Abstract
We have used oligonucleotides modified with biotin in the 5'-end as primers in the polymerase chain reaction (PCR)-amplification. This results in the synthesis of 5'-biotinylated DNA molecules, which are detected by hybridization to a labelled probe in solution. The formed hybrids are collected on an avidin-matrix by mediation of the biotin residue of the target molecules. The affinity-based hybrid collection method is quantitative and makes it possible to measure the amount of DNA produced in the PCR-amplification. At low concentrations of template the efficiency of the process is close to 100%, making it possible to detect the presence of a few molecules of target DNA in 25 cycles. With high template concentrations the efficiency of the process is low.
Full text
PDF



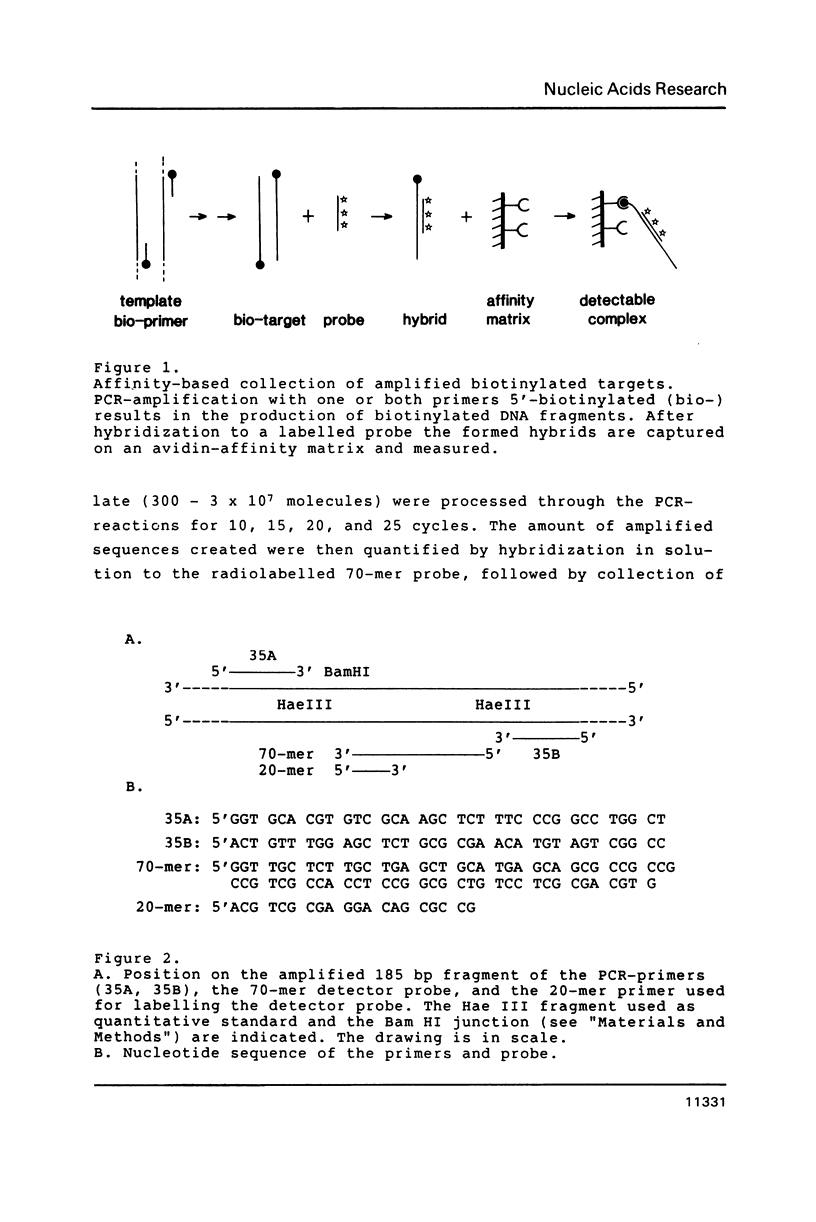
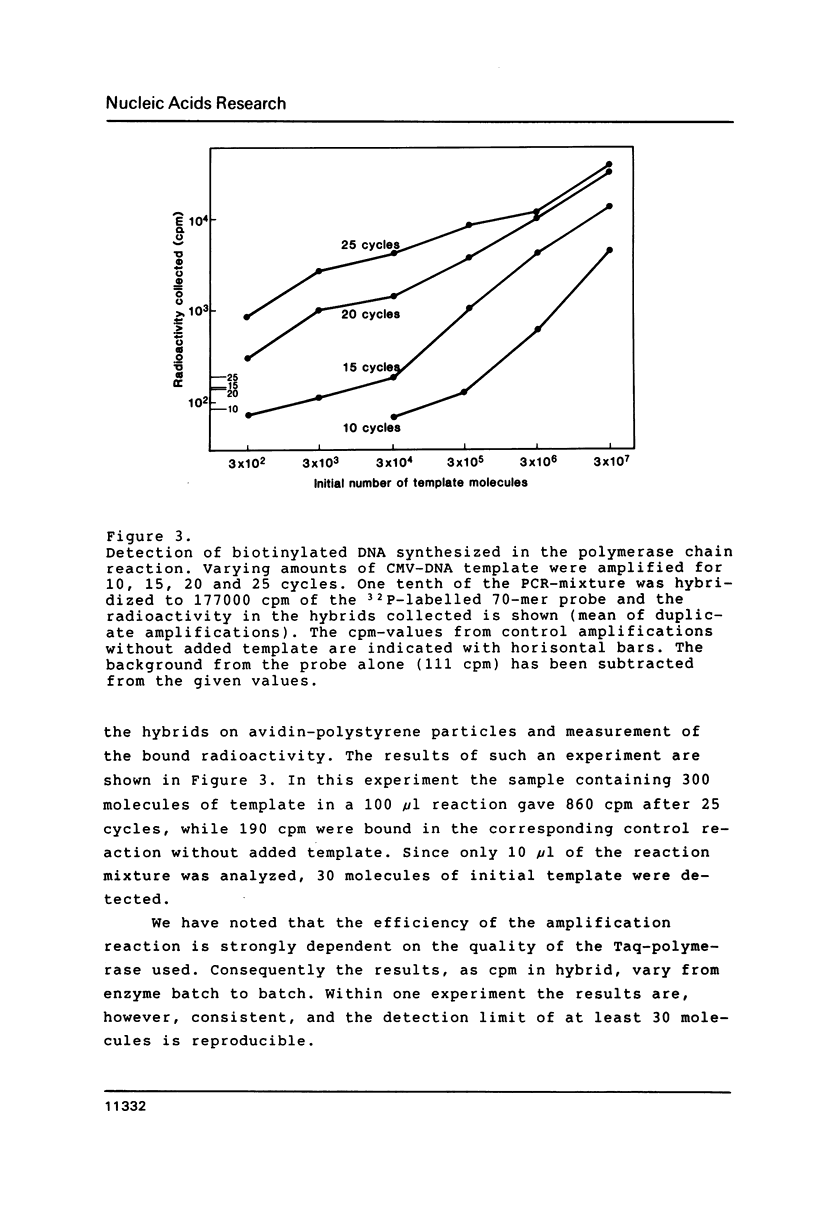

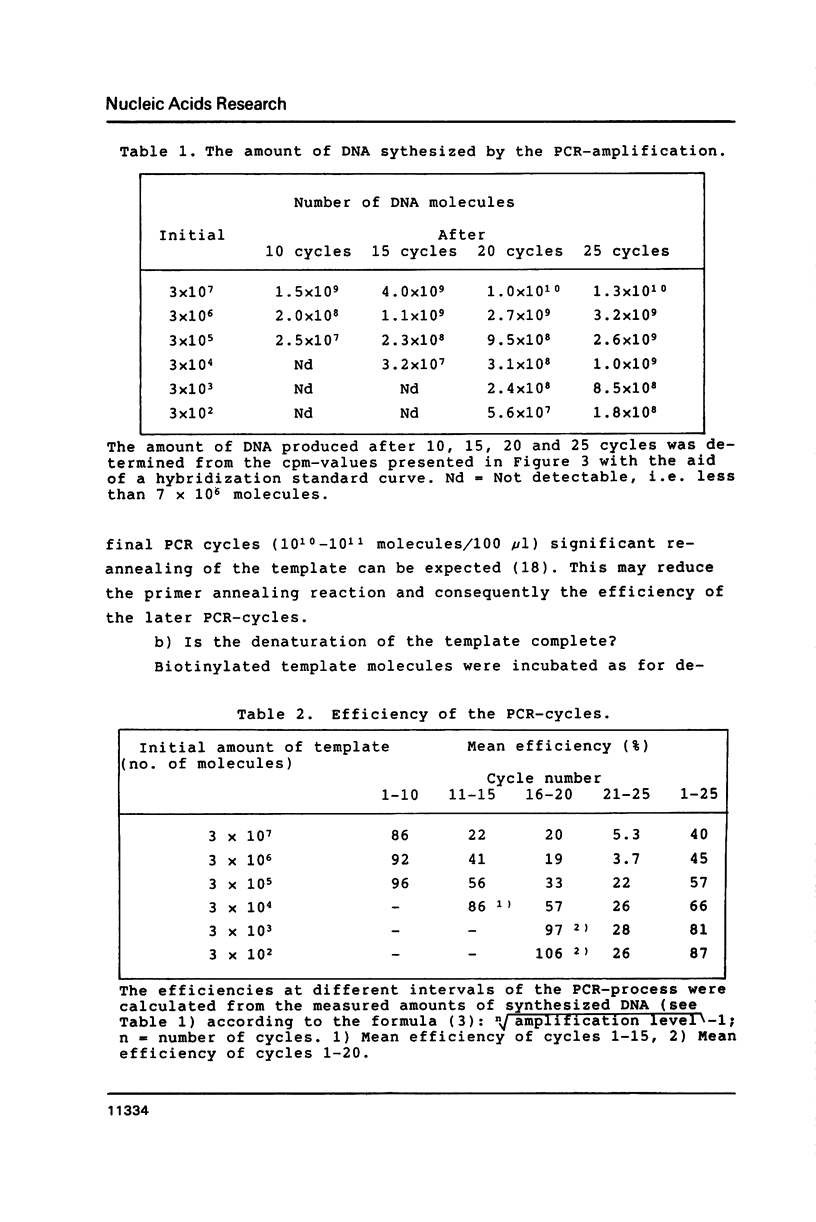
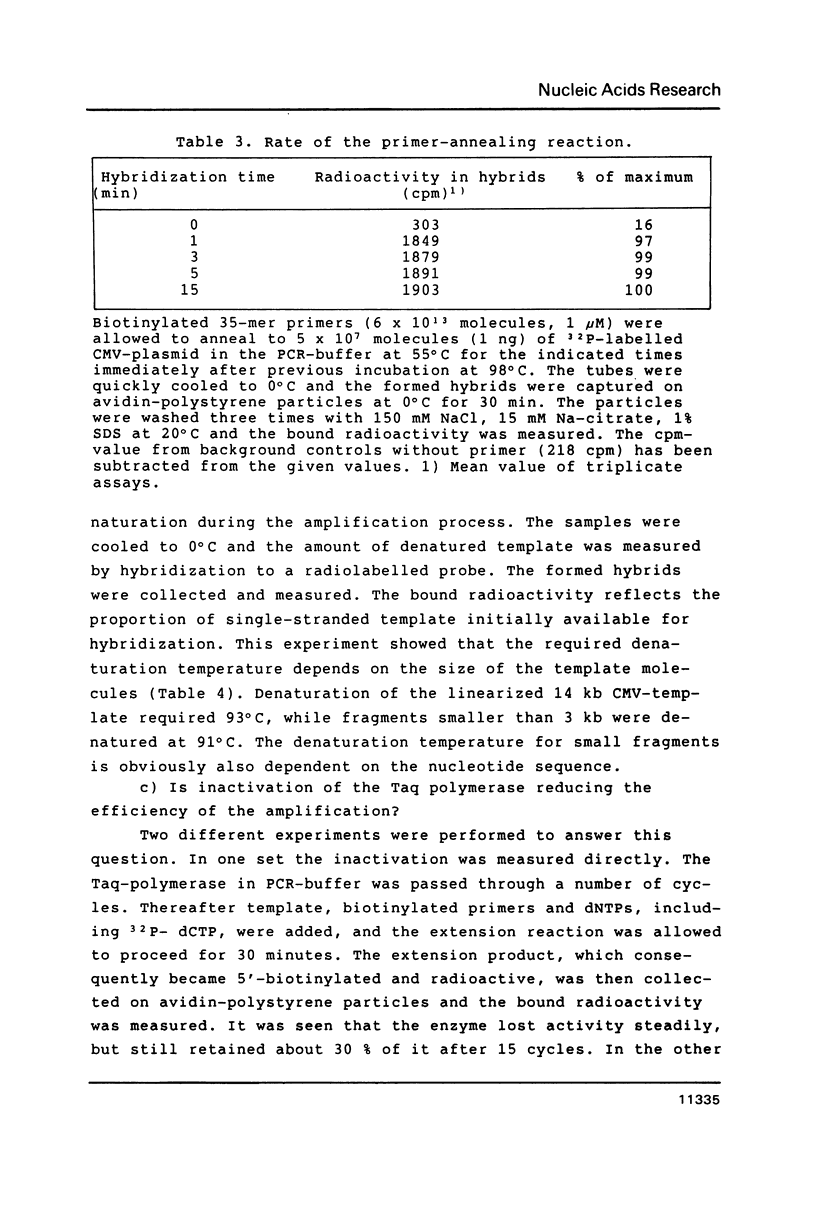


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin S., Popow-Kraupp T., Heinz F. X., Kunz C. Problems in detection of cytomegalovirus in urine samples by dot blot hybridization. J Clin Microbiol. 1987 Oct;25(10):1973–1977. doi: 10.1128/jcm.25.10.1973-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loche M., Mach B. Identification of HIV-infected seronegative individuals by a direct diagnostic test based on hybridisation to amplified viral DNA. Lancet. 1988 Aug 20;2(8608):418–421. doi: 10.1016/s0140-6736(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Murakawa G. J., Zaia J. A., Spallone P. A., Stephens D. A., Kaplan B. E., Wallace R. B., Rossi J. J. Direct detection of HIV-1 RNA from AIDS and ARC patient samples. DNA. 1988 May;7(4):287–295. doi: 10.1089/dna.1988.7.287. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Downing R. G., Akrigg A., Dollery A. A., Duggleby C. J., Wilkinson G. W., Greenaway P. J. Use of recombinant plasmids to investigate the structure of the human cytomegalovirus genome. J Gen Virol. 1982 Mar;59(Pt 1):111–129. doi: 10.1099/0022-1317-59-1-111. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Laaksonen M., Söderlund H. Fast quantification of nucleic acid hybrids by affinity-based hybrid collection. Nucleic Acids Res. 1986 Jun 25;14(12):5037–5048. doi: 10.1093/nar/14.12.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updyke T. V., Nicolson G. L. Immunoaffinity isolation of membrane antigens with biotinylated monoclonal antibodies and streptavidin-agarose. Methods Enzymol. 1986;121:717–725. doi: 10.1016/0076-6879(86)21070-8. [DOI] [PubMed] [Google Scholar]
- Virtanen M., Syvänen A. C., Oram J., Söderlund H., Ranki M. Cytomegalovirus in urine: detection of viral DNA by sandwich hybridization. J Clin Microbiol. 1984 Dec;20(6):1083–1088. doi: 10.1128/jcm.20.6.1083-1088.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



