Abstract
Cromolyn sodium (cromolyn) effectively inhibits both antigen- and exercise-induced asthma when used as an aerosol. Intranasal cromolyn is also recommended for preventing and treating allergic rhinitis. By inhibiting the degranulation of sensitized mast cells, cromolyn reduces the release of mediators that trigger inflammation and the allergic response. The precise pharmacologic activity of cromolyn has not been fully elucidated. This study evaluated the effect of cromolyn on isolated rat's trachea. The following assessments of cromolyn were performed: (1) effect on tracheal resting tension, (2) effect on contraction caused by 10−6 M of methacholine as a parasympathetic mimetic, and (3) effect of the drug on electrically induced tracheal contractions. The results indicated cromolyn could inhibit electrical field stimulation-induced spike contraction when the preparation was increased to 10−4M. Adding cromolyn at doses of ≥10−8 M did not elicit a relaxation or contraction response to 10−6 M of methacholine-induced contraction. It alone had a minimal effect on the basal tension of the trachea as the concentration increased. This study indicates cromolyn had no cholinergic or anticholinergic effect and high concentrations of cromolyn might actually inhibit parasympathetic function of the trachea. Inhibiting parasympathetic function of the trachea through stabilizing the presynaptic nerve by cromolyn may be responsible for protecting patients against antigen- and exercise-induced asthma.
Keywords: Cromolyn, in vitro study smooth muscle, trachea
Cromolyn sodium (cromolyn) was first introduced as a treatment for asthma in 1967 by Altounyan.1 Intranasal cromolyn is also recommended for preventing and treating allergic rhinitis.2,3 In a different form, it can be also used for allergic conjunctivitis, systemic mastocytosis, and eosinophilic gastroenteritis.4 When used as an aerosol, cromolyn effectively inhibits both antigen- and exercise-induced asthma, and chronic use (four times daily) reduces the overall level of bronchial reactivity.5 However, it has no effect on airway smooth muscle tone and is ineffective in reversing asthmatic bronchospasm; it is of value when taken prophylactically.5 Since its approval by the Food and Drug Administration in 1973 for treating asthma and in 1983 for treating allergic rhinitis, cromolyn has established an excellent safety record.3 Apart from the initial bronchoconstriction that can occur in some patients, no serious side effects or long-term toxicity problems have been reported.
Cromolyn has been shown to inhibit the allergen-induced early and late-phase responses in the upper and lower airways and conjunctiva, where mucosal mast cells are crucially involved in the allergic response.6 By inhibiting the degranulation of sensitized mast cells, cromolyn reduces the release of mediators that trigger inflammation and the allergic response.5 The precise pharmacologic activity of cromolyn has not been fully elucidated. Inhibiting calcium influx or altering the function of delayed chloride channels in the cell membrane might be responsible for it.5,6 There are reports on the effect of cromolyn in vitro or in vivo trying to find the possible mechanism of the drug against antigen- and exercise-induced asthma attacks. During an asthmatic attack, the trachea plays an important role in reducing pulmonary function as it contracts. Therefore, the effect of cromolyn on the trachea merits further exploration.
Our previous report developed a simple and rapid test for screening parasympathetic mimetic agents and potential tracheal contraction agents.7,8 The technique could identify how cromolyn affects the trachea directly. The purpose of this study was to determine the effects of cromolyn on the isolated trachea in vitro.
MATERIALS AND METHODS
Rat Trachea Tissue Preparation
Twenty rats were anesthetized by i.p. administration of pentobarbital (45 mg/kg), and two pieces of trachea ∼5 mm in length were removed from each rat. This study was approved by an animal experiment review board (IACUC-08-188). The equipment and process were designed based on our previous study.7,8 The tracheal specimen was mounted using two steel plates and submersed in a 30-mL muscle bath at 37°C. The bath was filled with 30 mL of Krebs solution consisting of (mmol/L): NaCl (118), KCl (4.7), CaCl2 (2.5), MgSO4 · 7H2O (1.2), KH2PO4 (1.2), NaHCO3 (25.0), and glucose (10.0). The upper side of the tracheal strip was attached to a Grass FT-03 force displacement transducer (AstroMed, West Warwick, RI) using a steel plate and a 3-0 silk ligature. The other side of the strip was fixed to a steel plate attached to a bath. A passive tension of 0.3 g was applied to the strips and subsequent changes in tension were recorded continuously using Chart V4.2 software (PowerLab; AD Instruments, CO Springs, CO). The chemicals used were of the highest purity available. All chemical reagents were obtained from Sigma (St. Louis, MO).
Methacholine Test
We tested methacholine as a tracheal contraction drug. Preliminary tests showed the tracheal strip immersed in the bath solution used for subsequent experiments did not contract when basal tension was applied. Before drug assays were conducted, isolated tracheas were equilibrated in the bath solution for 15–30 minutes, during which continuous aeration with a mixture of 95% O2 and 5% CO2 was applied. Stepwise increases in the amount of drugs used were used to study the contraction or relaxation responses of tracheal strips. When the concentration of cromolyn is 10−4M, we add atropine, which is classified as an anticholinergic drug, to thoroughly assay the effect of cromolyn on 10−6 M methacholine-induced contraction. All drugs were administered by adding a defined volume of stock solution to the tissue bath solution. In each experiment, one untreated strip served as a control.
Electrical Field Stimulation Test
Electrical field stimulation (EFS; 5 Hz, 5-ms pulse duration, at a voltage of 50 V, trains of stimulation for 5 seconds) was applied to the trachea strip with two wire electrodes placed parallel to the trachea strip and connected to a direct-current stimulator (Grass S44; Grass Instrument Co., Quincy, MA). An interval of 2 minutes was imposed between each stimulation period to allow recovery from the response. Stimulation was applied contiguously to the trachea at 37°C.
Cromolyn Assessments
The following assessments for cromolyn were performed: (1) effect on tracheal resting tension—this test examined the effect of the drug on the simulating condition of the resting trachea condition; (2) effect on contraction caused by 10−6 M of methacholine (a parasympathetic mimetic)—this procedure was concerned with examining postsynaptic events such as muscle receptor blockade, enhancement, and second messengers; and (3) effect of cromolyn on electrically induced contractions—electrical stimulation of this tissue causes parasympathetic nerve remnants in the trachea to release the transmitter acetylcholine. If there is interference with transmitter release, electrical stimulation does not cause contraction. Thus, presynaptic events were seen more easily with this procedure. Concentrations of drugs are expressed as concentrations present in the 30-mL bath solution. In each experiment, repetitions were performed at least six times.
Statistical Analysis
Data were presented as mean values and SD. The differences between mean values were compared using Student's t-test, and were assumed to be significant at p < 0.05.
RESULTS
The degree of contraction or relaxation of tracheal strips was estimated from the tension applied to the transducer. Tracheal contraction induced by a small dose of methacholine was easily detected (not shown), and the tissue remained in a contracted state until the drug was rinsed from the tissue.
The Pharmacologic Effects on Tracheal Contraction Caused by Methacholine
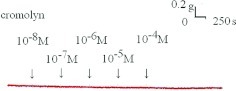
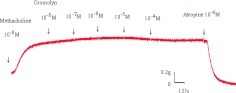
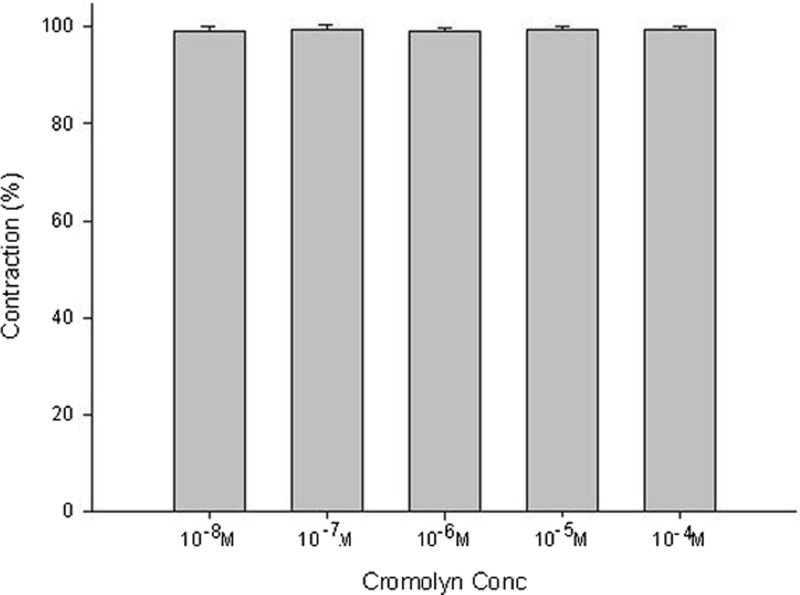
Adding the mast cell–stabilizing drug, cromolyn, to the basal tension had a negligible effect (Fig. 1). It resulted in no relaxation of the trachea when introduced after adding a constricting agent such as 10−6 M of methacholine (Fig. 2). As the concentration of cromolyn increased from 10−8 to 10−4 M, it had no effect on contraction (Figs. 2 and 3). At 10−8 M of cromolyn, the tension was 99 ± 0.8% of the control values (Fig. 3). At 10−5 and 10−4 M of cromolyn, the tensions were 100 ± 0.8% and 99 ± 0.8%, respectively (Fig. 3). The difference in tension among the specimens treated with 10−8 M of cromolyn and 10−5 or 10−4 M of cromolyn was not statistically significant. The total relaxation of the 10−6 M methacholine–induced contracted tracheal strip was observed when adding 10−6 M of atropine among the specimens treated with cromolyn (Fig. 2).
Figure 1.
Tension changes in rat trachea after applying various cromolyn concentrations. Cromolyn alone had a minimal effect on the basal tension of the trachea as the concentration increased. Original basal tension was 0.3 g.
Figure 2.
Original recording of the effects of cromolyn on 10−6 M of methacholine-induced contraction of rat trachea. Cromolyn had a minimal effect on 10−6 M of methacholine-induced contraction of rat trachea as the concentration increased. The total relaxation of the 10−6 M methacholine-induced contracted tracheal strip was observed when adding 10−6 M of atropine among the specimens treated with cromolyn.
Figure 3.
Effects of cromolyn on 10−6 M of methacholine-induced contraction (contraction area calculated at 100% with no addition of cromolyn) of rat trachea. The difference in tension between 10−8 M of cromolyn and 10−5 M of cromolyn or 10−4 M of cromolyn was not statistically significant. The results were mean ± SD (n = 6).
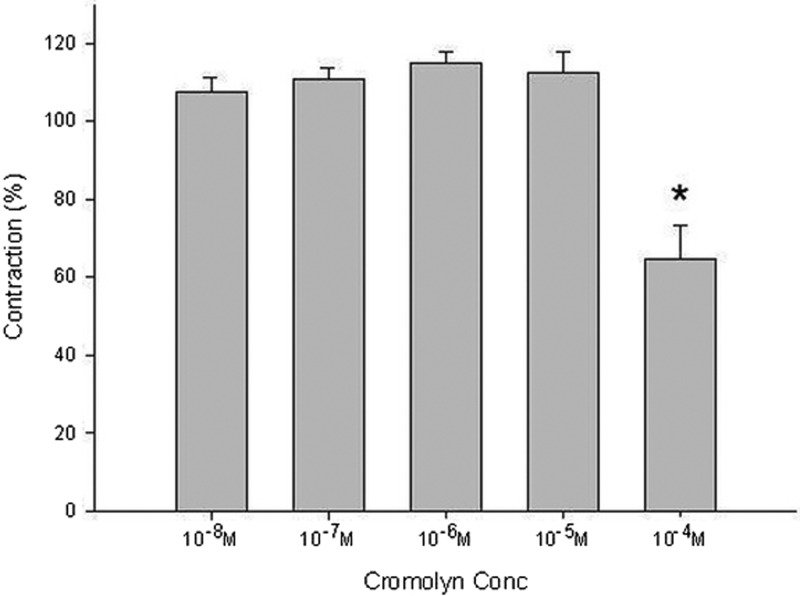
The Pharmacologic Effects on Electrically Induced Tracheal Contractions
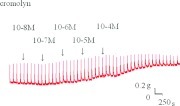
Lower doses of cromolyn slightly enhanced the spike contraction induced by EFS, and a high dose inhibited the spike contraction induced by EFS (Figs. 4 and 5). The peak tension of the tracheal strip evoked by EFS on adding 10−8 M of cromolyn was 108 ± 3.8%, whereas at 10−5 and 10−4 M of cromolyn the peaks were 112 ± 5.3% and 65 ± 8.7%, respectively (Fig. 5). The peak tension of the tracheal strip evoked by EFS at 10−4 M cromolyn addition was significantly less than that at 10−8 M of cromolyn (p < 0.05).
Figure 4.
Original recording of effects of cromolyn on electrically induced tracheal contractions was noted. A higher dose of cromolyn decreased the spike contraction induced by electrical field stimulation (EFS).
Figure 5.
Effects of cromolyn on electrically induced tracheal contractions (contraction area calculated at 100% with no addition of cromolyn). The peak tension of the tracheal strip evoked by electrical field stimulation (EFS) during the addition of 10−4 M of cromolyn was significantly less than that for the addition of 10−8 M of cromolyn (p < 0.05). The results were mean ± SD (n = 6).
DISCUSSION
The results of our experiments should be interpreted within the context of the test materials used. Our study was simple and effective. An intact tracheal ring was an important component of our technique.9,10 Such an intact tracheal ring is much more representative of a physiological setting than smooth muscle strips. Although it was difficult to determine which tissue component of the trachea was responsible for the drug-induced contraction, the nature of specific tissues and their responses to specific drugs provided some indication. First, the tracheal strips used in our study were crude preparations containing cartilage and tracheal smooth muscle. The smooth muscle of the trachea appeared to be the main tissue component responsible for contraction, because the other components (epithelium, glands, connective tissue, nerves, and cartilage) did not contract to a significant extent. Because this method involved cross-contraction, changes in tension were caused by radial contraction of the tracheal ring. Although responses to drugs and electrical stimulation have been verified for similar preparations,9–13 the contractile response observed in this study was probably an aggregate of the responses of various types of muscle tissue. Second, the isolated tracheal preparations used in our experiments were excised from rats without damaging the endothelium or smooth muscle. Therefore, it is reasonable to assume the tracheal responses to test agents in our study are comparable with those observed after applying an inhaler to the trachea during an asthma attack.
The cholinergic contracting agent tested in this preparation is commonly used for research purposes. Note, cromolyn resulted in no relaxation of the trachea smooth muscle when introduced after applying methacholine. However, the total relaxation of the contracted tracheal strip was observed when adding 10×6 M of atropine. The previously indicated cromolyn had no cholinergic or anticholinergic effect. Thus, it should be possible to assess the effects of common drugs and potential therapeutic agents supposedly not responsible for relieving acute asthma attacks. In addition, basal tension had a minimal effect at various concentrations of cromolyn. There are reports about initial mild bronchoconstriction when using cromolyn in some patients, and it was believed to be secondary to local irritation after inhaling cromolyn.4,5 There was no such effect in this study. This result was compatible with cromolyn basic and clinical entities: it has no effect on airway smooth muscle tone and was ineffective in reversing asthmatic bronchospasm.5
EFS is a common experimental tool activating the nerve terminals within the tissue to be tested and inducing the release of endogenous neurotransmitters, thereby causing the smooth muscle to contract. EFS-induced spike contraction of canine nasal mucosa, which is believed to result from the contraction of vascular smooth muscles, disappeared after ipsilateral cervical sympathetic ganglionectomy.14 Thus, EFS-induced spike contraction of isolated canine nasal mucosa has been proven to be mediated by sympathetic innervation.14 In this study, EFS-induced spike contraction of the tracheal smooth muscle was believed to be caused by stimulation of parasympathetic innervation. Therefore, EFS-induced contraction of the trachea decreased as the cromolyn concentration was high. These findings suggested a mast cell–stabilizing drug, cromolyn, could antagonize the parasympathetic innervation responsible for trachea smooth muscle contraction. Commercial Intal Nebulizer Solution (2 mL, King Pharmaceuticals, Inc, Bristol, TN) contains 20 mg of cromolyn sodium, which is ∼2 × 10−2 M cromolyn sodium. When applying an inhalation, one gets immediately a dilution resulting in a concentration of 2 × 10−3 M of cromolyn sodium at the nasal mucosal side. It remains to be shown that a concentration of 10−4 M can be reached at the tracheal smooth muscles. Therefore, commercial Intal Nebulizer Solution could inhibit parasympathetic function of the trachea. The actual concentration of cromolyn sodium in tracheal smooth muscle when used in an inhalation requires further investigation. Note, a low dose of cromolyn resulted in mildly increased EFS-induced contraction of the trachea. Clearly, what was observed in this study is very interesting, but further study is needed to clarify these phenomena.
Another interesting finding is the experimental responses for methacholine and the EFS differed in the study administering cromolyn. Cromolyn had no cholinergic or anticholinergic effect in the methacholine experiment and caused no further contraction in the contracted tracheal smooth muscle induced by methacholine. It revealed no effect in postsynaptic events. On the other hand, a higher dose of cromolyn inhibited EFS-induced spike contraction of the trachea. It can be concluded that there is interference with transmitter release, so electrical stimulation causes decreased contraction. The presynaptic events may be responsible for the pharmacologic mechanism, so cromolyn played a role in the prophylactic treatment of asthma, stabilizing the presynaptic nerve. It was not easy to obtain human tissue for similar studies. The effect of this drug on isolated human tracheal smooth muscle still requires further investigation. Because this was an in vitro study, there are reservations as to its comparability with an in vivo situation in humans. In the in vivo situation, the response might be much more complicated than that in the in vitro situation.
A generally believed mechanism of the action of cromolyn is its ability to inhibit allergen-induced mediator release from mast cells. The presence of mast cells in rat trachea had been reported in the previous investigation. In rat trachea, two types of mast cells have been identified: connective tissue mast cells and mucosal mast cells. They have been observed in the submucosa region and epithelial layer, respectively.15 However, there are other statements for its possible association or additional mechanisms. A neural pathway mechanism for its effectiveness in asthma has been proposed where cromolyn could suppress the excitatory actions of afferent vagal C-fiber endings, suggesting part of its inhibitory capacity in vivo may result from suppressing the vagal reflex pathways.16 Alvarez et al. suggested the existence of a double site of pharmacologic action of cromolyn, one on the mast cell system and the second on the smooth muscle of the trachea or intestinal tract.17 Cromolyn inhibits guinea pig ileum contractions induced by electrical stimulation, which is mediated by acetylcholine release from Auerbach's plexus. They concluded cromolyn has a protective effect on smooth muscle fibers as well as on myenteric plexus.17 In their further studies, cromolyn inhibited contractions induced by electrical stimulation in both guinea pig ileum and the trachea as well as atropine does. Either this shows an inhibition of acetylcholine release from postganglionic parasympathetic fibers or an anticholinergic effect is involved in the mode of action of cromolyn.18 Our study can illustrate their statement of the inhibition of acetylcholine release from postganglionic parasympathetic fibers by cromolyn.
CONCLUSION
This study indicates cromolyn had no cholinergic or anticholinergic effect and higher concentrations of cromolyn might actually inhibit parasympathetic function of the trachea. Inhibiting parasympathetic function of the trachea through stabilizing the presynaptic nerve by cromolyn may be responsible for protecting patients against antigen- and exercise-induced asthma.
Footnotes
Funded in part by Taipei Medical University-Shuang-Ho Hospital (100TMU-SHH-05) and the National Science Council, R.O.C. (NSC Grant 98-2314-B-016-018-MY2)
The authors have no conflicts to declare pertaining to this article
REFERENCES
- 1. Altounyan REC. Inhibition of experimental asthma by a new compound disodium cromoglycate “Intal.” Acta Allergol 22:487, 1967 [Google Scholar]
- 2. Okuda M. A survey of rhinitis in Japan and an evaluation of the treatment with sodium cromoglycate. Rhinology 20:63–72, 1982 [PubMed] [Google Scholar]
- 3. Ratner PH, Ehrlich PM, Fineman SM, et al. Use of intranasal cromolyn sodium for allergic rhinitis. Mayo Clin Proc 77:350–354, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Edwards AM. The discovery of cromolyn disodium and its effect on research and practice in allergy and immunology. J Allergy Clin Immunol 115:885–888, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Boushey HA. Drug used in asthma. In Basic and Clinical Pharmacology, 11th ed Katzung BG. (Ed). San Francisco, CA: McGraw-Hill, 333–349, 2009 [Google Scholar]
- 6. Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol 8:218–230, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Wang H-W, Wu C-C. Effects of oxymetazolin on isolated rat's tracheal smooth muscle. Eur Arch Otorhinolaryngol 265:695–698, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Wang H-W, Chou Y-L, Chu Y-H. Azelastine nasal spray inhibiting parasympathetic function of tracheal smooth muscle. Rhinology 48:211–215, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Bratton DL, Tanaka DT, Grunstein MM. Effects of temperature on cholinergic contractility of rabbit airway smooth muscle. J Appl Physiol 63:1933–1941, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Beny J, Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol 266:H1465–H1472, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Yau KI, Ko FN, Chien CH. Effects of prokinetic agents on contractile responses to electrical Weld stimulation of isolated guinea pig trachea. J Formos Med Assoc 98:567–572, 1999 [PubMed] [Google Scholar]
- 12. Gonzalez O, Santacana GE. Effect of low temperature on tracheal smooth muscle contractile and relaxing responses evoked by electrical field stimulation. Phys Res 20:237–243, 2001 [PubMed] [Google Scholar]
- 13. Yau KI, Hwang TL. The nonadrenergic noncholinergic system can modulate the effect of prokinetic agents on contractile response of isolated guinea pig trachea segments to electrical field stimulation. J Formos Med Assoc 101:695–699, 2002 [PubMed] [Google Scholar]
- 14. Wang H-W, Jackson RT. Do cholinergic neurons directly innervate nasal blood vessels? Rhinology 26:139–146, 1988 [PubMed] [Google Scholar]
- 15. Ikawati Z, Nose M, Maeyama K. Do mucosal mast cells contribute to the immediate asthma response? Jpn J Pharmacol 86:38–46, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Dixon M, Jackson DM, Richards IM. The action of sodium cromoglycate on “C” fibre endings in the dog lung. Br J Pharmacol 70:11–13, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arruzazabala ML, Alvarez RG. Further studies on the effects of disodium cromoglycate on guinea pig ileum. Agents Actions 12:596–600, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Alvarez RG, Arruzazabala ML. Effects of disodium cromoglycate (DSCG) on the guinea pig trachea and ileum. Allergol Immunopathol (Madr) 11:425–430, 1983 [PubMed] [Google Scholar]