Abstract
Inverted papilloma (IP) is a common benign tumor in the nose and sinus. Osteogenesis in sinonasal IP is extremely rare; to date, only five cases of IP with new bone formation appear in the literature. In addition, the mechanism of osteogenesis in IP remains unclear. Here, we describe three cases of IP with new bone formation and an investigation into a possible role for bone morphogenic protein (BMP) in osteogenesis. Of three patients with sinonasal IP with new bone formation, two were treated by endoscopic sinus surgery and one was followed up with watchful waiting. Tumor tissues were subjected to immunohistochemistry to detect BMP expression. The patients were successfully treated surgically and showed no evidence of recurrence postoperatively. Follow-up examination is ongoing. Immunohistochemically, the tumors expressed BMP-4 but not BMP-2 or BMP-7. ESS could be successfully used to achieve complete removal of the sinonasal IPs with new bone formation. BMP-4 might be associated with new bone formation in the tumor.
Keywords: Bone formation, bone morphogenic protein, endoscopic sinus surgery, inverted papilloma, sinonasal
Inverted papilloma (IP), a benign sinonasal tumor that is locally aggressive and destructive, tends to recur if incompletely removed and undergoes significant malignant changes. Bony thickening is often observed in the attachment of IPs1,2; however, osteogenesis in IP is extremely rare, and only five cases of IP with osteogenesis have been reported.3–5 In addition, the mechanism of osteogenesis in sinonasal IP remains unclear.
Bone morphogenic proteins (BMPs) are multifunctional growth factors belonging to the transforming growth factor β superfamily. It has been shown that BMPs are involved in the regulation of cell proliferation, survival, differentiation, and apoptosis.6,7 BMPs play a pivotal role in inducing formation of bone, cartilage, ligament, and tendon at both heterotopic and orthotopic sites. In this study, our objectives were to document three cases of IP with new bone formation and investigate a possible role of BMP in osteogenesis.
MATERIALS AND METHODS
Patients and Tissue Samples
Tumor tissues were obtained from patients with IP with new bone formation treated in our hospital.
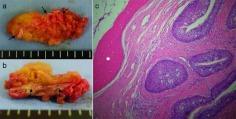
Case 1 was a 70-year-old woman who presented with a right nasal tumor that caused right nasal obstruction. Nasal endoscopy revealed a pinkish mass that filled the right nasal cavity. Enhanced computed tomography (CT) showed an inhomogeneously enhancing mass, which filled the right nasal cavity, with marked osteogenesis. The bone was thick and edged shaped (Fig. 1). Biopsy of the tumor was performed, and preoperative pathological examination revealed IP. Endoscopic sinus surgery (ESS) was selected based on the preoperative radiological findings and was performed under general anesthesia. We observed that the tumor had a broad attachment to the roof of the anterior ethmoid sinus. The tumor was thus successfully removed in one piece with the surgical margin free of disease. Newly generated bone tissue was surrounded within the tumor (Fig. 2). The procedure lasted 1 hour, and intraoperative blood loss was 20 mL. Histopathologically, papillomatous proliferation of tumor cells was observed and new bone formation consisting of randomly organized trabeculae lined by osteoblasts was observed in the tumor (Fig. 2). Thus, a definitive diagnosis of IP with new bone formation was established.
Figure 1.
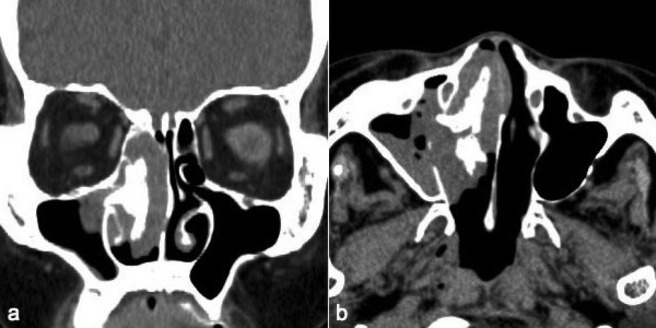
Case 1 showing (a) axial and (b) horizontal enhanced computed tomography (CT) with an inhomogeneously enhancing mass, which filled the right nasal cavity, with marked osteogenesis.
Figure 2.
Case 1. Macroscopic ([a] the whole; [b] the cross-section) and (c) microscopic (100× magnification) features of the excised tumor. Histological examination showed a papillomatous proliferation of tumor cells with new bone formation consisting of randomly organized trabeculae lined by osteoblasts. Black arrows and asterisk denote bone tissue.
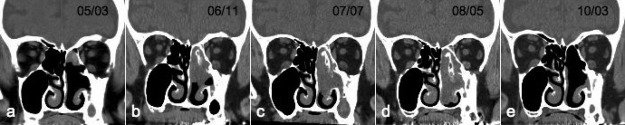
Case 2 was a 75-year-old man who had undergone ESS under local anesthesia with a preoperative diagnosis of a nasal polyp 5 years previously. Postoperative pathological examination revealed IP. The base of the tumor remained and the tumor gradually enlarged. Interestingly, CT showed new bone formation accompanying the tumor enlargement. As the tumor enlarged, the bone enlarged (Fig. 3). Although the patient had refused surgery for complete resection of the tumor after the first operation, the left nasal obstruction then worsened as the tumor enlarged: he agreed to the surgery finally in 2008, at which time he was successfully treated by ESS under general anesthesia. Postoperative histological examination revealed IP with new bone formation.
Figure 3.
Case 2. A series of axial computed tomography (CT) images over time showed a gradually enlarging tumor in the left nasal cavity. As the tumor enlarged, the bone enlarged. The patient was successfully treated by endoscopic sinus surgery. (a) March 2005, (b) November 2006, (c) July 2007, (d) May 2008, and (e) March 2010 (20 months postoperatively).
Case 3 was an 81-year-old man who presented with a left nasal tumor causing left nasal obstruction. Nasal endoscopy revealed a pinkish mass that filled the left nasal cavity and enhanced CT showed an inhomogeneously enhancing mass, which filled the left nasal cavity, with osteogenesis. The bone was a round shape, which was different from those of cases 1 and 2 (Fig. 4). Biopsy of the tumor revealed IP. Although ESS was recommended to the patient, he refused surgery. Follow-up examinations with watchful waiting have been continued for 24 months thus far. Although left nasal obstruction lasted, symptoms such as facial pain, swelling, and nasal bleeding, which was suspicious of malignancy, have not been shown.
Figure 4.
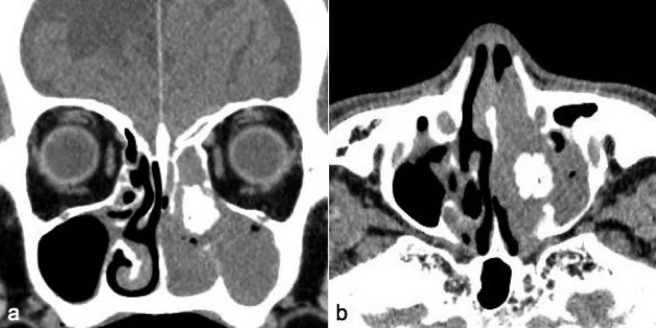
Case 3. (a) Axial and (b) horizontal enhanced computed tomography (CT) showed an inhomogeneously enhancing mass, which filled the left nasal cavity, with marked osteogenesis.
Samples that served as controls for BMP expression, consisting of five IPs without new bone formation (common type of IP) and five nasal polyps, were obtained from age- and sex-matched patients treated by ESS at our hospital during the same periods.
Immunohistochemistry
Rabbit polyclonal antibodies against human BMP-2, BMP-4 (LifeSpan BioSciences, Seattle, WA), and BMP-7 (AVIVA Systems Biology, San Diego, CA) were used for the detection of BMP expression in the IPs. IP tissues were fixed with 10% formalin and embedded in paraffin. Four-micrometer-thick paraffin sections were prepared for light microscopic examination. Specimens were dehydrated through a graded series of ethanol and treated with 1% hydrogen peroxide in absolute methanol for 30 minutes. Sections were exposed to Protein Block Serum-Free (Dako, Carpinteria, CA) for 5 minutes and then incubated with anti-human BMP-2, -4, and -7 antibodies (1:100 in 1% bovine serum albumin/phosphate-buffered saline [PBS]) for 24 hours. After rinsing with PBS, sections were incubated with biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for 1 hour. After they were rinsed with PBS, sections were incubated with ABC reagent (Vector Laboratories) for 30 minutes and developed in 0.05% 3,3′-diaminobenzidine-0.01% H2O2 substrate medium in 0.1 M of phosphate buffer for 8 minutes.
RESULTS
Clinical Outcome
In cases 1 and 2, the patients were surgically treated, the postoperative courses were uneventful, and recurrence was not observed at the 2-year follow-up examination. Case 3 has continued to be followed under a watchful waiting regimen. None of the cases showed any association with malignancy. All patients were diagnosed with IP with new bone formation based on the CT and histological findings.
Expression of BMP in IP
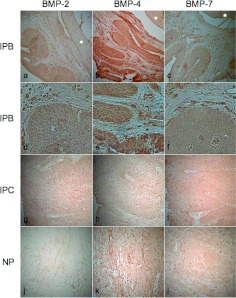
To further investigate the osteogenesis in the tumor, the expression of BMP was also examined. Interestingly, IP with bone formation expressed BMP-4 but not BMP-2 or -7. The tumor cells were homogeneously stained with BMP-4. IP without bone formation, a common type of IP, and nasal polyps were all stained negatively for BMP-2, -4, and -7 (Fig. 5; Table 1). These findings suggest that BMP-4 produced by IP tumor cells might be associated with new bone formation in the tumor.
Figure 5.
Immunohistochemistry for bone morphogenic protein (BMP; [a–c and g–l] 100× magnification; [d and f] 400× magnification). (a, d, g, and j) BMP-2, (b, e, h, and k) BMP-4, and (c, f, i, and l) BMP-7. IPB cells were strongly positively stained for BMP-4 and produced a negative result after staining for BMP-2 and BMP-7. IPB, inverted papilloma with new bone formation; IPC, inverted papilloma without bone formation (common type); NP, nasal polyp. *Bone tissue.
Table 1.
Expression of bone morphogenic protein (BMP)
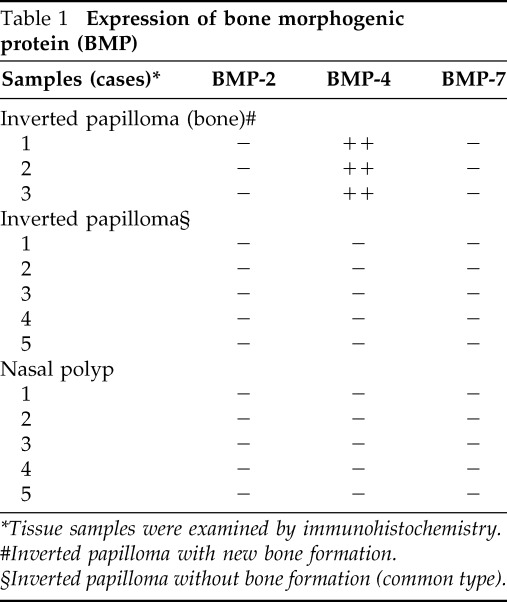
*Tissue samples were examined by immunohistochemistry.
#Inverted papilloma with new bone formation.
§Inverted papilloma without bone formation (common type).
DISCUSSION
IP with new bone formation is extremely rare: only five cases have been reported to date.3–5 In the present study, we document three additional cases of sinonasal IP with new bone formation, its clinical management, and a possible role for BMP in osteogenesis. Calcifications may be classified as entrapped bone structures or as primary tumoral calcifications. Essentially, entrapped bone is a bone fragment enclosed within the tumor that erodes because of pressure atrophy, whereas primary tumorous calcification is calcification created by the tumor itself. Both may appear as calcifications on CT.1,2,4 The histopathological examination revealed no entrapped bone or primary tumor calcification in the current bone structure. The examination showed new bone formation consisting of randomly organized trabeculae lined by osteoblasts. These randomly organized trabeculae were divided with prominent capillaries and mesenchymal cells. In general, however, the entrapped bone should be in a lamellar structure; however, in our case, the bone trabeculae were woven and represented active osteoblast production of newly formed bone. In addition, intraoperative findings showed that the newly generated bone in the tumor was independent of anatomically normal anatomic bone structure. Thus, the current cases were diagnosed as IP with new bone formation. Differential diagnosis for IP with new bone formation might be osteoma or fungal ball. A pathological examination by preoperative biopsy is important for the treatment.
Because IP is rare with accompanying new bone formation, the clinical difference between this disease and common IP is unclear. Aggressiveness has not been shown in the reported cases and in our cases. Generally, the treatment of choice for sinonasal IPs is surgery. To date, many series of sinonasal IPs have been reported,8–11 and different approaches, including lateral rhinotomy with external ethmoidectomy, the Caldwell-Luc approach, midfacial degloving, or ESS, have been used depending on tumor extension.9,10 ESS is now widely accepted and commonly performed in cases requiring nose or paranasal sinus surgery. It provides excellent magnification, illumination, and angled visualization, thereby allowing the surgeon to isolate the base of the tumor and accurately define the extent of disease. Among the five reported cases of IP with new bone formation, two were treated by ESS and three by external approaches. No recurrences have been observed in these cases.3–5 In our cases, two were successfully treated by ESS and one is being followed with watchful waiting. Long-term follow-up of our patients will help in the optimal clinical management of IP with new bone formation.
Another important finding in this report is the appearance of BMP expression in the tumor. This is the first report showing BMP expression in sinonasal IPs. BMPs belong to the transforming growth factor β superfamily that mediates a multitude of developmental processes in various tissues.6 This class of >20 proteins has been shown to have roles in cellular lineage commitment, differentiation, proliferation, patterning/morphogenesis, cellular maintenance/survival, and apoptosis.7,12 BMPs facilitate intramembranous and endochondral bone formation as well as formation of cartilage.13 Although numerous growth factors such as platelet-derived growth factor and vascular endothelial growth factor may be involved in new bone formation, available studies suggest only BMPs are capable of initiating the process.14 By induction differentiation of pluripotent progenitor cells along an osteogenic line, BMPs are able to stimulate osteogenesis at tissues distant from bone, a process termed “osteoinduction.”15 Extensive studies have indicatted that the BMPs with greatest osteogenic capacity are BMP-2, -4, -5, -6, -7, and -9.7,12 BMP-2 plays a key role in osteoblast differentiation, osteogenesis, and chondrogenesis. It is potentially a retinoid mediator and plays a possible role in apoptosis. BMP-2 is also involved in dorsoventral patterning, craniofacial development, and heart development.7 BMP-4 regulates the formation of teeth, limbs, lung, eye, and bone from mesoderm and also plays a role in fracture repair and is involved in dorsoventral patterning and craniofacial development.7 BMP-7 plays a crucial role in osteoblast differentiation, eye development, renal development/repair, and craniofacial development and may play a role in cerebral protection from ischemic stroke.7 In addition to their multifunctional roles in the regulation of cell proliferation, survival, differentiation, and apoptosis, BMPs have shown to be involved in tumorigenesis of various types of tumors by in vivo and in vitro studies.12 Expression of BMPs in tumor tissues has been reported.12,16 BMP-2/-4 was localized predominantly to the cytoplasm of malignant cells with primitive mesenchymal features; no or little BMP is detected in the more differentiated elements of bone and soft tissue sarcomas.16 Different levels of BMP-2/-4 expressions in bone and soft tissue sarcomas have been considered to be associated with the stage of mesenchymal differentiation. The ectopic formation of cartilage and bone mediated by BMP recapitulates the developmental processes that occur in the limb bud.17 BMP is synthesized as precursor molecule consisting of a signal peptide. Mature BMP is then secreted in an active form, with dimerization taking place either intracellulary or extracellulary on secretion. These active molecules bind to BMP type I and type II extracellular surface protein receptors, which have serine–threonine kinase activity. Mesenchymal stem cells have a number of BMP receptors, and through their action, BMPs become potent inducers of osteoblast differentiation from these cells, as shown in vitro.18 The process of endochondral bone formation is responsible for the majority of the limb skeleton. During this process, mesenchymal stem cells migrate via chemotaxis, condense, and then differentiate into chondrocytes, which, in turn, lay the extracellular matrix elements of cartilage. Invasion by osteoblasts, osteoclasts, blood vessels, and hematopoietic cells follows. The resulting cartilaginous matrix is then degraded and replaced by bone, completing the endochondral bone formation process.19 BMPs have been shown to facilitate this process, particularly at the mesenchymal stem cell chemotaxis and differentiation stage.19 In addition, BMPs induce proliferation and secretion of extracellular matrix elements in these cells. In this manner, BMPs may self-regulate via extracellular matrix–ligand binding in addition to their growth functions. The antagonism of BMP activity has particular importance during the osteogenic and chondrogenic embryonic developmental processes.20 Intramembranous bone formation, in which mesenchymal stem cells condense and differentiate into osteoblasts without the formation of a cartilaginous scaffold, is also facilitated by BMPs.20,21 In this manner, BMPs have extensive roles not only in mediating the development of the bone skeleton and its supporting structures, but also in craniofacial development.21 In the present study, IPs with new bone formation expressed BMP-4 but not BMP-2 or BMP-7. These findings suggest that BMP-4 might be involved in the osteogenesis in IPs. IP cells might induce new bone in the stroma via BMP-4/BMP receptor signaling.
In conclusion, we have described an additional three cases of sinonasal IP with new bone formation. ESS was successful in achieving complete removal of the tumor. BMP-4 might be associated with new bone formation in the tumor.
Footnotes
The authors have no conflicts to declare pertaining to this article
REFERENCES
- 1. Chiu AG, Jackman AH, Antunes MB, et al. Radiographicand histologic analysis of the bone underlying inverted papilloma. Laryngoscope 116:1617–1620, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Lee DK, Chung SK, Dhong HJ, et al. Focal hyperostosis on CT of sinonasal inverted papilloma as a predictor of tumor origin. Am J Neuroradiol 28:618–621, 2007 [PMC free article] [PubMed] [Google Scholar]
- 3. Dadas B, Ercan I, Basak T. Inverted papilloma with new bone formation in ethmoidofrontal region. Otolaryngol Head Neck Surg 134:343–344, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Unlu HH, Songu M, Ovali GY, Nese N. Inverted papilloma with new bone formation: Report of three cases. Am J Rhinol 21:607–610, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Tsuzuki K, Nishigami T, Takebayashi H, et al. Inverted papilloma with osteogenesis in the anterior ethmoid and frontal sinuses. J Laryngol Otol 124:230–233, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Hogan BL. Bone morphogenic proteins: Multifunctional regulators of vertebrate development. Genes Dev 10:1580–1594, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Xiao YT, Xiang LX, Shao JZ. Bone morphogenic protein. Biochem Biophys Res Commun 362:550–553, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Busquets JM, Hwang PH. Endoscopic resection of sinonasal inverted papilloma: A meta-analysis. Otolaryngol Head Neck Surg 134:476–482, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Tomenzoli D, Castelnuovo P, Pagella F, et al. Different endoscopic surgical strategies in the management of inverted papilloma of the sinonasal tract: Experience with 47 patients. Laryngoscope 114:193–200, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Krouse JH. Endoscopic treatment of inverted papilloma: Safety and efficacy. Am J Otolaryngol 22:87–99, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Wormald PJ, Ooi E, van Hasselt A, Nair S. Endoscopic removal of sinonasal inverted papilloma including endoscopic medial maxillectomy. Laryngoscope 113:867–873, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Thawani JP, Wang AC, Than KD, et al. Bone morphogenic proteins and cancer: Review of the literature. Neurosurgery 66:233–246, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Wang EA, Rosen V, D'Alessandeo JS, et al. Recombinant human bone morphogenic protein induces bone formation. Proc Natl Acad Sci USA 87:2220–2224, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wozney JM, Rosen V. Bone morphogenic protein and bone morphogenic protein gene family in bone formation and repair. Clin Orthop Relat Res 346:26–37, 1998 [PubMed] [Google Scholar]
- 15. Ludwig SC, Boden SD. Osteoinductive bone graft substitutes for spinal fusion: A basic science summary. Orthop Clin North Am 30:635–645, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Yoshikawa H, Rettig WJ, Lane JM, et al. Immunohistochemical detection of bone morphogenic proteins in bone and soft-tissue sarcomas. Cancer 74:842–847, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Reddi AH. Bone morphogenic proteins: An unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev 8:11–20, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Tsumaki N, Yoshikawa H. The role of bone morphogenic proteins in endochondral bone formation. Cytokine Growth Factor Rev 16:279–285, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci 1968:19–25, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hoffmann A, Gross G. BMP signaling pathways in cartilage and bone formation. Crit Rev Eukaryot Gene Expr 11:23–45, 2001 [PubMed] [Google Scholar]
- 21. Nie X, Luukko K, Kettunen P. BMP signaling in craniofacial development. Int J Dev Biol 50:511–521, 2006 [DOI] [PubMed] [Google Scholar]