Abstract
There is a paucity of data regarding prevalence and characteristics of adult seafood allergy in United States cohorts. This study was designed to determine the characteristics of patient-reported seafood allergy in a large allergy referral adult population. Retrospective analysis was performed of laboratory and clinical characteristics of seafood-allergic patients in three allergy clinics in the Texas Medical Center between January 1, 1997 and January 30, 2010. Of 5162 patients seen in this adult allergy referral population, 159 had physician-diagnosed seafood allergy with an average age of diagnosis of 50.2 (18–81 years) years. Shellfish allergy (59.1%) was more frequent than fish allergy (13.8%). Crustacean allergy (82.6%) was more frequent than mollusk allergy (7.2%). Shrimp (72.5%), crab (34.8%), and lobster (17.4%) were the most common shellfish allergies and tuna (28.6%), catfish (23.8%), and salmon (23.8%) were the most common fish allergies. One-third of seafood-allergic patients reported reactions to more than one seafood. Shellfish-allergic adults were more likely to experience respiratory symptoms than fish-allergic adults (p < 0.05). The likelihood of having anaphylaxis (32%) was not statistically different between shellfish- and fish-allergic subjects. Severe reactions were 12.9 times more likely to occur within the 1st hour of ingestion compared with nonsevere reactions (p < 0.005). The percentage of seafood allergy in this adult allergy referral population was 3.08%.
Keywords: Anaphylaxis, fish, food allergy, hypersensitivity, seafood, shrimp, urticaria
Seafood is a major source of food considering that the United States is the third largest seafood consumer after China and Japan with the average citizen consuming 16.0 lb of seafood yearly.1,2 Seafood is categorized as fish or shellfish, with shellfish divided into crustaceans and mollusks. Crustaceans include shrimp, lobster, crab, and crawfish and mollusks include scallops, oysters, clams, and squid.3 Seafood allergy is one of the most common food allergies in adults,4,5 with shellfish being the most common cause of adult food allergy.6 Allergic reactions to any food range from mild to severe and potentially life-threatening.4,7 Crustaceans and fish are considered most likely to cause severe anaphylaxis. Seafood allergy is thought to be lifelong.5,8,9
Although there have been studies of seafood allergy prevalence in pediatric allergy referral populations,10 population-based cohorts of fish-allergic children,11 and food-induced anaphylaxis populations,12,13 there is scarce data regarding prevalence of seafood allergy in U.S. adults in the allergy referral population.14 Additionally, there have been very few large studies detailing the clinical characteristics of physician-diagnosed adult seafood allergy. Food allergy is the third largest reason for outpatient allergy/immunology (A/I) consultation.15 The prevalence of adult seafood allergy has primarily been determined in the general population by survey data through self-report.16–19 A meta-analysis investigating the prevalence of food allergies found that 0–2% of persons reported fish allergy and 0–10% of persons reported shellfish allergy.20 The goals for this study were to determine the percentage and characteristics of seafood allergy in adults attending the allergy office–based clinics in a large tertiary care medical center. We aimed to compare the clinical characteristics of adults with shellfish allergy versus fish allergy as well as to describe the distribution of specific seafood allergies in this population. We also analyzed the co-occurrence of atopic diseases, asthma, and other food allergies. Determination of these aspects of adult seafood allergy will allow us to better understand this particular food hypersensitivity in our adult office-based population giving physicians a better understanding regarding the diagnosis and treatment of this important allergy.
METHODS
Study Subjects
This study was a retrospective chart review of all adult patients (≥18 years) with seafood allergy in three adult allergy/immunology (A/I) clinics of the Texas Medical Center (TMC) over a 13 year period. In 2009, there were 6 million patients and 160,000 daily visitors to the TMC.21 Ben Taub Hospital Outpatient A/I Clinic (BT), the only A/I service within the entire Harris County Hospital District System; the Michael E. Debakey Veteran's Affairs Medical Center Outpatient A/I Clinic (VA); and the Baylor College of Medicine Outpatient A/I Clinic (Baylor Clinic) were included. Less than 5% of the patients seen at the VA and BT clinics are referred by an outside allergist for evaluation, but ∼50% of the Baylor Clinic patients are referred by an outside allergist.
Data Collection
The VA electronic medical records, Baylor and BT clinics' paper charts, and electronic medical records were searched to identify all patients seen between January 1, 1997 and January 30, 2010. The charts were searched for ICD codes for food and seafood allergy (ICD-9 codes 693.1 and V15.04, respectively). All patients identified were included in the analysis. Records were reviewed for symptoms suggestive of an IgE-mediated allergic reaction (defined in the next section) after exposure to the seafood(s) and specific IgE testing, including both immediate hypersensitivity skin testing and serum-specific IgE testing.22 If multiple serum-specific IgE tests to different kinds of seafood were performed, the test was considered positive if any one of the multiple tests were positive for at least one seafood (crab, shrimp, lobster, or fish [≥ 3 mm greater than negative control]).
Definitions
IgE-mediated reactions including anaphylaxis were defined based on the current consensus definition.23 Respiratory symptoms included dyspnea, chest tightness, wheezing, cough, and throat swelling. Skin symptoms included urticaria, angioedema (AE), flushing, and pruritus. Eye/nasal symptoms consisted of sneezing, rhinorrhea, nasal congestion, nasal itching, ocular pruritus, and coryza. Gastrointestinal (GI) symptoms involved nausea, vomiting, mouth tingling, and/or itching. Vascular symptoms included syncope, headache, and/or dizziness/presyncope.
The severity of the reactions to seafood was also defined: mild reactions included cutaneous and/or mucosal symptoms only (not including AE); moderate reactions included AE, GI, and/or respiratory only or any three systems together; and severe reactions involved the cardiovascular system, neurological system, or any four systems together.
Statistical Analysis
The prevalence of patients with seafood allergy in the total clinic population and the total food allergy clinic population was determined. Demographics were compared with the general demographics of the individual clinics.
Further analyses were performed using SPSS (Statistics 17.0, IBM) and STATA statistical software (Stata Corp LP). Chi-square and Fisher's exact tests were applied to analyze categorical variables. Logistic regression and odds ratios were also performed. Analysis of patients with both shellfish and fish allergy or an unknown seafood allergy included both exclusion and consideration as a separate group.
RESULTS
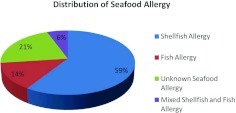
There were 5162 patients seen in the A/I clinics of the TMC in the 13 years between January 1, 1997 and January 30, 2010. Of these, 640 (12.4%) had physician-recorded food allergy. Of the A/I clinic patients with food allergy, 159 (24.8%) had seafood allergy, giving a prevalence of 3.08% in the total allergy clinic population. Nine seafood-allergic adults of 159 (5.66%) reported both shellfish and fish allergy. Fifty-nine percent were only allergic to shellfish (n = 94/159) with 13.8% (n = 22/159) allergic only to fish. Thirty-four (21.4%) had unspecified seafood allergy (Fig. 1). Of the entire A/I clinic population, 1.8% (94/5162) had only shellfish allergy, 0.43% (22/5162) had only fish allergy, 0.17% (9/5162) had combined shellfish/fish allergy, and 0.66% (34/5162) had unknown seafood allergy.
Figure 1.
Distribution of seafood allergy in 159 seafood-allergic patients.
Demographics
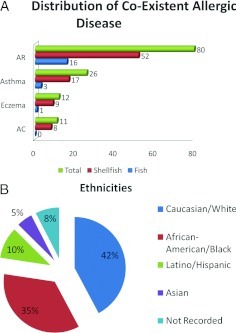
The average seafood-allergic patient age was 50.2 (range, 18–81 years) years old and there was no significant difference between the three clinics (VA, 52.7 years [range, 22–81 years]; BT, 47.5 years [range, 22–77 years]; Baylor Clinic, 48.5 years [range, 18–76 years]). The VA patient population made up 45% of the whole patient cohort and had a uniquely high female ratio of 29.6%. Seafood allergy was described slightly more by women (52%; n = 82) than by men (48%; n = 77). Of female seafood-allergic patients, 57.3% (n = 47) reported shellfish allergy, 13.4% (n = 11) had fish allergy, 6.1% (n = 5) reported both fish and shellfish allergy, and 23.2% (n = 19) were unspecified. Of male seafood-allergic patients, 61.0% (n = 47) reported shellfish allergy, 14.3% (n = 11) had fish allergy, 5.2% (n = 4) reported allergy to both fish and shellfish, and 19.5% (n = 15) were unspecified. Allergic rhinitis (51%), asthma (16.6%), and eczema (7.6%) were the most common allergic diseases in the cohort (Fig. 2 A). White and black American ethnicities were dominant (42 and 35%, respectively) with only 10 and 5% of the total number of patients representing Hispanic and Asian ethnic groups, respectively. The remaining patients had no ethnicity recorded (Fig. 2 B).
Figure 2.
(A) Number of seafood-allergic, shellfish-allergic, and fish-allergic individuals with coexistent atopic diseases including allergic rhinitis (AR), asthma, eczema, and allergic conjunctivitis (AC). (B) Ethnicity distribution of 159 seafood-allergic individuals.
Prevalence of Specific Seafood Allergies
Shellfish allergy (shellfish only and combined shellfish/fish-allergic individuals) was found in 2% of the total clinic population (n = 103/5162). Fish allergy (fish only and combined shellfish/fish-allergic individuals) was noted in 0.6% (n = 31/5162). Shellfish-allergic patients were significantly less likely than fish-allergic patients to report another nonseafood food allergy (p = 0.011).
In patients reporting one or more specific shellfish allergy (n = 69), shrimp (72.5%), crab (34.8%), and lobster (17.4%) were the most frequent allergens. Other reported shellfish included crawfish (14.5%), oyster (8.7%), clam (5.8%), scallop (2.9%), mussel (1.4%), squid (1.4%), and octopus (1.4%; Table 1). Of shellfish-allergic patients, 82.6% were allergic to crustaceans, 7.2% to mollusks, and 10.1% to crustaceans and mollusks. Of 64 crustacean-allergic subjects (including crustacean only and combined crustacean/mollusk-allergic individuals), 24 (37.5%) were allergic to more than one crustacean. Of 12 mollusk-allergic subjects (including mollusk only and combined crustacean/mollusk-allergic individuals), 2 (16.7%) were allergic to more than one mollusk. Twenty-seven shellfish-allergic patients reported reactions to multiple shellfish (39.1%; n = 27/69).
Table 1.
Number of patients reporting specific symptoms to specific shellfish
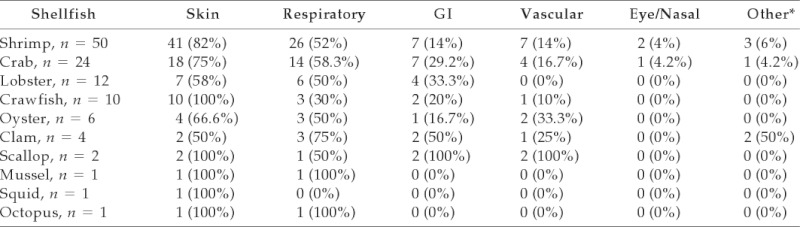
When looking at individual shellfish, skin and respiratory symptoms were most common overall.
*Other symptoms included diaphoresis, myalgia, agitation, and anxiety.
GI = gastrointestinal.
Of patients who reported one or more fish allergies (n = 21), tuna (28.6%), catfish (23.8%), and salmon (23.8%) were the most frequent allergens. Other reported fish included whitefish (14.3%), cod (9.52%), sardine (9.52%), soulfish (4.76%), trout (4.76%), snapper (4.76%), redfish (4.76%), and caviar (4.76%; Table 2). Seven fish-allergic patients reported allergic reactions to multiple fish (33.3%; n = 7/21).
Table 2.
Number of patients reporting specific symptoms to specific fish
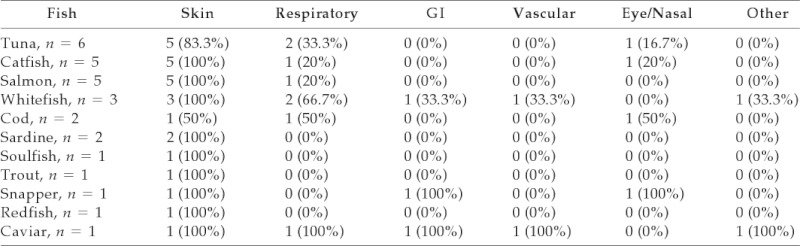
When looking at individual fish, skin symptoms were most common overall.
GI = gastrointestinal.
Clinical Symptoms of Seafood Allergy
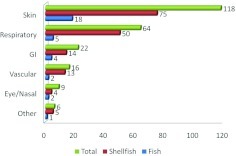
Skin, respiratory, and GI symptoms were most frequently recorded in allergic reactions (Fig. 3). The most frequent clinical manifestations of the 103 shellfish-allergic patients were skin symptoms (77.7%; n = 80). Respiratory symptoms were the second most common (50.5%; n = 52) and GI symptoms were third. Among 46 crab-, lobster-, and crawfish-allergic patients, 13 (28.3%) developed GI symptoms. Vascular symptoms were less common and were seen in 10–33% of patients with shellfish allergy and both scallop-allergic patients. Eye/nasal symptoms were the least common, occurring in 4% of shrimp- and crab-allergic patients (Table 1).
Figure 3.
Number of seafood-allergic, shellfish-allergic, and fish-allergic subjects reporting skin, respiratory, gastrointestinal (GI), vascular, eye/nasal, and other symptoms.
Fish-allergic patients reported skin manifestations most frequently (n = 23/24; 95.8%) with respiratory manifestations second most common (14/24 tuna-, salmon-, and whitefish-allergic patients). Sardine-, soulfish-, trout-, and redfish-allergic patients had no other organ involvement other than skin. The single patient with codfish allergy had skin, respiratory, and eye/nasal involvement. The snapper-allergic subject had skin, GI, and eye/nasal symptoms (Table 2).
Twenty-four of 50 shrimp-allergic patients (48%) had reactions involving more than one system. Reactions involving more than one organ system were also found in 54% (n = 13/24) crab-allergic, 50% (n = 6/12) lobster-allergic, 40% (n = 4/10) crawfish-allergic, 33.3% (n = 2/6) oyster-allergic, and 75% (n = 3/4) clam-allergic patients. Both scallop-allergic patients had more than one system involved. Each mussel- and octopus-allergic patient had more than one involved organ system. Fish-allergic individuals with tuna, salmon, whitefish, cod, snapper, and caviar allergies had symptoms involving more than one organ system.
The likelihood of having a severe reaction (47%) or anaphylaxis (32%) was not statistically different between shellfish- and fish-allergic subjects. Subjects with severe reactions were 12.9 times more likely to have the reaction in ≤60 minutes compared with those subjects who had nonsevere reactions (p = 0.002).
Chi-square analysis showed a significant difference in respiratory reactions between shellfish- (50%) and fish-allergic individuals (5%; p < 0.05). Logistic regression suggested that shellfish-allergic subjects were six times more likely to experience respiratory symptoms during a reaction than fish-allergic subjects, but this result only approached significance (p =0 .056). Seafood-allergic subjects with respiratory symptoms were 6.5 times more likely to experience that reaction in ≤60 minutes than subjects who did not experience a respiratory reaction (p = 0.021).
Specific IgE Testing
Sixty-eight percent of serum-specific IgE tests to seafood (n = 31) were positive. Of recorded positive tests, only 12 values were recorded (Table 3). In this limited number of patients with specific IgE testing, shellfish-allergic patients were more likely to have a positive specific IgE than fish-allergic patients or patients who were allergic to both fish and shellfish, but this finding only approached significance (p = 0.06). Interestingly, 100% of seafood-allergic patients who did not have skin symptoms had a positive specific IgE. Conversely, 58% of patients with skin symptoms had a positive specific IgE. Only 31% of seafood-allergic patients with vascular symptoms had a positive specific IgE.
Table 3.
Specific IgE to seafood
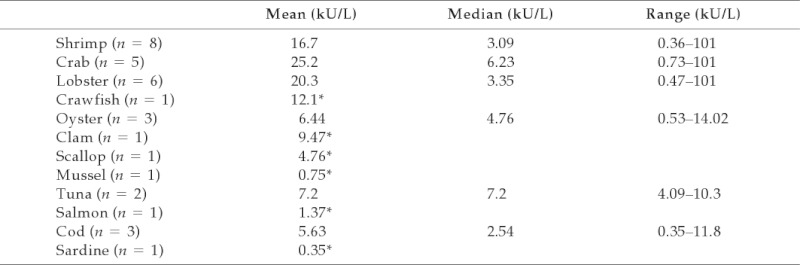
Mean, median, and ranges of specific IgE for seafood-allergic patients.
*Only one test result recorded.
DISCUSSION
Given the lack of detailed information about adult food allergy in the literature, the objectives of this study were to describe the clinical characteristics of seafood allergy in an adult U.S. allergy referral population. Most of the clinic patients came for primary allergy care so these results may be generalizable to other U.S. allergy referral populations.
Demographics
Women are reported to have more food allergies than men23–25 and we found that seafood allergy was also slightly more frequent in women in our cohort. There was an overrepresentation of female patients visiting the VA A/I Clinic (29.6%) for seafood allergy in our cohort compared with the number of females visiting VA A/I clinics in Los Angeles in 2005 (7.8%).26 This difference may be a result of study location. Nevertheless, our data support the finding that women are more likely to have seafood allergy than men. The average age of seafood allergy diagnosis was 50.2 years old, consistent with survey data reporting any seafood allergy in adults most prevalent between 41 and 60 years.17 This age represented the age at which the patient reported the allergy in the clinic.
Prevalences
Physician-recorded food allergy was present in 12.4% of this referral population. Self-reported doctor-diagnosed food allergy was found in 5.3% of adults in a recent food allergy survey study18 and Sampson et al. reported a food allergy prevalence of 3.5–4.0% of the adult population.26 Our data indicate a similar percentage of food allergy in the allergy referral population with physician-reported seafood allergy present in 3.08%. This is higher than a questionnaire-based study reporting 2.8% prevalence of seafood allergy in the general adult population.17 This prevalence of food and seafood allergy is not unexpected because these referral patients are more likely to have allergic disease. Our analyses may have underestimated the true prevalence in this population, however, given the retrospective nature of this study. Geographic location may also explain these differences, because the patients seen at TMC clinics live in the U.S. Gulf Coast with potentially higher exposure to seafood.24,26,27
A population-based questionnaire survey in a Western population in the Philippines and Singapore reported a prevalence of shellfish allergy as 3.22% in teenagers.28 The 2% prevalence of shellfish allergy in our cohort is consistent with prior data.17,18 The 1.24% prevalence of crustacean allergy in our cohort is higher than prior reports of 0.3% in the general adult population17 but the 0.6% prevalence of fish allergy in our cohort is consistent with earlier reports.17,18,27 In contrast to a smaller Asian cohort, our study population showed more allergies to fish than mollusks. Our population likely had many dietary and ethnic/cultural differences, explaining the reversal of frequency of mollusk and fish allergies compared with the cohort from Singapore.14 Although salmon has been reported as the major fish allergen, tuna allergy was the most frequently diagnosed in our cohort.17
Clinical Cross-Reactivities
In our cohort, 66.2% of seafood-allergic adults had an isolated seafood allergy. Shrimp was the most frequently reported allergic seafood, consistent with other studies.29–31 Our data showed that most shellfish-allergic individuals were only allergic to one specific shellfish (60.9%). This was also true for fish-allergic individuals being allergic to only one specific fish (71.4%). These data support the fact that many species-specific proteins can serve as potential allergens. Cross-reactivity seems to occur with multiple allergenic epitope recognition in adults.32 Waring et al. reported 50–100% of young adult shrimp-sensitive subjects reacted to more than one type of shellfish.26 These differences may be explained by the fact that our study has a much larger subject number and all shellfish-allergic patients are included in our cohort.
Of mollusk-allergic individuals, 16.7% were allergic to more than one mollusk. In a study by Sicherer et al.,17 49% of mollusk-allergic patients reported reactions to other mollusks. Our study is retrospective, which may account for the differences. However, allergenic protein cross-reactivities in mollusks needs further study to determine clinical relevance. In our cohort, 10.1% persons had a combined crustacean and mollusk hypersensitivity. Although crustaceans and mollusks share common epitopes in tropomyosin, the homologies between the two are not as high as those within the crustacean group and that within the mollusk group33; hence, a lower level of cross-reactivity between these two types of shellfish is not unexpected.
In our cohort, 29% fish-allergic subjects reacted to more than one fish. The literature describes the prevalence of fish cross-reactivity ranging from 50 to 67%.17,29,34 One possible reason for this discrepancy is the lack of food challenge in our cohort or the phenomenon of patient self-restriction after one reaction to one kind of fish. Six percent of subjects had both shellfish and fish allergy in our cohort, which is in general agreement with Borrego et al.29 who reported that shellfish-allergic patients have more likelihood of reactivity with other shellfish (75%) than fish-allergic patients (50%).31
Clinical Symptoms of Seafood Allergy
Skin manifestations such as urticaria and AE and respiratory symptoms were found to be the first and second most common manifestations of seafood allergy, respectively. This finding is in agreement with other studies.14,17,29,30,34 However, our data showed that respiratory reactions were six times more likely in shellfish-allergic than in fish-allergic patients and these symptoms occur more rapidly than nonrespiratory reactions. Severe seafood-allergic reactions are also more likely to occur rapidly after ingestion. Importantly, shellfish- and fish-allergic reactions did not differ with regard to risk of severity of reactions and anaphylaxis in our cohort.
Specific IgE Testing
There are no predictive values for serum-specific IgE to shrimp, crab, or lobster.7 However, our data suggest shellfish-allergic patients are more likely to have a positive specific IgE than fish-allergic patients or combined shellfish/fish-allergic patients. Standardized studies are necessary to determine cutoff values for a variety of seafood allergens.
Our study does have the distinct advantage of reporting the percentage and characteristics of seafood-allergic adults in a large diverse urban referral population. Strength of this study is shown in the description of specific characteristics of U.S. patients and their reactions to common and lesser-recognized seafood allergens. The limitations of our study included the retrospective design with the lack of specific coding for seafood allergy in some records, incomplete charting, and inconsistent specific IgE testing.
CONCLUSION
Seafood allergy was diagnosed in 159 (3.08%) adult patients from a 5162 allergy referral patient population. The average age of diagnosis was 50.2 years. Shellfish allergy was more common than fish and crustacean allergy was more common than mollusk. The three most common shellfish allergies were shrimp, crab, and lobster and the most common fish allergies were tuna, catfish, and salmon. Approximately one-third of seafood-allergic patients had reactions to more than one seafood. Shellfish- and fish-allergic subjects had the same likelihood of having a severe reaction or anaphylaxis. Severe reactions were 12.9 times more likely to occur within the 1st hour of ingestion compared with nonsevere reactions.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Broadhurst CL, Wang Y, Crawford MA, et al. Brain specific lipids from marine, lacustrine, or terrestrial food resources: Potential impact on early African Homo sapiens. Comp Biochem Physiol B Biochem Mol Biol 131:653–673, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Current Fishery Statistics No. 2008. Available online at www.noaanews.noaa.gov/stories2008/20080717_seafood.html; last accessed May 24, 2011
- 3. Lehrer SB, Ayuso R, Reese G. Seafood allergy and allergens: A review. Mar Biotechnol 5:339–348, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Taylor SL. Molluscan shellfish allergy. Adv Food Nutr Res 54:139–177, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Wood RA. The natural history of food allergy. Pediatrics 111:1631–1637, 2003 [PubMed] [Google Scholar]
- 6. Wild LG, Lehrer SB. Fish and shellfish allergy. Curr Allergy Asthma Rep 5:74–79, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Kandyil RM, Davis CM. Shellfish allergy in children. Pediatr Allergy Immunol 20:408–414, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Lopata AL, Lehrer SB. New insights into seafood allergy. Curr Opin Allergy Clin Immunol 9:270–277, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Daul CB, Morgan JE, Lehrer SB. The natural history of shrimp-specific immunity. J Allergy Clin Immunol 86:88–93, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Crespo JF, Pascual C, Burks AW, et al. Frequency of food allergy in a pediatric population from Spain. Pediatr Allergy Immunol 6:39–43, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Eggesbo M, Halvorsen R, Tambs K, Botten G. Prevalence of parentally perceived adverse reactions to food in young children. Pediatr Allergy Immunol 10:122–132, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Pumphrey RSH, Stanworth SJ. The clinical spectrum of anaphylaxis in north-west England. Clin Exp Allergy 26:1364–1370, 1996 [PubMed] [Google Scholar]
- 13. Kemp SF, Lockey RF, Wolf BL, Lieberman P. Anaphylaxis: A review of 266 cases. Arch Intern Med 155:1749–1754, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Thong BYH, Cheng YK, Leong KP, et al. Immediate food hypersensitivity among adults attending a clinical immunology/allergy centre in Singapore. Singapore Med J 48:234–240, 2007 [PubMed] [Google Scholar]
- 15. Dietrich JJ, Quinn JM, England RW. Reasons for outpatient consultation in allergy/immunology. Allergy Asthma Proc 30:69–74, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Woods RK, Abramson M, Bailey M, Walters EH. International prevalences of reported food allergies and intolerances. Comparisons arising from the European Community Respiratory Health Survey (ECRHS) 1991–1994. Eur J Clin Nutr 55:298–304, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the US determined by random telephone survey. J Allergy Clin Immunol 114:159–165, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Vierk KA, Koehler KM, Fein SB, Street DA. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol 119:1504–1510, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Jones RB, Hewson P, Kaminski ER. Referrals to a regional allergy clinic—An eleven year audit. BMC Public Health 29:10:790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: A meta-analysis. J Allergy Clin Immunol 120:638–646, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Lieberman JA, Sicherer SH. The diagnosis of food allergy. Am J Rhinol Allergy 24:439–443, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Available online at www.texasmedicalcenter.org/root/en/GetToKnow/FactsandFigures/Facts+and+Figures.htm; accessed May 24,2011
- 23. Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: Summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 117:391–397, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Ross MP, Ferguson M, Street D, et al. Analysis of food-allergic and anaphylactic events in the national electronic injury surveillance system. J Allergy Clin Immunol 121:166–171, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Gupta S, Klaustermeyer WB. Demographics and long-term follow-up in a Veterans Affairs allergy/immunology clinic: A 10-year analysis. Allergy Asthma Proc 26:310–315, 2005 [PubMed] [Google Scholar]
- 26. Sampson HA. Update on food allergy. J Allergy Clin Immunol 113:805–819, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Waring NP, Daul CB, deShazo RD, et al. Hypersensitivity reactions to ingested crustacean: Clinical evaluation and diagnostic studies in shrimp-sensitive individuals. J Allergy Clin Immunol 76:440–445, 1985 [DOI] [PubMed] [Google Scholar]
- 28. Shek LP, Cabrera-Morales EA, Soh SE, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol 126:324–331, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Castillo R, Carrilo T, Blanco C, et al. Shellfish hypersensitivity: Clinical and immunological characteristics. Allergol Immunopathol (Madr) 22:83–87, 1994 [PubMed] [Google Scholar]
- 30. Morgan JE, O'Neil CE, Daul CB, Lehrer SB. Species-specific shrimp allergens: RAST and RAST-inhibition studies. J Allergy Clin Immunol 83:1112–1117, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Torres-Borrego J, Martinez-Cuevas JF, Tejero-Garcia J. Cross reactivity between fish and shellfish. Allergol Immunopathol 31:146–151, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Ayuso R, Sánchez-Garcia S, Lin J, et al. Greater epitope recognition of shrimp allergens by children than by adults suggests that shrimp sensitization decreases with age. J Allergy Clin Immunol 125:1286–1293, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Leung PS, Chow WK, Duffey S, et al. IgE reactivity against a cross-reactive allergen in crustacean and mollusca: Evidence for tropomyosin as the common allergen. J Allergy Clin Immunol 98:954–961, 1996 [DOI] [PubMed] [Google Scholar]
- 34. American College of Allergy, Asthma, and Immunology Food allergy: A practice parameter. Ann Allergy Asthma Immunol 96:S1–S68, 2006 [PubMed] [Google Scholar]