Abstract
Oral curcumin is recognized to have anti-inflammatory properties and has been used by ancient traditional medicine for centuries to treat a variety of diseases. In vitro studies have confirmed the ability of curcumin to inhibit allergic inflammatory cytokine responses from lymphocytes; however, there are no in vivo studies of curcumin to treat inflammation associated with allergic asthma. This study was designed to determine the effect of oral curcumin supplementation on patients with stable, persistent, atopic asthma. Adult patients with stable, persistent asthma with evidence of allergic sensitization were randomized to receive 1000 mg of curcumin twice daily or placebo. Subjects were followed for 6 months and performed monthly spirometry (pre- and postbronchodilator); Asthma Control Test (ACT) scoring; and measurements for fractional excretion of nitric oxide (NO), serum eosinophil count, leukocyte count, total IgE, specific IgE to Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farinae (Der f), use of rescue albuterol, and dose of inhaled corticosteroid. Nine patients were randomized into the treatment arm and six were randomized into the placebo group. No differential response was seen in the treatment and placebo groups regarding the primary end point, postbronchodilator forced expiratory volume in 1 second (FEV1). Similarly, all secondary end point evaluations were not significantly different. Despite in vitro evidence that curcumin has anti-inflammatory properties and can inhibit allergic cytokine responses from lymphocytes in vitro, curcumin, 1000-mg, twice daily supplementation did not significantly affect postbronchodilator FEV1, ACT scores, use of rescue bronchodilator, dose of inhaled corticosteroid, exhaled NO, serum IgE, total white blood cell count specific IgE to Der p or Der f, and blood eosinophils in patients with persistent atopic asthma.
Keywords: Allergy, asthma, curcumin, herbal
Curcumin (diferuloylmethane) is a naturally occurring polyphenolic molecule that is derived from the root of the Curcuma longa plant. It has been used for many centuries, particularly in India and China, for homeopathic medical treatment of conditions including arthritis, urinary afflictions, and asthma.
Laboratory evaluation of curcumin indicates antioxidant properties, and numerous molecular targets, including transcription factors AP-1 and NF-κβ, have been identified. As such, it inhibits the secretion of both proinflammatory (TNF-α and IL-6) and anti-inflammatory (IL-10) cytokines.1 Curcumin also decreases the expression and release of eotaxin, monocyte chemotactic protein 1, and monocyte chemotactic protein 3 from IL-1β–stimulated human airway smooth muscle cells.2 When added to Dermatophagoides farinae (Der f)–stimulated lymphocyte cell cultures from allergic asthmatic patients, curcumin inhibits Der f–induced lymphocyte proliferation and production of IL-2, IL-4, IL-5, and granulocyte macrophage colony-stimulating factor.3 Ram et al. showed that curcumin decreases airway constriction and hyperreactivity in guinea pigs (20 mg/kg) when coadministered with ovalbumin.4
There are no in vivo studies using curcumin to treat asthma inflammation. Therefore, we performed a randomized, double-blinded, placebo-controlled pilot study to evaluate the effects of oral curcumin supplementation versus placebo, on adult patients with a history of stable persistent asthma and allergic sensitization. A daily dose of 2000 mg was chosen based on previously published data of potential side effects, drug absorption, and biological effects when given as an oral supplement in patients with Alzheimer's dementia.5 Curcumin capsules were purchased from Sabinsa Corp. (Piscataway, NJ) and their appearance was identical to placebo.
This study enrolled adult males and nonpregnant female patients aged 18–60 years with a history of physician-diagnosed asthma for 1 year or longer, forced expiratory volume in 1 second (FEV1) of ≥60%, current use of low-or-medium–dose inhaled corticosteroids,6 ≥1 puff of short-acting β-agonist in the past 30 days, and a ≥2+ reaction by prick-puncture test (erythema larger than a nickel in diameter with wheal <3 millimeter) to Dermatophagoides pteronyssinus (Der p) or Der f. Subjects were excluded if there was a history of allergy or intolerance to curcumin, other chronic respiratory conditions (chronic obstructive pulmonary disease, pulmonary hypertension, interstitial lung disease, etc.), FEV1< of 60%, and history of smoking in the past year or cumulative smoking of ≥10 pack-years. Other exclusive criteria included the use of oral corticosteroid in the preceding 1 month, high-dose inhaled corticosteroid for ≥2 weeks during the 4 weeks preceding the screening visit, long-acting β-agonists at screening visit, and use of short-acting β-agonist at >4 puffs/day on average during the preceding 2 weeks (other than before exercise). Additional exclusive criteria included the current use of allergen immunotherapy or any immunotherapy in the past year; current use of antileukotrienes; diseases, i.e., medical or psychiatric or social problems that, in the investigator's opinion, would interfere with participation in the study or place the subject at risk; inability to swallow the study capsule; personal history of alcohol or illicit drug dependence in the last year; and inability to correctly use a peak flow meter.
The study was conducted between November 2008 through January 2010. All study methods and documents were approved by the Institutional Review Board of the University of South Florida, Tampa, FL.
During screening visit 1, subjects underwent baseline spirometry, Asthma Control Test (ACT) scoring, and skin-prick testing to Der f and Der p allergen extracts. Average ACT score for treatment and placebo groups were 19 and 18, respectively. Average postbronchodilator FEV1 was 82% in the treatment and 78% in the placebo groups.
After a 2-week run-in period to confirm stable disease, subjects were then randomized; nine were entered into the treatment arm (curcumin at 1000 mg twice daily), and six were entered into the placebo group. Mean prebronchodilator FEV1 at visit 1 for subjects in treatment and placebo arms were 2.29 and 2.87 L, respectively. Subjects also underwent induced sputum collection, exhaled nitric oxide (eNO), another ACT and phlebotomy to measure serum total IgE, total blood eosinophil count, and serum-specific IgE to Der p1 and Der f1.
Two subjects in the treatment arm withdrew consent and were not included in the final analysis. The remaining subjects then returned for three follow-up visits, each 1 month apart, at which time they underwent spirometry, eNO, ACT scoring, phlebotomy for measurement of serum total IgE, blood eosinophils, specific antibody to Der f and Der p, and total white blood cell count. Subjects also maintained a daily diary to record the use of their rescue inhaler, symptoms such as coughing and wheezing, peak expiratory flow rates, dose of inhaled corticosteroids, and/or antibiotic or systemic corticosteroid. Bottles containing study drug were weighed to monitor for medication compliance.
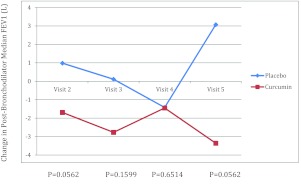
Postbronchodilator FEV1 was chosen to be the primary end point because it measures the best lung function that can be achieved by bronchodilator therapy on the day of the visit and therefore is a more stable measure in asthmatic patients than comparing visit-to-visit baseline FEV1.7 Primary end point analysis was done using Wilcoxon sum rank test instead of t-test because the data did not follow a normal distribution. Compared with baseline, the FEV1 value at visit 5 in the treatment group decreased (mean = 4.6; SD = 5.8), whereas it increased in the placebo group (mean = 3.85; SD = 3.07; p = 0.0562). Overall, there was no statistically significant improvement in median values of FEV1 among those subjects taking curcumin compared with placebo (Fig. 1; Table 1). Similarly, secondary end point evaluations of eNO, ACT scores, all of the serum analyses, rescue albuterol use, and dose of inhaled corticosteroid were also not significantly different (data not shown).
Figure 1.
Plot of median change in postbronchodilator forced expiratory volume in 1 second (FEV1; percent predicted) of subjects receiving curcumin or placebo by visit (30-day intervals).
Table 1.
Change in FEV1 at each follow-up visit compared with baseline
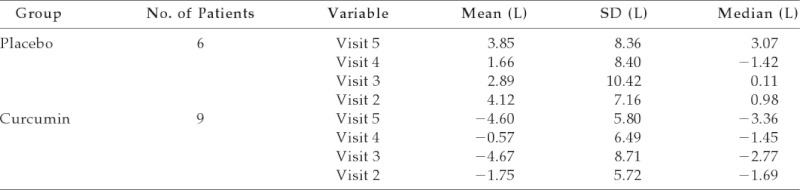
FEV1 = forced expiratory volume in 1 s.
In conclusion, despite in vitro evidence that curcumin has anti-inflammatory properties and can inhibit allergic cytokine responses from lymphocytes in vitro, curcumin at 1000 mg twice daily supplementation did not significantly affect postbronchodilator FEV1, ACT scores, use of rescue bronchodilator, dose of inhaled corticosteroid, eNO levels, or levels of serum IgE, total white blood cells, antibody specific to Der p or Der f, and blood eosinophils in patients with persistent atopic asthma. Future studies may benefit from a larger sample size, longer study duration, higher dose of curcumin, and/or improvements in oral bioavailability.
Footnotes
Funded by the University of South Florida, College of Medicine and James A. Haley Department of Veterans' Affairs Hospital, Department of Internal Medicine, Division of Allergy and Immunology
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Wong CK, Li ML, Wang CB, et al. House dust mite allergen Der p 1 elevates the release of inflammatory cytokines and expression of adhesion molecules in co-culture of human eosinophils and bronchial epithelial cells. Int Immunol 18:1327–1335, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Wuyts WA, Vanaudenaerde BM, Dupont LJ, et al. Involvement of p38 MAPK, JNK, p42/p44 ERK and NF-kappaB in IL-1beta-induced chemokine release in human airway smooth muscle cells. Respir Med 97:811–817, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi T, Hashimoto S, Horie T. Curcumin inhibition of Dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem Pharmacol 54:819–824, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull 26:1021–1024, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Baum L, Lam CW, Cheung SK, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 28:110–113, 2008 [DOI] [PubMed] [Google Scholar]
- 6. National Heart, Lung, and Blood Institute Guidelines for the diagnosis and management of asthma. Expert Panel Report 3, p. 349, 2007. Available online at www.nhlbi.nih.gov/guidelines/asthma/; accessed May 12, 2010
- 7. Enright PL, Lebowitz MD, Cockroft DW. Physiologic measures: Pulmonary function tests. Asthma outcome. Am J Respir Crit Care Med 149:S9–S18, 1994 [DOI] [PubMed] [Google Scholar]