Abstract
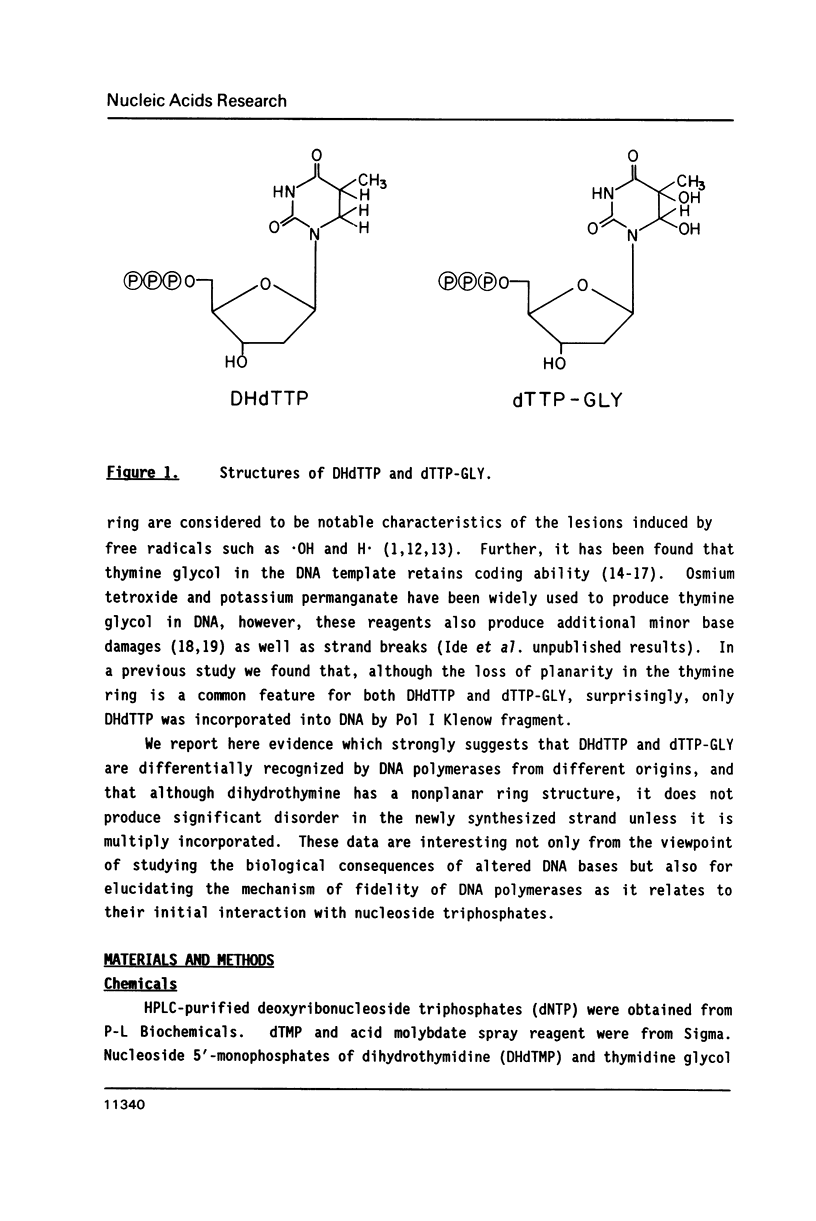
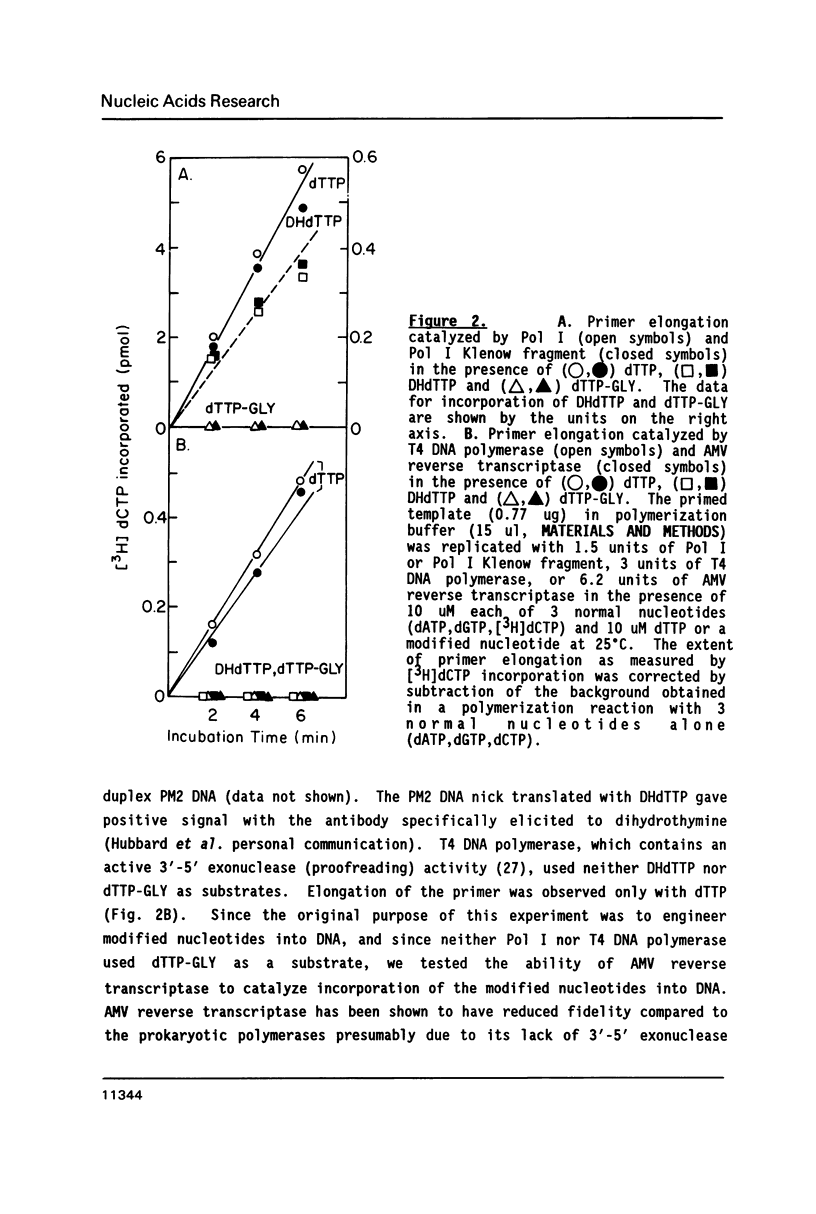
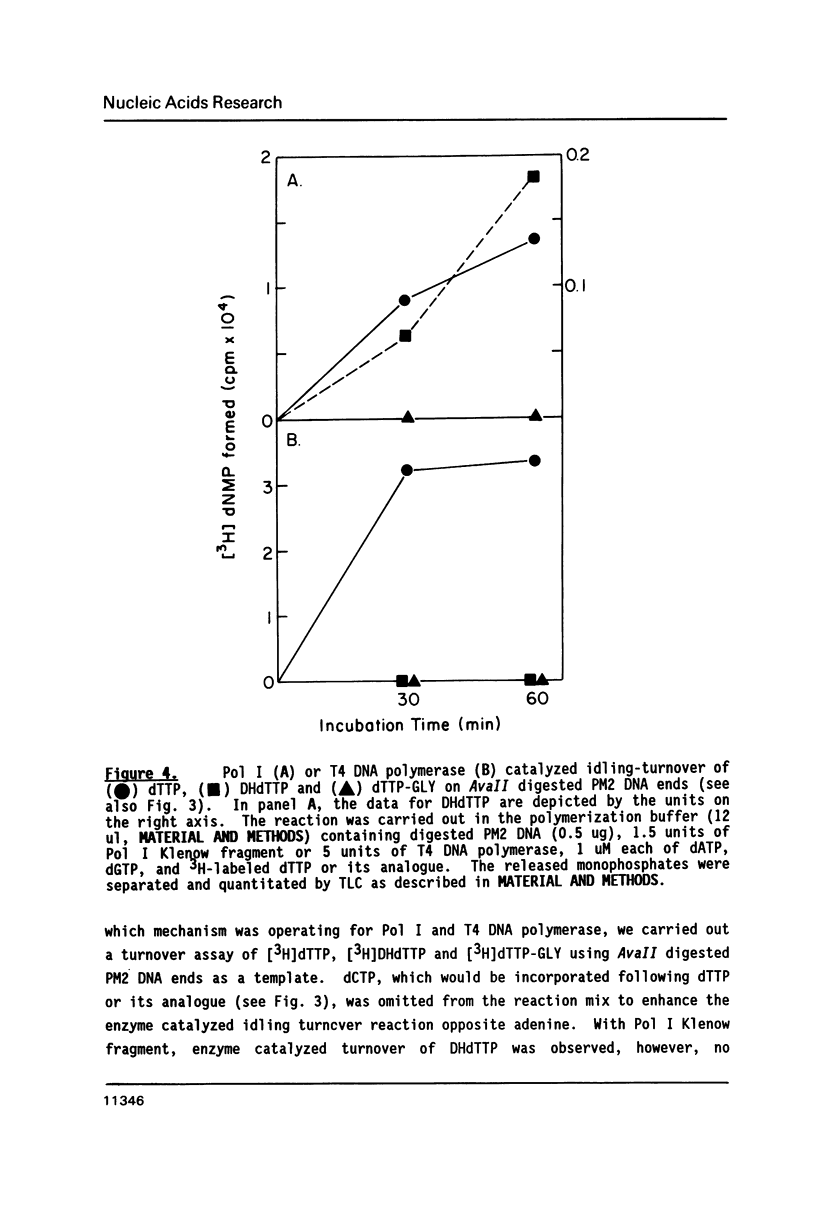
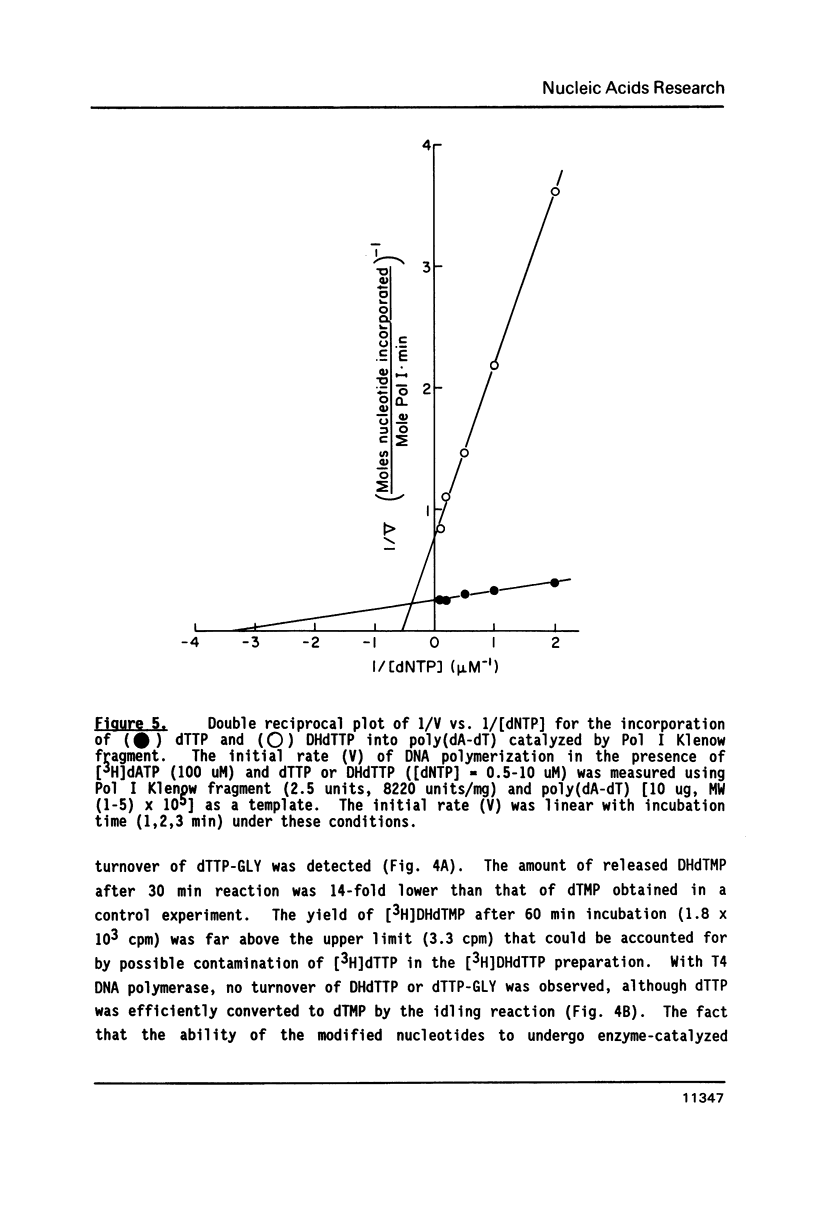
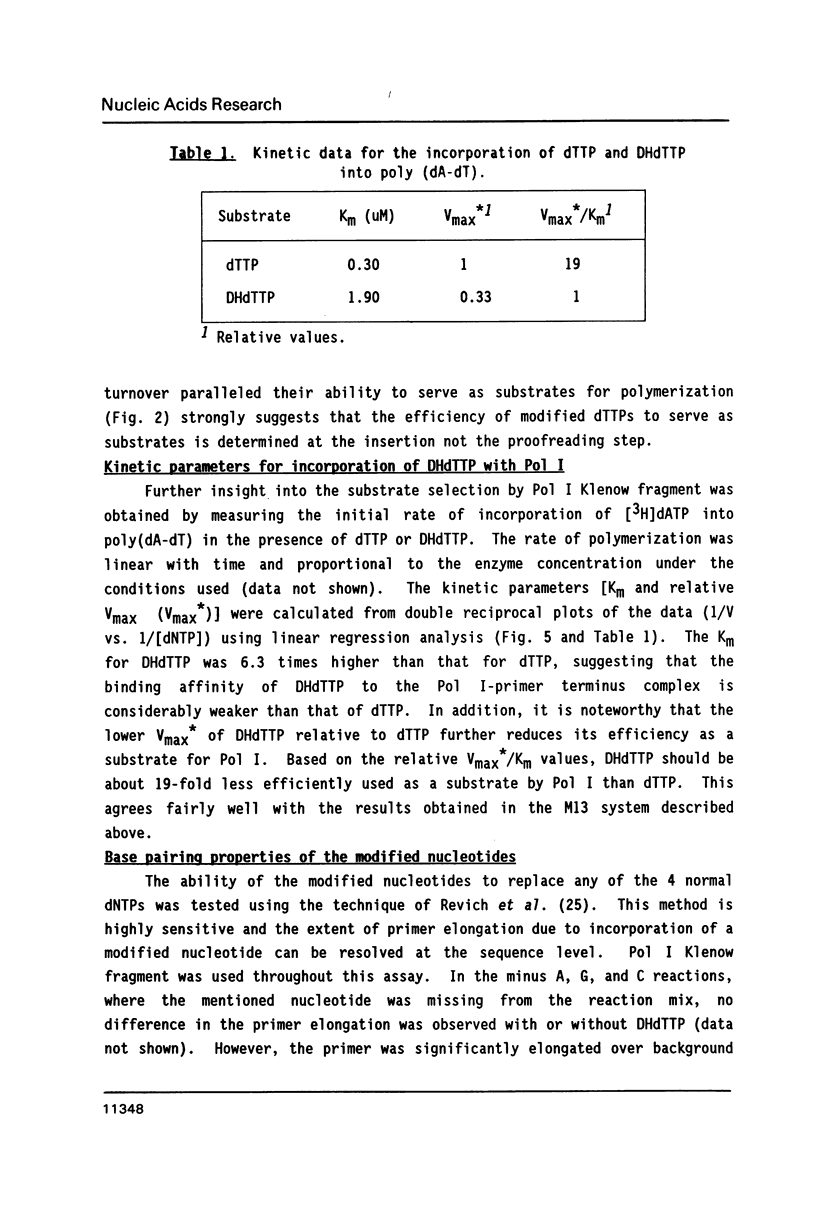
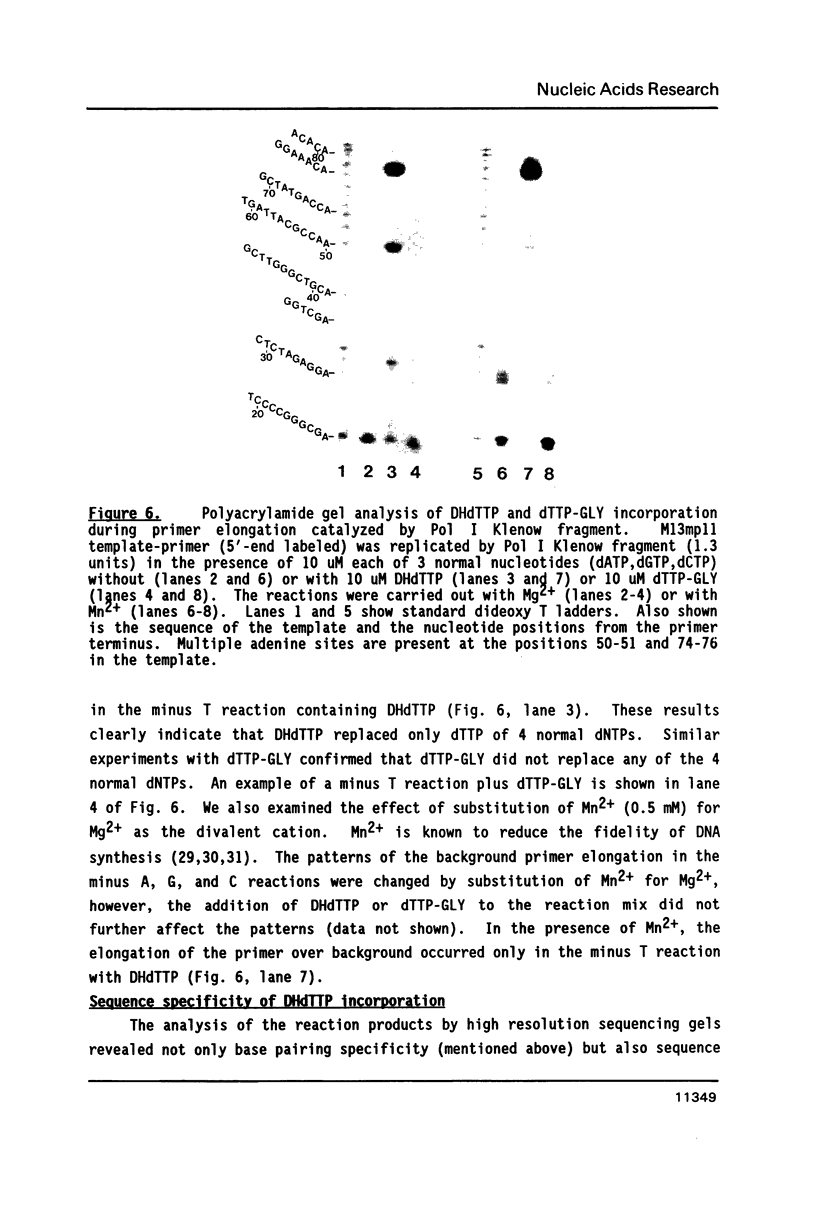
The ability of dihydrothymidine (DHdTTP) and thymidine glycol (dTTP-GLY) 5'-triphosphates to serve as substrates for different DNA polymerases was investigated. DHdTTP but not dTTP-GLY was used as a substrate by E. coli DNA polymerase I (Pol I). Within the detection limit of the assay used, neither T4 DNA polymerase nor avian myeloblastosis virus (AMV) reverse transcriptase used DHdTTP or dTTP-GLY as substrates. The ability of DHdTTP and dTTP-GLY to undergo enzyme-catalyzed turnover to the monophosphate paralleled their ability to serve as substrates for polymerization. These results, along with kinetic parameters for the incorporation of DHdTTP with Pol I, strongly suggest that the saturation of thymine C5-C6 bond and the substituent groups at C5 and C6 differentially exert effects on binding to DNA polymerases. DNA sequencing gel analysis of the polymerization products revealed that most single adenine sites were capable of templating DHdTTP, however, DNA synthesis was partially arrested at multiple adenine sites, suggesting that sequential incorporation of DHdTTP produced significant disorder in the primer terminus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Medina A., Vicuña R., Venegas A., Valenzuela P., Yudelevich A. Molecular cloning and physical map of bacteriophage PM2 DNA. Gene. 1981 Jan-Feb;13(1):115–118. doi: 10.1016/0378-1119(81)90049-4. [DOI] [PubMed] [Google Scholar]
- Basu A., Modak M. J. Identification and amino acid sequence of the deoxynucleoside triphosphate binding site in Escherichia coli DNA polymerase I. Biochemistry. 1987 Mar 24;26(6):1704–1709. doi: 10.1021/bi00380a032. [DOI] [PubMed] [Google Scholar]
- Battula N., Loeb L. A. On the fidelity of DNA replication. Lack of exodeoxyribonuclease activity and error-correcting function in avian myeloblastosis virus DNA polymerase. J Biol Chem. 1976 Feb 25;251(4):982–986. [PubMed] [Google Scholar]
- Beckman R. A., Mildvan A. S., Loeb L. A. On the fidelity of DNA replication: manganese mutagenesis in vitro. Biochemistry. 1985 Oct 8;24(21):5810–5817. doi: 10.1021/bi00342a019. [DOI] [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. Thymine lesions produced by ionizing radiation in double-stranded DNA. Biochemistry. 1985 Jul 16;24(15):4018–4022. doi: 10.1021/bi00336a032. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Thymine glycol lesions terminate chain elongation by DNA polymerase I in vitro. Nucleic Acids Res. 1986 Jan 24;14(2):737–749. doi: 10.1093/nar/14.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Goodman M. F., Branscomb E. W., Galas D. J. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J Biol Chem. 1979 Mar 25;254(6):1902–1912. [PubMed] [Google Scholar]
- Demple B., Linn S. 5,6-Saturated thymine lesions in DNA: production by ultraviolet light or hydrogen peroxide. Nucleic Acids Res. 1982 Jun 25;10(12):3781–3789. doi: 10.1093/nar/10.12.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Loeb L. A. Manganese as a mutagenic agent during in vitro DNA synthesis. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1041–1046. doi: 10.1016/0006-291x(75)90779-2. [DOI] [PubMed] [Google Scholar]
- Ferrin L. J., Mildvan A. S. NMR studies of conformations and interactions of substrates and ribonucleotide templates bound to the large fragment of DNA polymerase I. Biochemistry. 1986 Sep 9;25(18):5131–5145. doi: 10.1021/bi00366a023. [DOI] [PubMed] [Google Scholar]
- Ferrin L. J., Mildvan A. S. Nuclear Overhauser effect studies of the conformations and binding site environments of deoxynucleoside triphosphate substrates bound to DNA polymerase I and its large fragment. Biochemistry. 1985 Nov 19;24(24):6904–6913. doi: 10.1021/bi00345a024. [DOI] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Hayes R. C., LeClerc J. E. Sequence dependence for bypass of thymine glycols in DNA by DNA polymerase I. Nucleic Acids Res. 1986 Jan 24;14(2):1045–1061. doi: 10.1093/nar/14.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R. C., Petrullo L. A., Huang H. M., Wallace S. S., LeClerc J. E. Oxidative damage in DNA. Lack of mutagenicity by thymine glycol lesions. J Mol Biol. 1988 May 20;201(2):239–246. doi: 10.1016/0022-2836(88)90135-0. [DOI] [PubMed] [Google Scholar]
- Hentosh P., Henner W. D., Reynolds R. J. Sequence specificity of DNA cleavage by Micrococcus luteus gamma endonuclease. Radiat Res. 1985 Apr;102(1):119–129. [PubMed] [Google Scholar]
- Huang W. M., Lehman I. R. On the exonuclease activity of phage T4 deoxyribonucleic acid polymerase. J Biol Chem. 1972 May 25;247(10):3139–3146. [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Ide H., Kow Y. W., Wallace S. S. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985 Nov 25;13(22):8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide H., Melamede R. J., Wallace S. S. Synthesis of dihydrothymidine and thymidine glycol 5'-triphosphates and their ability to serve as substrates for Escherichia coli DNA polymerase I. Biochemistry. 1987 Feb 10;26(3):964–969. doi: 10.1021/bi00377a042. [DOI] [PubMed] [Google Scholar]
- Jorgensen T. J., Kow Y. W., Wallace S. S., Henner W. D. Mechanism of action of Micrococcus luteus gamma-endonuclease. Biochemistry. 1987 Oct 6;26(20):6436–6443. doi: 10.1021/bi00394a021. [DOI] [PubMed] [Google Scholar]
- Joyce C. M., Kelley W. S., Grindley N. D. Nucleotide sequence of the Escherichia coli polA gene and primary structure of DNA polymerase I. J Biol Chem. 1982 Feb 25;257(4):1958–1964. [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Mossoba M. M., Riesz P. Formation of .OH and .H in aqueous solutions by ultrasound using clinical equipment. Radiat Res. 1983 Nov;96(2):416–421. [PubMed] [Google Scholar]
- Otvös L., Sági J., Kovács T., Walker R. T. Substrate specificity of DNA polymerases. I. Enzyme-catalysed incorporation of 5-'1-alkenyl)-2'-deoxyuridines into DNA. Nucleic Acids Res. 1987 Feb 25;15(4):1763–1777. doi: 10.1093/nar/15.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Endonuclease-resistant apyrimidinic sites formed by neocarzinostatin at cytosine residues in DNA: evidence for a possible role in mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3182–3186. doi: 10.1073/pnas.82.10.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8501–8505. doi: 10.1073/pnas.83.22.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan R., Melamede R. J., Laspia M. F., Erlanger B. F., Wallace S. S. Properties of antibodies to thymine glycol, a product of the radiolysis of DNA. Radiat Res. 1984 Mar;97(3):499–510. [PubMed] [Google Scholar]
- Randall S. K., Eritja R., Kaplan B. E., Petruska J., Goodman M. F. Nucleotide insertion kinetics opposite abasic lesions in DNA. J Biol Chem. 1987 May 15;262(14):6864–6870. [PubMed] [Google Scholar]
- Reeves S. T., Beattie K. L. Base-pairing properties of N4-methoxydeoxycytidine 5'-triphosphate during DNA synthesis on natural templates, catalyzed by DNA polymerase I of Escherichia coli. Biochemistry. 1985 Apr 23;24(9):2262–2268. doi: 10.1021/bi00330a021. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revich G. G., Beattie K. L. Utilization of 1,N6-etheno-2'-deoxyadenosine 5'-triphosphate during DNA synthesis on natural templates, catalyzed by DNA polymerase I of Escherichia coli. Carcinogenesis. 1986 Sep;7(9):1569–1576. doi: 10.1093/carcin/7.9.1569. [DOI] [PubMed] [Google Scholar]
- Revich G. G., Hillebrand G. G., Beattie K. L. High-performance liquid chromatographic purification of deoxynucleoside 5'-triphosphates and their use in a sensitive electrophoretic assay of misincorporation during DNA synthesis. J Chromatogr. 1984 Dec 28;317:283–300. doi: 10.1016/s0021-9673(01)91667-x. [DOI] [PubMed] [Google Scholar]
- Rouet P., Essigmann J. M. Possible role for thymine glycol in the selective inhibition of DNA synthesis on oxidized DNA templates. Cancer Res. 1985 Dec;45(12 Pt 1):6113–6118. [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Fraenkel-Conrat H., Kuśmierek J. T. Preparation and template activities of polynucleotides containing O2- and O4-alkyluridine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1722–1726. doi: 10.1073/pnas.75.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Spengler S. J., Fraenkel-Conrat H., Kuśmierek J. T. O4-Methyl, -ethyl, or -isopropyl substituents on thymidine in poly(dA-dT) all lead to transitions upon replication. Proc Natl Acad Sci U S A. 1986 Jan;83(1):28–32. doi: 10.1073/pnas.83.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. M., Goodman M. F. Kinetic measurement of 2-aminopurine X cytosine and 2-aminopurine X thymine base pairs as a test of DNA polymerase fidelity mechanisms. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6429–6433. doi: 10.1073/pnas.79.21.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]