Abstract
Background. Gastric cancer (GC) is an important malignant disease around the world. Abnormalities of microRNAs (miRNAs) have been implicated in carcinogenesis of various cancers. In the present study, we examined miR-21 expression in human gastric cancer with lymph node metastasis and attempted to uncover its relationship with clinicopathologic data, especially with lymph node metastasis. Materials and Methods. The expression levels of miR-21 in the tumor specimens of GC patients were quantified by RT-PCR. The correlation between miR-21 level and multiple clinicopathological factors was then examined by Mann-Whitney test, Kaplan-Meier survival analysis, and operating characteristic (ROC) analysis. Results. The expression level of miR-21 was higher in GC patients with lymph node metastasis than in those without lymph node metastasis (P < 0.05). Expression level of miR-21 was significantly correlated with histologic type, T stage, lymph node metastasis and pTNM stage. The overall survival rates in GC patients with low upregulated miR-21 expression were significantly higher than those with high upregulated miR-21 (P < 0.05). Conclusion. A close association is implicated between the elevated miR-21and lymph node metastasis, which could potentially be exploited as a practical biomarker for lymph node metastasis in patients with GC.
1. Introduction
Gastric cancer (GC) is the fourth most prevalent malignant cancer worldwide and is the second most frequent cause of cancer death [1]. Despite many advances made in GC therapy, the prognosis for patients with GC remains unsatisfying. Because of early detection in screening programmes in Japan, survival is good (52%), whereas survival in the USA, Europe, and China generally is only 20–25% for the delayed diagnosis [2]. Five-year survival rate for advanced or metastatic gastric cancer is nearly 5–20%, with median overall survival being less than 1 year [3, 4]. Therefore, it is necessary to find prognostic markers that could accurately indicate biological characteristics of GC and supply the evidence for early diagnosis and predicting the clinical outcome so as to improve the clinical management of GC patients. With the advances in diagnostic and operative technologies, surgical decisions have been made based on tumor stage [5, 6]. In order to tailor surgical therapy, it is necessary to assess clinical stage and depth of tumor invasion. However, the method for prediction of lymph node metastasis, another important determinant for prognosis, has not yet been well established.
MicroRNAs (miRNAs), a species of small noncoding RNA of about 21–23 nucleotides, could interact with their target mRNAs to interfere with the translation by promoting mRNA degradation or to block translation by binding to partial homology to target mRNA in the 39-untranslated region [7, 8]. It has been reported that miRNAs play important roles in various human biological processes such as metabolism, differentiation, cell proliferation, and apoptosis. Abnormalities of miRNA are suggested in carcinogenesis of various cancers, indicating that miRNAs could be used as molecular biomarkers for diagnosis of cancer and prediction of prognosis [9–12]. MiR-21 has been reported to be elevated in multiple human solid tumors including lung, breast, stomach, prostate, colon, and pancreatic cancer and their respective normal adjacent tissue. Meanwhile, serum miR-21 was also found to be overexpressed in many cancers such as diffuse large B-cell lymphoma, ovarian cancer, prostate cancer, or breast cancer. Evidence supports the hypothesis that miR-21 is a central oncomiR [13–16].
In the present study, we explore miR-21 expression and its correlation with clinicopathological factors in gastric cancer. Furthermore, we also determine whether miR-21 expression in lymph node might be a molecular biomarker for predicting the lymph node metastasis of GC patients.
2. Materials and Methods
2.1. Patients and Samples
Tumor specimens and normal control tissues were collected from 86 patients who underwent surgical treatment for histologically proven gastric adenocarcinoma at the Department of Surgery, Nanjing Second People's Hospital, Shanghai Sixth People's Hospital, and Shanghai Putuo Hospital, from January 2006 to December 2008. None of the patients had been administered by chemotherapy or radiotherapy prior to undergoing surgical resection. Metastatic lymph nodes were also harvested during gastrectomy. Clinical stage of GC was assessed on the basis of the tumor node metastasis (TNM) classification system recommended by the International Union against Cancer. For accurate N staging, more than 15 lymph nodes in one patient were collected by means of a careful manual palpation. Demographic and clinicopathological details of patients were collected from electronic patient records. The study was approved by the Institutional Review Board of Nanjing Second People's Hospital, Shanghai Sixth People's Hospital, and Shanghai Putuo Hospital. Written informed consent was obtained from each participant.
2.2. RNA Extraction and qRT-PCR
Total RNA was extracted from frozen specimens using Trizol (Invitrogen) following the manufacturer's guide. Total RNA was eluted in 100 mL and stored at −20°C. 5 mL of RNA was used to measure the expression of miR-21 by quantitative RT-PCR (qRT-PCR) with the TaqManH miRNA reverse transcription kit and the TaqManH miRNA assay-specific RT primers for miR-21 according to the instructions of the manufacturer (Applied Biosystems, Foster City, CA). The expression of Let-7a was used as internal control. Real-time PCR was performed with 3 mL of each cDNA on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) in duplicates. C T value is defined as the number of PCR cycles at which the fluorescent signal crosses the threshold. The difference of C T values between internal control and miR-21was presented as −ΔC T. ΔΔC T is the difference of ΔC T values between paired specimens. 2ΔΔCT represents the exponential value of ΔC T, which means fold change in expression.
2.3. Statistical Analysis
The correlation between up-regulated miR-21 and different clinical factors was assessed by Mann-Whitney analysis, paired t-test linear regression, and Kaplan-Meier survival analysis. To determine to which extent the obtained −ΔC T value of miR-21 could efficiently separate different clinical subsettings, operating characteristic (ROC) analysis was generated, and the sensitivity and specificity of the optimum cut-off point were defined as those values that maximized the area under the ROC curve (AUC). Data analysis was done using R software statistical environment (version 2.14.1; R Development Core Team, Vienna, Austria). All statistical tests were two-sided, and P value of less than .05 was considered statistically significant.
3. Results
3.1. Expression of miR-21 in GC Specimens
The expression level of miR-21 was analyzed by qRT-PCR. The expression level of internal control, Let-7a, showed no significant difference between the three groups (data not shown). We found significant difference between normal controls and patients without lymph node metastasis (P < 0.05). Furthermore, there is significant increase of miR-21 expression levels in patients with lymph node metastasis compared to normal controls and patients without lymph node metastasis (Figure 1(a)). A cut-off value of 5.12 was best distinguished in patients with lymph node metastasis and without lymph node metastasis, and the AUC value was 0.79 (Figure 1(b)).
Figure 1.
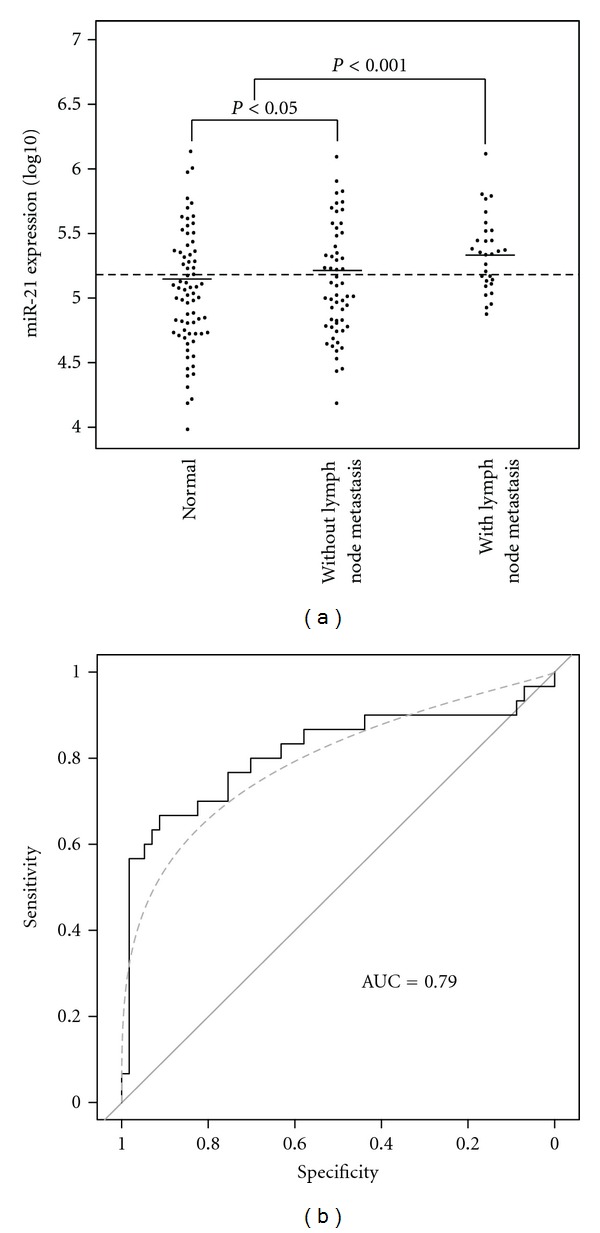
Comparison of levels of miR-21 in samples from gastric cancer (GC) patients without lymph node metastasis (n = 56), GC patients with lymph node metastasis (n = 30), and normal controls (n = 72). The lines denote the medians. Dot lines at the y-axis denote cut-off values. A significant difference between all histological groups was examined by ANOVA test. NS denotes no significant difference between groups. Receiver operating characteristics (ROCs) curves were used to discriminate patients with and without lymph node metastasis.
3.2. Correlation of miR-21 Expression with Clinicopathological Factors of GC Patients
The expression of miR-21 was found to be highly upregulated in 30 (34.9%) of 86 cases, whereas the remaining 56 cases (65.1%) were classified as having low up-regulated expression. To investigate the clinical significance of up-regulated miR-21, the relationship between miR-21 and clinicopathological factors was further assessed. As shown in Table 1, high up-regulated miR-21 expression appeared to be significantly associated with more histologic type (P < .001), T stage (P < .001), lymph node metastasis (P < .001) and pTNM stage (P < .05) in univariate analysis. Multivariate analysis found that pTNM stage (P = .001) and lymph node metastasis (P = .001) were statistically significant.
Table 1.
Relationship between clinicopathological factors and miR-21 expression in patients with GC.
| Parameters | miR-21 expression | P value∗ | |
|---|---|---|---|
| Low (n = 56) | High (n = 30) | ||
| Age (means ± SD, years) | 56.03 ± 12.86 | 56.26 ± 11.14 | 0.436 |
| Gender | 0.128 | ||
| Male | 35 (62.3%) | 20 (66.1%) | |
| Female | 21 (37.7%) | 10 (33.9%) | |
| Tumor location | 0.526 | ||
| Proximal | 3 (5%) | 1 (3%) | |
| Body | 24 (42.6%) | 12 (41%) | |
| Distal | 28 (50.4%) | 17 (56%) | |
| Histologic type | <0.001 | ||
| Differentiated | 19 (34.3%) | 7 (22.5%) | |
| Undifferentiated | 37 (65.7%) | 23 (77.5%) | |
| T stage | <0.001 | ||
| T1 | 37 (66.7%) | 15 (49.3%) | |
| T2 | 12 (21.4%) | 7 (23.6%) | |
| T3 | 7 (11.9%) | 5 (18.1%) | |
| T4 | 0 (0%) | 3 (9%) | |
| Lymph node metastasis | <0.001 | ||
| Present | 17 (30.5%) | 26 (85.3%) | |
| Absent | 39 (69.5%) | 4 (14.7%) | |
| Liver metastasis | 0.266 | ||
| Present | 27 (47.8%) | 15 (50.3%) | |
| Absent | 29 (52.2%) | 15 (49.7%) | |
| Peritoneal metastasis | 0.137 | ||
| Present | 32 (57.8%) | 18 (61.2%) | |
| Absent | 24 (42.2%) | 12 (38.8%) | |
| pTNM stage | <0.05 | ||
| I | 29 (52.6%) | 12 (41.1%) | |
| II | 16 (27.8%) | 7 (21.8%) | |
| III | 6 (11.3%) | 6 (19.3%) | |
| IV | 5 (8.3%) | 5 (17.8%) | |
*P value was tested in univariate analysis.
3.3. Correlation of miR-21 Expression with Prognosis of GC Patients
The overall survival rates in the patients with or without lymph node metastasis and with low or high up-regulated expression of miR-21 were statistically estimated. As shown in Figure 2, the median overall survival time in patients with high and low up-regulated miR-21 expression levels was nearly 11.2 and 13.8 months, respectively. The survival difference between these two groups was statistically significant (P < 0.05). We found that there were no significant differences between the patients with lymph node metastasis and high up-regulated expression of miR-21 and without lymph node metastasis and low up-regulated expression of miR-21 (P > 0.05).
Figure 2.
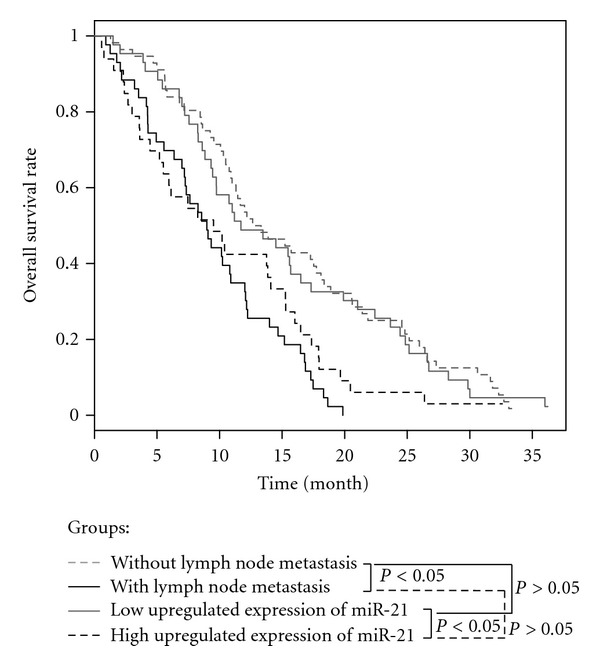
Kaplan-Meier survival curves of GC patients. The overall survival rate in patients with high miR-21 expression was significantly lower than that in those with low miR-21 expression (P < 0.05).
4. Discussion
To develop sensitive and specific minimally invasive molecular biomarkers for tailored management of cancers is a major challenge in clinical oncology. Dozens of studies have shown that miRNAs might be used as potential molecular biomarkers for human malignancies. Although numerous studies have been published for investigating the effect of the abnormal expression levels of miRNAs on the tumorigenesis, there is only a paucity of reports dealing with clinical impact of miRNAs in patients with lymph node metastasis [17–21]. Therefore, identification of miRNAs expression in patients with lymph node metastasis will be helpful to facilitate the clinical management of GC.
In this study, we have established the potentiality of miR-21 in the tumor tissue as a biomarker for lymph node metastasis. Using qRT-PCR, miR-21 was identified to be highly upregulated in GC patients with lymph node metastasis compared to patients with no lymph node metastasis (P < 0.05). ROC curve showed that the AUC value was nearly 0.8, which indicated that the expression level of miR-21 might be used to predict the lymph node metastasis in patients with GC. A previous study reported by Chan et al. also showed that miR-21 was overexpressed in most of the gastric cancer patients [22]. In addition, miR-21 and its precursor have been reported to be upregulated in many human malignancies, including pancreatic cancer, breast cancer, colorectal cancer, glioblastomas, and lung cancer [20, 23, 24].
The molecular mechanisms of regulating the expression of miR-21 in GC are rarely reported, especially the reports of evaluating the correlation between miR-21 expression level and clinical stage, lymph node metastasis, and prognosis of GC patients. The evidence was derived from the recently published reports. Suppression of miR-21 in MCF-7 cell, which overexpressed miR-21, could increase apoptosis and decrease cell proliferation, and knockdown of miR-21 in glioblastoma cells also showed that this miRNA has an anti-apoptotic function [25, 26]. Furthermore, two direct targets of miR-21, PDCD4 and maspin, which could decrease the metastasis of malignancies, have been found [27]. Taken other line of evidence together, a possible role of miR-21 as an oncogene has been hypothesized, including proliferation, cell cycle, metastasis, and chemosensitivity of tumor cells by targeting several tumor suppressor genes such as PTEN, MARCKS, PDCD4, and Cdc25A [28–31]. However, additional studies should be designed to investigate the molecular mechanisms of both the cause and effect of altered expression of miR-21 in GC.
Our present study next focused on the potential relationship between the expression level of up-regulated miR-21 and various GC clinicopathological factors, as well as the prognosis of the patients. It is worth noting that high up-regulated expression level of miR-21 was significantly correlated with histologic type, T stage, lymph node metastasis, pTNM stage, and poor overall survival of the patients with GC. High up-regulated expression of miR-21 in GCs with lymph node metastases indicates that its up-regulation was acquired in the course of tumor progression and, in particular, during the acquisition of metastatic potential. These results suggest that miR-21 could serve as a prognostic marker for prognosis of GC patients. However, the reports published by chan et al. did not show that higher expression of miR-21 affected the clinical prognosis of gastric cancer patients [22]. A potential important reason is that the patient cohort is too small to find the statistically significant difference, which only included 24 and 13 patients in group of <2-fold and ⩾2-fold elevated expression, respectively. Meanwhile, the design of our study was also different from the report by chan et al. In our study, we mainly examined the miR-21 expression in GC with lymph node metastasis, 56 and 30 GC patients without and with lymph node metastasis were included in the present study, respectively, and in the study reported by chan et al., only 2 and 35 GC patients without and with lymph node metastasis were included, respectively. Other researchers also reported the clinical significance of miR-21expression in human cancers. Yan et al. reported that miR-21 overexpression was correlated with specific breast cancer clinicopathological features, advanced tumor stage, lymph node metastasis, and poor survival of the patients [20]. Other reports showed that miR-21 expression in lung cancer was significantly correlated with advanced TNM stage, presence of lymph node metastasis, and overall survival [24]. This result indicates that miR-21 expression level could be used for the prediction of the clinical outcome. Of course, further prospective studies with a larger cohort are needed to confirm its prognostic significance in GC patients.
In conclusion, high up-regulated expression of miR-21 in GC was correlated with lymph node metastasis. Furthermore, the expression level of miR-21 might be also a potential prognostic factor for GC patients. Further studies are needed to verify the impact of miR-21 expression on gastric cancer, including the metastasis and prognosis.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. The Lancet. 2009;374(9688):477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of Clinical Oncology. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 4.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. Journal of Clinical Oncology. 2006;24(18):2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 5.Hsu JT, Liu MS, Wang F, et al. Standard radical gastrectomy in octogenarians and nonagenarians with gastric cancer: are short-term surgical results and long-term survival substantial? Journal of Gastrointestinal Surgery. 2012;16(4):728–737. doi: 10.1007/s11605-012-1835-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Liang H. Some problems in the surgical treatment of gastric cancer. Chinese Journal of Cancer. 2010;29(4):369–373. doi: 10.5732/cjc.009.10629. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Ke XS, Liu CM, Liu DP, Liang CC. MicroRNAs: key participants in gene regulatory networks. Current Opinion in Chemical Biology. 2003;7(4):516–523. doi: 10.1016/s1367-5931(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 9.Luo X, Burwinkel B, Tao S, Brenner H. MicroRNA signatures: novel biomarker for colorectal cancer? Cancer Epidemiology Biomarkers and Prevention. 2011;20(7):1272–1286. doi: 10.1158/1055-9965.EPI-11-0035. [DOI] [PubMed] [Google Scholar]
- 10.Skrzypski M, Dziadziuszko R, Jassem J. MicroRNA in lung cancer diagnostics and treatment. Mutation Research. 2011;717(1-2):25–31. doi: 10.1016/j.mrfmmm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Chen X, Cao Y. New role of microRNA: carcinogenesis and clinical application in cancer. Acta Biochimica et Biophysica Sinica. 2011;43(11):831–839. doi: 10.1093/abbs/gmr080. [DOI] [PubMed] [Google Scholar]
- 12.Munker R, Calin GA. MicroRNA profiling in cancer. Clinical Science. 2011;121(4):141–158. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 13.Moore LM, Zhang W. Targeting miR-21 in glioma: a small RNA with big potential. Expert Opinion on Therapeutic Targets. 2010;14(11):1247–1257. doi: 10.1517/14728222.2010.527334. [DOI] [PubMed] [Google Scholar]
- 14.Sekuklu SD, Donoghue MTA, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochemical Society Transactions. 2009;37(4):918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 15.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. Journal of Cellular and Molecular Medicine. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467(7311):86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZL, Zhao XH, Wang JW, et al. microRNA-92a promotes lymph node metastasis of human esophageal squamous cell carcinoma via E-cadherin. Journal of Biological Chemistry. 2011;286(12):10725–10734. doi: 10.1074/jbc.M110.165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. Journal of Digestive Diseases. 2010;11(1):50–54. doi: 10.1111/j.1751-2980.2009.00413.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang ZM, Yang J, Shen XY, et al. MicroRNA expression profile in non-cancerous colonic tissue associated with lymph node metastasis of colon cancer. Journal of Digestive Diseases. 2009;10(3):188–194. doi: 10.1111/j.1751-2980.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, He YL, Cai SR, et al. Expression and prognostic impact of PRL-3 in lymph node metastasis of gastric cancer: its molecular mechanism was investigated using artificial microRNA interference. International Journal of Cancer. 2008;123(6):1439–1447. doi: 10.1002/ijc.23643. [DOI] [PubMed] [Google Scholar]
- 22.Chan SH, Wu CW, Li AFY, Chi CW, Lin WC. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Research. 2008;28(2):907–911. [PubMed] [Google Scholar]
- 23.Pereira PM, Marques JP, Soares AR, Carreto L, Santos MAS. Microrna expression variability in human cervical tissues. PLoS ONE. 2010;5(7) doi: 10.1371/journal.pone.0011780.e11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. Journal of Surgical Oncology. 2011;104(7):847–851. doi: 10.1002/jso.22008. [DOI] [PubMed] [Google Scholar]
- 25.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 26.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Research. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 27.Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochemical and Biophysical Research Communications. 2009;388(3):539–542. doi: 10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 28.Lou Y, Yang X, Wang F, Cui Z, Huang Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. International Journal of Molecular Medicine. 2010;26(6):819–827. doi: 10.3892/ijmm_00000530. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Li D, Sha J, Sun P, Huang Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochemical and Biophysical Research Communications. 2009;383(3):280–285. doi: 10.1016/j.bbrc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 30.Fassan M, Pizzi M, Giacomelli L, et al. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Archiv. 2011;458(4):413–419. doi: 10.1007/s00428-011-1046-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Zou F, Xiaodong Z, et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Research. 2009;69(20):8157–8165. doi: 10.1158/0008-5472.CAN-09-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


