned function. In particular, the strict conservation of an aspartate within the proposed nucleoside binding site suggests a role in ATP binding for this residue. Comparison of 5 herpes TKs with the thymidylate kinase of yeast revealed significant similarity which was strongest in those regions important to catalytic activity of the herpes TKs, and, therefore we propose that the herpes TK may be derived from a cellular thymidylate kinase. The implications for the evolution of enzyme activities within a pathway of nucleotide metabolism are discussed.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., McGowan J. J., Bryans J. T., Randall C. C., Gentry G. A. Induction of deoxythymidine kinase activity in several mammalian cell lines after infection with six different strains of equine herpesvirus type 1 (EHV-1). Virology. 1978 Oct 15;90(2):351–359. doi: 10.1016/0042-6822(78)90319-7. [DOI] [PubMed] [Google Scholar]
- Allen G. P., McGowan J. J., Gentry G. A., Randall C. C. Biochemical transformation of deoxythymidine kinase-deficient mouse cells with UV-irradiated equine herpesvirus type 1. J Virol. 1978 Oct;28(1):361–367. doi: 10.1128/jvi.28.1.361-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., McGowan J. J., Randall C. C., Mancini W., Cheng Y. C., Gentry G. A. Purification and characterization of deoxythymidine kinase (dTK) induced in dTK- 3T3 mouse cells by equine herpesvirus type 1 (EHV-1). Virology. 1979 Jan 30;92(2):367–374. doi: 10.1016/0042-6822(79)90141-7. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bello L. J., Whitbeck J. C., Lawrence W. C. Map location of the thymidine kinase gene of bovine herpesvirus 1. J Virol. 1987 Dec;61(12):4023–4025. doi: 10.1128/jvi.61.12.4023-4025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemer W., Niller H. H., Nitsche N., Scholz B., Fleckenstein B. Organization of the thymidylate synthase gene of herpesvirus saimiri. J Virol. 1986 Oct;60(1):114–123. doi: 10.1128/jvi.60.1.114-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E., Gibbs A. J., Seigman L. J., Both G. W. Fowlpox virus thymidine kinase: nucleotide sequence and relationships to other thymidine kinases. Virology. 1987 Feb;156(2):355–365. doi: 10.1016/0042-6822(87)90415-6. [DOI] [PubMed] [Google Scholar]
- Breen E. J., Browne L. H., Glue L., Williams K. L. The DNA rodent: a portable hand held DNA sequence reader. Comput Appl Biosci. 1988 Mar;4(1):217–217. doi: 10.1093/bioinformatics/4.1.217. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Chen M. S., Summers W. P., Walker J., Summers W. C., Prusoff W. H. Characterization of pyrimidine deoxyribonucleoside kinase (thymidine kinase) and thymidylate kinase as a multifunctional enzyme in cells transformed by herpes simplex virus type 1 and in cells infected with mutant strains of herpes simplex virus. J Virol. 1979 Jun;30(3):942–945. doi: 10.1128/jvi.30.3.942-945.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Walker J., Prusoff W. H. Kinetic studies of herpes simplex virus type 1-encoded thymidine and thymidylate kinase, a multifunctional enzyme. J Biol Chem. 1979 Nov 10;254(21):10747–10753. [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute J. J., Mocarski E. S., Lehman I. R. A DNA helicase induced by herpes simplex virus type 1. Nucleic Acids Res. 1988 Jul 25;16(14A):6585–6596. doi: 10.1093/nar/16.14.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Location and orientation of homologous sequences in the genomes of five herpesviruses. J Gen Virol. 1983 Sep;64(Pt 9):1927–1942. doi: 10.1099/0022-1317-64-9-1927. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristensky B., Lis J., Wu R. Portable microcomputer software for nucleotide sequence analysis. Nucleic Acids Res. 1982 Oct 25;10(20):6451–6463. doi: 10.1093/nar/10.20.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., McKee S. A., Keller P. M. Altered thymidine-thymidylate kinases from strains of herpes simplex virus with modified drug sensitivities to acyclovir and (E)-5-(2-bromovinyl)-2'-deoxyuridine. Mol Pharmacol. 1983 Sep;24(2):316–323. [PubMed] [Google Scholar]
- Gentry G. A. Locating a nucleotide-binding site in the thymidine kinase of vaccinia virus and of herpes simplex virus by scoring triply aligned protein sequences. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6815–6819. doi: 10.1073/pnas.82.20.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Graham D., Larder B. A., Inglis M. M. Evidence that the 'active centre' of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986 Apr;67(Pt 4):753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haarr L., Flatmark T. Evidence that deletion of coding sequences in the 5' end of the thymidine kinase gene of herpes simplex virus type 1 affects the stability of the gene products. J Gen Virol. 1987 Nov;68(Pt 11):2817–2829. doi: 10.1099/0022-1317-68-11-2817. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Smiley J. R. Effects of deletions on expression of the herpes simplex virus thymidine kinase gene from the intact viral genome: the amino terminus of the enzyme is dispensable for catalytic activity. J Virol. 1984 Jun;50(3):733–738. doi: 10.1128/jvi.50.3.733-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. E., Glaser R., Hewetson J., O'Callaghan D. J. Expression of altered ribonucleotide reductase activity associated with the replication of the Epstein-Barr virus. Virology. 1978 Aug;89(1):262–271. doi: 10.1016/0042-6822(78)90058-2. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis M. M., Darby G. Analysis of the role of the cysteine 171 residue in the activity of herpes simplex virus type 1 thymidine kinase by oligonucleotide-directed mutagenesis. J Gen Virol. 1987 Jan;68(Pt 1):39–46. doi: 10.1099/0022-1317-68-1-39. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Jong A. Y., Kuo C. L., Campbell J. L. The CDC8 gene of yeast encodes thymidylate kinase. J Biol Chem. 1984 Sep 10;259(17):11052–11059. [PubMed] [Google Scholar]
- Kit S., Ichimura H., De Clercq E. Phosphorylation of nucleoside analogs by equine herpesvirus type 1 pyrimidine deoxyribonucleoside kinase. Antiviral Res. 1987 Jan;7(1):53–67. doi: 10.1016/0166-3542(87)90039-8. [DOI] [PubMed] [Google Scholar]
- Kit S. Thymidine kinase. Microbiol Sci. 1985 Dec;2(12):369–375. [PubMed] [Google Scholar]
- Kolb E., Hudson P. J., Harris J. I. Phosphofructokinase: complete amino-acid sequence of the enzyme from Bacillus stearothermophilus. Eur J Biochem. 1980 Jul;108(2):587–597. doi: 10.1111/j.1432-1033.1980.tb04754.x. [DOI] [PubMed] [Google Scholar]
- Kwoh T. J., Engler J. A. The nucleotide sequence of the chicken thymidine kinase gene and the relationship of its predicted polypeptide to that of the vaccinia virus thymidine kinase. Nucleic Acids Res. 1984 May 11;12(9):3959–3971. doi: 10.1093/nar/12.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. P., Katan M. Viral aspects of protein phosphorylation. J Gen Virol. 1988 Jul;69(Pt 7):1441–1464. doi: 10.1099/0022-1317-69-7-1441. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Littler E., Zeuthen J., McBride A. A., Trøst Sørensen E., Powell K. L., Walsh-Arrand J. E., Arrand J. R. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986 Aug;5(8):1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. Y., Summers W. C. Site-directed mutagenesis of a nucleotide-binding domain in HSV-1 thymidine kinase: effects on catalytic activity. Virology. 1988 Apr;163(2):638–642. doi: 10.1016/0042-6822(88)90308-x. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Dolan A., McNab D., Perry L. J., Taylor P., Challberg M. D. Structures of herpes simplex virus type 1 genes required for replication of virus DNA. J Virol. 1988 Feb;62(2):444–453. doi: 10.1128/jvi.62.2.444-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 1986 Feb 25;14(4):1765–1777. doi: 10.1093/nar/14.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Michelson S., Tardy-Panit M., Bârzu O. Properties of a human cytomegalovirus-induced protein kinase. Virology. 1984 Apr 30;134(2):259–268. doi: 10.1016/0042-6822(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M. A new DNA-exonuclease in cells infected with herpes virus: partial purification and properties of the enzyme. J Gen Virol. 1968 Dec;3(3):337–347. doi: 10.1099/0022-1317-3-3-337. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Kit S. Nucleotide sequence of the marmoset herpesvirus thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide. Virology. 1984 Jun;135(2):316–330. doi: 10.1016/0042-6822(84)90189-2. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M. A., Galloway D. A. Nucleotide sequence of the herpes simplex virus type 2 thymidine kinase gene. J Virol. 1983 Jun;46(3):1045–1050. doi: 10.1128/jvi.46.3.1045-1050.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Wade R. D., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Guanosine cyclic 3',5'-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry. 1984 Aug 28;23(18):4207–4218. doi: 10.1021/bi00313a030. [DOI] [PubMed] [Google Scholar]
- Thompson R., Honess R. W., Taylor L., Morran J., Davison A. J. Varicella-zoster virus specifies a thymidylate synthetase. J Gen Virol. 1987 May;68(Pt 5):1449–1455. doi: 10.1099/0022-1317-68-5-1449. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Davison A. J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981 Dec;57(Pt 2):307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]



