Abstract
Invasive fungal sinusitis (IFS) is a highly aggressive infection that can affect hematologic patients. The classically described general risk factors, however, do not fully explain the development of IFS in a small percentage of cases. This study examined the impact of anatomic sinonasal factors and environmental factors on the development of IFS in high-risk patients. Medical records and computed tomography (CT) scans of patients admitted to our institution who were at high risk of developing IFS were retrospectively reviewed. Twenty-seven patients of 797 fulfilled the inclusion criteria. Patients affected by IFS were compared with patients not affected to identify possible sinonasal and environmental risk factors of IFS. Seven patients were excluded because of the lack of adequate radiological images. Six of the 20 eligible patients were assigned to the study group of patients affected by IFS and the remaining 14 patients were assigned to the control group. All but one case developed the infection during the summer with a significantly higher mean environmental temperature (p = 0.002). Anatomic nasal alterations were found in all patients affected by IFS and were significantly more frequent than in the control group (p = 0.014). It would be advisable to have patients with hematologic risk factors of IFS, especially during the summer period, undergo endoscopic nasal assessment. Furthermore, a CT finding of anatomic nasal alterations, such as anterior nasal septum deviation causing nasal obstruction, should increase the suspicion of IFS in case of the occurrence of nasal symptoms.
Keywords: fungal infection, hematologic malignancy, invasive fungal sinusitis, nasal endoscopy, sinonasal risk factors, sinus endoscopic surgery
Invasive fungal sinusitis (IFS) is extremely rare in immunocompetent individuals.1,2 IFS almost exclusively affects immunocompromised patients, such as those suffering of hematologic malignancy and undergoing allergenic bone marrow transplantation (BMT) with an incidence ranging from 1.7 to 2.6%.3,4 Moreover, IFS can be characterized by a rapid vascular spread from the nasal and sinus mucosa into the orbit, skull base, and brain.
Multiple fungal species have been identified in patients with IFS, in particular, Aspergillus spp. (flavus, fumigatus, terreus, and niger) and Zygomyces (Rhizomucor, Rhizopus, and Mucor).5,6 The most common classification was defined by de Shazo et al.7,8 and it groups IFS into three forms: granulomatous invasive, chronic invasive, and acute fulminant. The severity of immunodepression appears to be related to the clinical presentation and the rapidity of the disease progression. In hematologic patients, the most frequent presentation is the acute fulminant form.8
Surgery combined with antifungal therapy has been shown to be the gold standard of IFS treatment,4,5,9 but mortality still remains elevated (47–80%).9–11 Because of the rapid progression of the disease, it is commonly accepted that promptness of treatment affects outcome,9,12 but diagnosis still requires histopathological evidence of fungal invasion of the nasal tissue.5 Hence, the rapidity of disease progression makes clinical, endoscopic, and radiologic data fundamental diagnostic tools to initiate prompt treatment. On the other hand, radiological findings specific for IFS, such as bone destruction, occur late in the course of the disease. Some authors have recently advocated the use of nasal endoscopy5,10,12 and frozen sections13 as a highly predictive diagnostic tool.
Because of the rarity of this disease, it is difficult to define proper treatment. Furthermore, the lack of knowledge regarding the pathogenesis of IFS explains the limited effectiveness of methods of prevention.
Although fungi are generally ubiquitous,14,15 only a small fraction of hematologic patients develop IFS. For this reason, the authors tried to point out possible causes present at the level of the sinonasal tract that are capable of fostering the development of IFS.
PATIENTS AND METHODS
Medical records of patients at high risk of developing IFS (acute myeloid leukemia, acute lymphoblastic leukemia, severe aplastic anemia, and allogeneic BMT) and admitted to the Institute of Hematology and Clinical Oncology “Seràgnoli,” Sant'Orsola-Malpighi Hospital, University of Bologna, Italy, between December 2005 and August 2009 were retrospectively reviewed. Twenty-seven of 797 patients (3.4%) fulfilled the inclusion criteria: presence of signs and symptoms possibly related to IFS and/or severe and prolonged neutropenia (<500/mm3, >10 days) and/or long-term corticosteroid therapy (0.5 mg/kg per day for >30 days). Patients affected by IFS (histopathological evidence) were assigned to the study group and patients without sinonasal fungal infection were assigned to the control group. Patients without a sinus computed tomography (CT) scan were excluded.
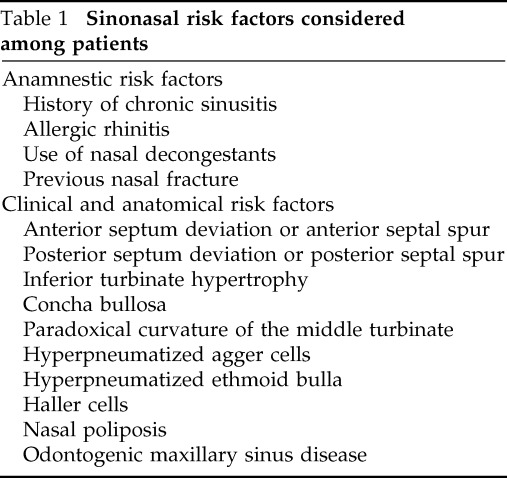
Previous sinus disease, allergic rhinitis, anatomic features predisposing nasal obstruction, and rhinosinusitis were considered local risk factors (Table 1). All anatomic predisposing factors were defined by radiological (CT) assessment. Septum deviation was classified in the anterior (deviation peak in the cartilage septum) and posterior (deviation peak in the bony septum). The septum deviation was considered clinically relevant in case of (1) medical history of nasal obstruction with clinical and radiological evidence of septum deviation and/or (2) ostiomeatal complex obstruction related to a septal deviation (or spur). Moreover, the environmental features (i.e., temperature and humidity) and the period of the year in which the infection started were considered general risk factors. In particular, the authors collected data regarding the average daily mean temperature, minimum and maximum temperatures, and the average daily mean relative humidity retrieved from the Regional Meteorological Service of Agenzia Regionale Prevenzione e Ambiente Emilia Romagna, Italy, including the 2 weeks preceding the occurrence of symptoms. This time cutoff was set considering all patients included to be exposed to environmental conditions before their hospital admission and symptoms development.
Table 1.
Sinonasal risk factors considered among patients
In all cases, the diagnosis of IFS was confirmed by histopathological examination of biopsy specimens. This group of IFS patients (6 cases) was compared with the case control group (14 cases), which, although affected by acute lymphoblastic leukemia, acute myeloid leukemia, BMT, and severe aplastic anemia and suffering from nasal symptoms such as nasal obstruction, rhinorrhea, facial pain, and pressure, did not develop IFS.
In all patients, the treatment consisted of surgery and systemic antifungal therapy. Surgery was characterized by transnasal endoscopic debridement of the necrotic tissue until healthy and bleeding tissue was reached. The study was approved by the Ethical Committee of our institution.
Statistical Analysis
Statistical analysis was performed using SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL). The chi-squared test or the Fisher's exact test was applied, when appropriate, to evaluate the statistical significance of the association between the categorical variables. The odds ratio (OR) was obtained for statistically significant risk factors by means of a 2 × 2 contingency table. The data regarding temperature and humidity did not follow a normal distribution function and, therefore, we used the Mann-Whitney U test to detect significant differences between the two groups. A value of p < 0.05 was regarded as statistically significant. The confidence intervals (CIs) were set at a 95% level.
RESULTS
We retrospectively reviewed 27 patients presenting nasal symptoms possibly related to rhinosinusitis who were admitted in the Hematology Unit. Seven patients were excluded due to the lack of radiological images. Six of the 20 eligible patients were assigned to the study group resulting to be affected by IFS and the remaining 14 patients were assigned to the control group.
The study group consisted of one woman (16.7%) and five men (83.3%) having a mean age of 49.3 years (range, 28–69 years) as is shown in Table 2. The etiologic agents were Zygomycetes in three (50%) patients, Aspergillus spp. in two patients (35%) and Fusarium solanii in one patient (15%). IFS began as acute IFS in five patients and one case started as the granulomatous invasive form.
Table 2.
Study group characteristics
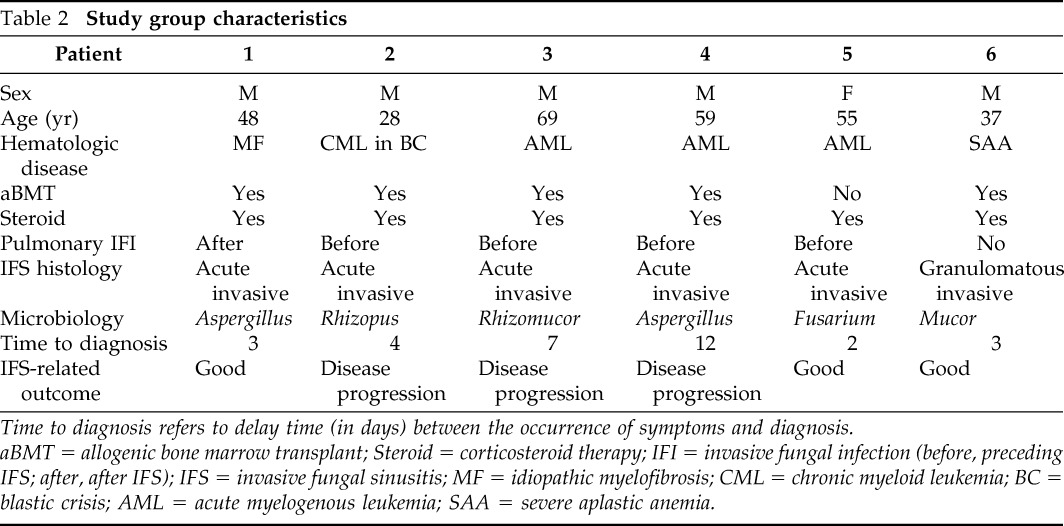
Time to diagnosis refers to delay time (in days) between the occurrence of symptoms and diagnosis.
aBMT = allogenic bone marrow transplant; Steroid = corticosteroid therapy; IFI = invasive fungal infection (before, preceding IFS; after, after IFS); IFS = invasive fungal sinusitis; MF = idiopathic myelofibrosis; CML = chronic myeloid leukemia; BC = blastic crisis; AML = acute myelogenous leukemia; SAA = severe aplastic anemia.
General predisposing factors in all patients of the IFS group were severe neutropenia (five patients underwent allogenic BMT and one had recurrent acute myelogenous leukemia unresponsive to treatment) and high-risk corticosteroid therapy that ranged from 9 to 34 days (mean duration of 16 days) before the IFS development. None of the patients had diabetes. Five patients had a concomitant invasive fungal pulmonary infection. Of these, four cases had developed a pulmonary infection before IFS and, in one case, it had developed after IFS. Previous pulmonary infections had been successfully treated in three cases before IFS occurrence.
The main symptoms of IFS were characterized by nasal obstruction (four cases), headache (three cases), and retro-orbital pain (two cases), and the diagnosis was generally achieved with a mean delay of 5.2 days (range, 2–12 days). Preoperative diagnosis was performed in all patients by a transnasal endoscopy and radiological assessment with a CT scan (Figs. 1 and 2). Microbiological assessment was performed in all cases using a nasal swab but only case 5 (Table 2) had a positive result (Fusarium spp.).
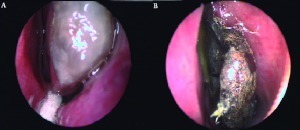
Figure 1.
(A) Endoscopic view of the left middle turbinate with invasive fungal infection (Fusarium spp) involving the head of the turbinate (patient 5). (B) Endoscopic view of invasive fungal infection (Rhizomucor) involving the left middle turbinate, having a necrotic appearance (patient 3).
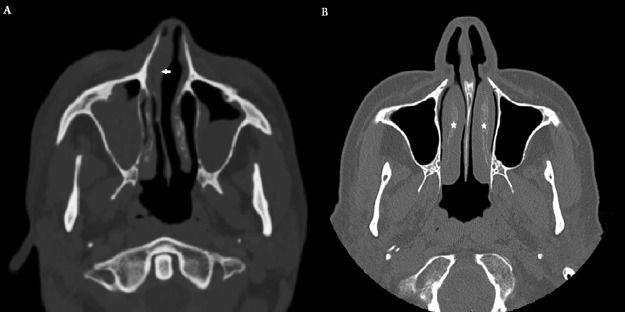
Figure 2.
(A) Computed tomography (CT) scan of a patient affected by right invasive fungal infection with notable anterior septum deviation (arrow) causing nasal obstruction. (B) CT scan of a control group patient (not affected by IFS), complaining of symptoms of nasal obstruction. Nasal obstruction was related to bilateral inferior turbinate hypertrophy (stars). Note the absence of nasal septum deviation.
All but case 4 developed the infection over the summer. The average daily mean temperature and humidity of the 15 days preceding the infection are reported in Table 3. All patients of the IFS group were exposed to outdoor environmental conditions during the 15 days before the symptoms occurrence.
Table 3.
Sinonasal and environmental risk factors
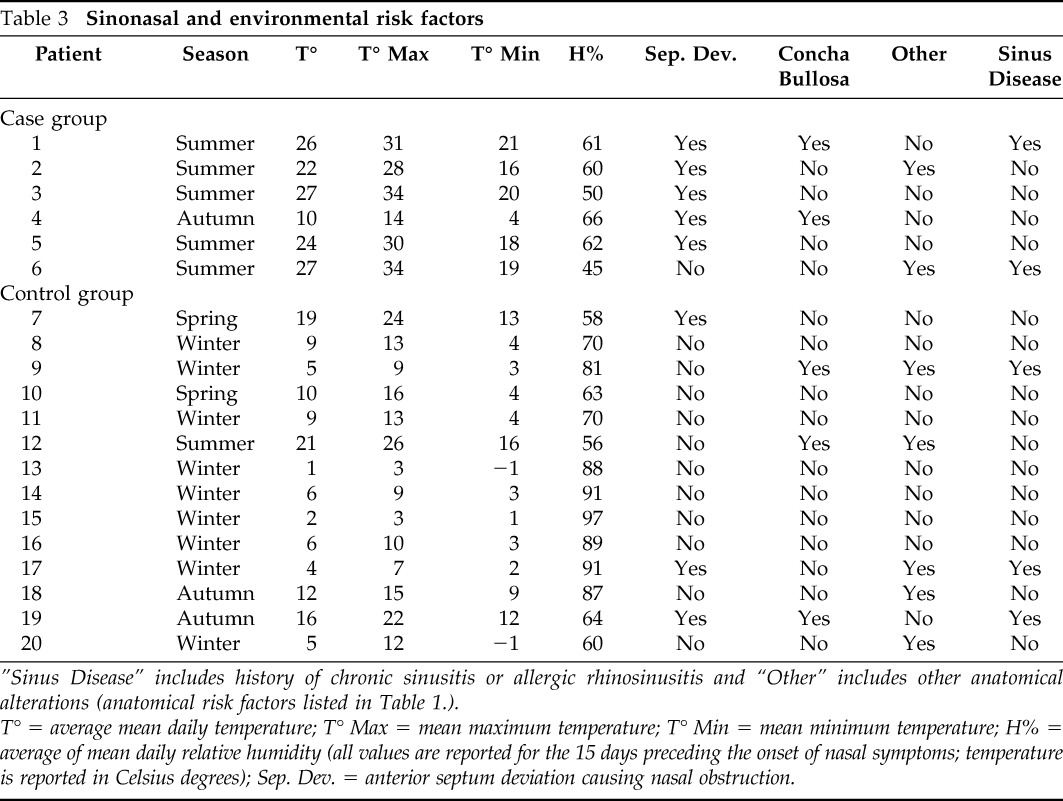
”Sinus Disease” includes history of chronic sinusitis or allergic rhinosinusitis and “Other” includes other anatomical alterations (anatomical risk factors listed in Table 1.).
T° = average mean daily temperature; T° Max = mean maximum temperature; T° Min = mean minimum temperature; H% = average of mean daily relative humidity (all values are reported for the 15 days preceding the onset of nasal symptoms; temperature is reported in Celsius degrees); Sep. Dev. = anterior septum deviation causing nasal obstruction.
Anatomic nasal alterations were found in all patients affected by IFS (Table 3). Anterior nasal septum deviation causing nasal or sinus obstruction was found in all but one case. The possible initial location of the disease could be identified in three cases at the level of the head of the middle turbinate (Fig. 1 A). In these three cases, a positive association between the side in which the disease started and the side of the anatomic nasal alteration was observed.
All patients were treated surgically and by antifungal therapy. The surgical treatment was characterized by a transnasal endoscopic approach, which permitted resection of the anatomic structures presenting necrosis until healthy tissue was reached. Case 6 underwent a combined transnasal endoscopic–external approach (orbital exenteration) to remove diseased tissue located at the level of either the lateral wall or the orbit.
Disease-free survival was 50% (three patients died due to progression of IFS). However, the overall survival rate was 16.7% (1/6). Patients who were diagnosed before the 4th day after the occurrence of symptoms had a good disease-related outcome. All patients who were diagnosed on the 4th day or later died due to progression of IFS.
The control group included seven men (50%) and seven women (50%) with a mean age of 41.4 years (range, 22–57 years). General predisposing factors for invasive fungal infection were severe neutropenia in all patients and long-term corticosteroid therapy in 12 cases (85.4%). Three of 14 cases (21%) had a possible or probable pulmonary fungal invasive infection, successfully treated with specific antifungal therapy in all patients.
The nasal symptoms reported in this group were nasal obstruction in 14 cases (100%), rhinorrhea in 4 cases (28.6%), and headache in 4 cases (28.6%). In all cases, a trans-nasal endoscopy and CT were performed but no evidence of disease was found. Symptoms occurred in nine cases (64.3%) during the winter, in two cases (14.3%) during the spring, in two cases (14.3%) during the autumn, and only one case (7.1%) during the summer. All of the 14 patients of the control group were exposed to outdoor environmental conditions during the 15 days before the symptom occurrence. The average temperature and humidity of the 15 days preceding the nasal symptoms and the anatomic nasal alterations noted are reported in Table 3.
Comparison between the groups showed a significant association of anterior septum deviation causing nasal obstruction and IFS (p = 0.018; OR, 18.3; CI, 17.3–19.4), and other nasal anatomic alterations and previous sinus disease did not reach statistical significance. Age and previous pulmonary fungal invasive infection were not significantly associated with IFS (p = 0.17 and p = 0.12, respectively). Summer was significantly associated with IFS (p = 0.002; OR, 65.0; CI, 63.1–66.9). There was a significant difference between average daily mean temperatures (p = 0.002), average maximum temperatures (p = 0.002), and average minimum temperatures (p = 0.003) of the 15 days preceding the infection in the two groups. The average mean humidity did not show a significant difference between the two groups (p = 0.15).
DISCUSSION
IFS is a rare infection having a poor prognosis, especially in severe immunocompromised patients. General predisposing factors are poorly controlled diabetes, severe neutropenia (<500/mm3) with a duration of neutropenia of >10 days, and long-term corticotherapy.5,11,14
Severe neutropenia and long-term corticosteroid therapy frequently seen in hematologic disease render these patients more prone to IFS in comparison with the chronic and granulomatous IFS forms, which are generally more frequently observed in diabetic patients.8 Common antifungal therapy is generally insufficient for controlling an aggressive disease course. Therefore, methods of prevention should be improved by the identification of potential risk factors. In our series, disease-related survival was improved in those patients receiving an early diagnosis whereas it was poor (100% related mortality) in those receiving a delayed diagnosis, delayed treatment, and suffering from nasal symptoms for at least 4 days.
Previously described general risk factors, however, do not fully explain the development of IFS in only a small percentage of cases. In our study, we considered either anatomic sinonasal factors, classically described as predisposing for sinusitis, or environmental factors that probably contributed to fungal growth. Our results showed a greater incidence of anatomic nasal alterations observed at CT scans in the IFS group, with a statistically significant difference between the groups (p = 0.014). In particular, anterior septum deviation causing nasal or sinus obstruction was the primary nasal alteration among the anatomic alterations considered and was the only one significantly associated with IFS (p = 0.018; Fig. 2).
Several studies have shown a seasonal variation in outdoor and indoor air concentration of airborne fungal spores during the summer period, such as those of Aspergillus species (A. terreus, A. flavus, and A. niger),15–18 Fusarium species,14,15 and Mucor species.17,19 Furthermore, other authors20,21 have found a positive correlation between average temperatures and airborne spore concentration. Epidemiological studies19,22 confirmed an increased seasonal rate of occurrence of IFS during the summer caused by the Mucor species. Finally, a relationship between environmental fungal concentration and incidence of invasive fungal infections in hematologic patients has been noted.23–25
The average daily mean temperature and mean maximum and mean minimum temperatures of the 15 days preceding the occurrence of symptoms was greater in the IFS group with a statistically significant difference between the groups (p = 0.002, p = 0.002 and p = 0.003, respectively). Our findings, such as the greater incidence of IFS during the summer with significantly superior mean temperatures in comparison with the other group, may support the hypothesis of an association between IFS development and exposure to environmental conditions, such as mean and maximum temperature.
Nasal function consists of humidifying and warming the air during inspiration and also in filtering the larger particles, such as airborne fungal spores.26 Although the fluid dynamics of the nasal cavity are not completely known, some studies have pointed out that the greatest airflow and the greatest air turbulence during normal inspiration develop in the limited area between the nasal septum and middle turbinate.26–28 Consequently, this area may be the initial point of accumulation of the fungal spores. This hypothesis could explain the finding of a middle turbinate involvement at the beginning of the IFS course. Furthermore, some anatomic anomalies, such as anterior septum deviation, could create or increase an airflow turbulence facilitating fungal spore deposition.28,29 Finally, the associated nasal obstruction leads to impaired ventilation with consequent inflammation and alteration of mucociliary clearance.26,27 The association between the site of the infection and the site of the anatomic alteration in all of the cases in our study is another finding that supports this hypothesis and the unilaterality at the onset of IFS.
CONCLUSION
In conclusion, it would be advisable to have the patients with hematologic risk factors of IFS, especially during the summer, undergo endoscopic nasal assessment. Furthermore, a CT finding of anatomic nasal alterations, such as nasal septum deviation causing nasal obstruction, should increase the suspicion of IFS in case of the occurrence of nasal symptoms. However, our retrospective study was based on a small group of patients because of the rarity of the disease. A multicentric cohort study should be performed to confirm our findings.
ACKNOWLEDGMENTS
The authors thank the Regional Meteorological Service of Agenzia Regionale Prevenzione e Ambiente Emilia Romagna, Italy for the supplied data.
Footnotes
The authors have no conflicts to declare pertaining to this article
REFERENCES
- 1. Chopra H, Dua K, Malhotra V, et al. Invasive fungal sinusitis of isolated sphenoid sinus in immunocompetent subjects. Mycoses 49:30–36, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Gupta AK, Ghosh S, Gupta AK. Sinonasal aspergillosis in immunocompetent Indian children: An eight-year experience. Mycoses 46:455–461, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Drakos PE, Nagler A, Or R, et al. Invasive fungal sinusitis in patients undergoing bone marrow transplantation. Bone Marrow Transplant 12:203–208, 1993 [PubMed] [Google Scholar]
- 4. Kennedy CA, Adams GL, Neglia JP, et al. Impact of surgical treatment on paranasal fungal infections in bone marrow transplant patients. Otolaryngol Head Neck Surg 116:610–616, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Shubert MS. Fungal rhinosinusitis: Diagnosis and therapy. Curr Allergy Asthma Rep 1:268–276, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Michael RC, Michael JS, Ashbee RH, et al. Mycological profile of fungal sinusitis: An audit of specimens over a 7-year period in a tertiary care hospital in Tamil Nadu. Ind J Pathol Microbiol 51:493–496, 2008 [DOI] [PubMed] [Google Scholar]
- 7. deShazo RD, Chaplin K, Swain RE. Fungal sinusitis. N Engl J Med 337:254–259, 1997 [DOI] [PubMed] [Google Scholar]
- 8. deShazo RD. Syndromes of invasive fungal sinusitis. Med Mycol 47(suppl 1):S309–S314, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Hachem RY, Boktour MR, Hanna HA, et al. Sinus surgery combined with antifungal therapy is effective in the treatment of invasive Aspergillus sinusitis in neutropenic patients with cancer. Infection 36:539–542, 2008 [DOI] [PubMed] [Google Scholar]
- 10. DelGaudio JM, Swain RE, Jr, Kingdom TT, et al. Computed tomographic findings in patients with invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg 129:236–240, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Epstein VA, Kern RC. Invasive fungal sinusitis and complications of rhinosinusitis. Otolaryngol Clin North Am 41:497–524, 2008 [DOI] [PubMed] [Google Scholar]
- 12. DelGaudio JM, Clemson LA. An early detection protocol for invasive fungal sinusitis in neutropenic patients successfully reduces extent of disease at presentation and long term morbidity. Laryngoscope 119:180–183, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Ghadiali MT, Deckard NA, Farooq U, et al. Frozen-section biopsy analysis for acute invasive fungal rhinosinusitis. Otolaryngol Head Neck Surg 136:714–719, 2007 [DOI] [PubMed] [Google Scholar]
- 14. VandenBergh MFQ, Verweij PE, Voss A. Epidemiology of nosocomial fungal infections: Invasive aspergillosis and the environment. Diagn Microbiol Infect Dis 34:221–227, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Aydogdu H, Asan A. Airborne fungi in child day care centers in Edirne City, Turkey. Environ Monit Assess 147:423–444, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fang ZG, Ouyang ZY, Hu LF, et al. Culturable airborne fungi in outdoor environments in Beijing, China. Sci Total Environ 350:47–58, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Lee T, Grinshpun SA, Kim KY, et al. Relationship between indoor and outdoor airborne fungal spores, pollen, and (1fi3)-b-d-glucan in homes without visible mold growth. Aerobiologia 22:227–236, 2006 [Google Scholar]
- 18. Herbarth O, Schlink U, Müller A, et al. Spatiotemporal distribution of airborne mould spores in apartments. Mycol Res 107:1361–1371, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Al-Ajam MR, Bizri AR, Mokhbat J, et al. Mucormycosis in the Eastern Mediterranean: A seasonal disease. Epidemiol Infect 134:341–346, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren P, Jankun TM, Belanger K, et al. The relation between fungal propagules in indoor air and home characteristics. Allergy 56:419–424, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kalyoncu F. Relationship between airborne fungal allergens and meteorological factors in Manisa City, Turkey. Environ Monit Assess 165:553–558, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Shpitzer T, Keller N, Wolf M, et al. Seasonal variations in rhino-cerebral mucor infections. Ann Otol Rhinol Laryngol 114:695–698, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Alberti C, Bouakline A, Ribaud P, et al. Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J Hosp Infect 48:198–206, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Leenders A, Van Belkum A, Behrendt M, et al. Density and molecular epidemiology of Aspergillus in air and relationship to outbreaks of Aspergillus infection. J Clin Microbiol 37:1752–1757, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pini G, Donato R, Faggi E, et al. Two years of a fungal aerobiocontamination survey in a Florentine haematology ward. Eur J Epidemiol 19:693–698, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Wolf M, Naftali S, Schroter RC, et al. Air-conditioning characteristics of the human nose. J Laryngol Otol 118:87–92, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Zhao K, Dalton P. The way the wind blows: Implications of modelling of the nasal airflow. Curr Allergy Asthma Rep 7:117–125, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Grützenmacher S, Robinson DM, Gräfe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—A model study. ORL J Otorhinolaryngol Relat Spec 68:199–205, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Garcia GJ, Tewksbury EW, Wong BA, et al. Interindividual variability in nasal filtration as a function of nasal cavity geometry. J Aerosol Med Pulm Drug Deliv 22:139–155, 2009 [DOI] [PubMed] [Google Scholar]