Abstract
Caffeic, syringic, and protocatechuic acids are phenolic acids derived directly from food intake or come from the gut metabolism of polyphenols. In this study, the antioxidant activity of these compounds was at first evaluated in membrane models, where caffeic acid behaved as a very effective chain-breaking antioxidant, whereas syringic and protocatechuic acids were only retardants of lipid peroxidation. However, all three compounds acted as good scavengers of reactive species in cultured cells subjected to exogenous oxidative stress produced by low level of H2O2. Many tumour cells are characterised by increased ROS levels compared with their noncancerous counterparts. Therefore, we investigated whether phenolic acids, at low concentrations, comparable to those present in human plasma, were able to decrease basal reactive species. Results show that phenolic acids reduced ROS in a leukaemia cell line (HEL), whereas no effect was observed in normal cells, such as HUVEC. The compounds exhibited no toxicity to normal cells while they decreased proliferation in leukaemia cells, inducing apoptosis. In the debate on optimal ROS-manipulating strategies in cancer therapy, our work in leukaemia cells supports the antioxidant ROS-depleting approach.
1. Introduction
In the scientific literature, the term polyphenols refers also to phenolic acids, although from the strict structural point of view of purely chemically based definition they are monophenolic compounds, as widely stated by Quideau et al. [1]. The reason for including phenolic acids in the family of plant polyphenols lies in the fact that they are bioprecursors of polyphenols and, more importantly, they are metabolites of polyphenols. Many papers and reviews describe studies on bioavailability of phenolic acids, emphasizing both the direct intake through food consumption and the indirect bioavailability derived by gastric, intestinal, and hepatic metabolism of “true” polyphenols [2–8].
Two aspects have to be taken into account when dietary phenolic compounds are tested in cell cultures as models: seldom the free form, that is, aglycone, is the actual molecule reaching blood and tissues; and the concentration used should be similar to that found in the circulatory system, that is, nmol/L to low mmol/L [6, 9].
More than ten years ago, we began studying the antioxidant activity of phenolic acids deriving from the intestinal metabolism of polyphenols or coming directly from food, representative of the two major classes of phenolic acids, that is, hydroxycinnamic and hydroxybenzoic acids. We have focused on antioxidant properties of these phenolic acids, tested at low micromolar concentration, starting from experiments conducted in homogeneous solution and in membrane models. In particular, we have studied caffeic acid (CAF), a derivate of cinnamic acid carrying two hydroxyl groups in ortho position, syringic (SYR), and protocatechuic (PRO) acids, with a hydroxybenzoic structure: SYR with methoxyl groups in positions 3 and 5 and hydroxyl group in 4, and PRO with hydroxyl groups in positions 3 and 4.
Direct sources of CAF are dates, berries, fruit such as apricot, apple and kiwi, spices, herbs, vegetables, beverages such as coffee, wine, and, at a lesser extent, beer [10–13]. The main sources of SYR are swiss chard, olives, walnuts, dates, spices, and pumpkin [10, 14]. PRO, besides being one of the main anthocyanin metabolite [15], is present in cocoa powder, dates, chicory, olives, and onions [10].
These compounds have been studied mainly for their properties against oxidative damage leading to various degenerative diseases, such as cardiovascular diseases, inflammation, and cancer. Indeed, tumour cells, including leukaemia cells, typically have higher levels of reactive oxygen species (ROS) than normal cells so that they are particularly sensitive to oxidative stress. Differences in ROS levels between normal and cancer cells are due to the dysregulation of redox balance in neoplastic cells that develop when, for instance, intracellular production of ROS increases, or when antioxidant defences are depleted [16–22]. The importance of ROS in cancer is unequivocal, but scientists still not exactly understand how reactive species act [23]. Many reviews extensively describe sources, mechanisms, and involvement of ROS in cancer, and illustrate the role of cellular redox regulation in cancer therapy development [24–30]. In particular, recently Wang and Yi [31] well reviewed “two paradoxical ROS-manipulation strategies in cancer treatment,” that is the opposite proantioxidant and antioxidant approaches. They propose the development of “redox signaling signature,” a combinational set of parameters such as redox status, antioxidant enzymes expression, cell signalling, and transcription factor activation profiles in a given type of cancer cells, to be used as an index for choosing one of the two faces of the coin: ROS-elevating or ROS-depleting specific therapy against certain type of cancer cells. A way to kill cells is the induction of apoptosis, the programmed cell death. Whether this goal should be reached by diminishing or increasing ROS is controversial, likely depending on the “redox signaling signature” of each cell type. The advantage of such a strategy, that is, the induction of apoptosis via death signalling pathways, is that normal cells are not significantly affected since their basal ROS levels are lower and, therefore, they are less susceptible to redox changes. Recently, Halliwell observed that one of the various contribution to cancer played by reactive species is their ability to suppress apoptosis [23]. Akt is the prototypic kinase which promotes cellular survival: Akt enhances survival by directly phosphorylating key regulatory proteins of the apoptotic cascades [32]. In fact, the phosphoinositide 3-kinase (PI3K)/Akt pathway is constitutively active in many tumours. The phosphorylation of PI3K activates Akt and phosphorylation by p-Akt activates key survival proteins and inactivates proapoptotic substrates. Phosphorylation of these proteins decreases tumour susceptibility to apoptotic stresses. In other words, activation of the PI3K/Akt pathway is one of the mechanisms by which ROS modulate cell survival during carcinogenesis.
The inhibition of ROS through the use of antioxidants decreases the antiapoptotic pathways that are activated by ROS in cancer cells [29, 33]. Although some studies report that high levels of ROS turn on cell death signalling [25] and that Akt dephosphorylation leading to apoptosis is induced by ROS [34], many papers demonstrate the link between the increased redox stress in tumours with the PI3K/Akt pathway [35, 36].
Our previous studies investigating the effect of IL-3 on the M07e human acute myeloid leukaemia (AML) cell line noted that the prosurvival effect of this cytokine was mediated by NAD(P)H oxidase isoform Nox2-derived ROS production, and was suppressed by antioxidants, NAD(P)H oxidase (Nox) inhibitors or specific knockdown of Nox2 expression [37, 38]. In a different AML cell line, B1647, expressing Nox2 and Nox4 [39], we showed that VEGF signalling and Nox activity are coupled, and that inhibitors of both Nox and VEGF receptor 2 are able to induce apoptosis in these leukaemia cell line [40].
Starting from our experience on antioxidant properties studied in model systems and on the role of ROS and their inhibitors in leukaemia cell proliferation, the aim of this work was to explore the antioxidant activity of three dietary phenolic acids in membrane models and in the leukaemia cell line, HEL, as well as to investigate the relationship among reactive species, cell proliferation, and apoptosis.
2. Materials and Methods
2.1. Materials
Egg yolk lecithin (phosphatidylcholine, PC) was purchased from Lipid Products (Redhill, UK). The thermolabile azo compound 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), caffeic acid (CAF), syringic acid (SYR), protocatechuic acid (PRO), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox, TRO), α-Tocopherol (Tocoph), hydrogen peroxide, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, dichlorofluorescin diacetate), 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Trypan Blue, Igepal CA-630, the fluorogenic substrates N-acetyl Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) for Caspase 3, N-Acetyl-Ile-Glu-Thr-Asp-7-amido-4-methylcoumarin (Ac-IETD-AMC) for Caspase 8, and N-Acetyl-Leu-Glu-His-Asp-7-amido-4-trifluoromethylcoumarin (Ac-LEHD-AFC) for Caspase 9, sodium orthovanadate, phenylmethanesulfonyl fluoride (PMSF), N-tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK), N-p-tosyl-L-phenylalanine chloromethyl ketone (TPCK), protease inhibitor cocktail, Laemmli sample buffer containing 2-mercaptoethanol, mouse monoclonal antitubulin antibody were obtained from Sigma-Aldrich. HEL (Human erythroleukaemia) cell culture was from DSMZ (Braunschweig, Germany); HUVEC (Human Umbilical Vein Endothelial Cells) were kindly donated by Professor Claudio Muscari, Department of Biochemistry, University of Bologna; RPMI 1640 (with Hepes, with L-glutamine), foetal calf serum, Penicillin/Streptomycin, were purchased from PAA. ATPlite 1step luminescence kit was from PerkinElmer. Nitrocellulose membranes and Amersham ECL Plus Western Blotting Detection Reagents were from GE-Healthcare. Anti-caspase 3, anti-Bax, anti-Bcl-2, and anti-p-Akt antibodies were purchased from Cell Signaling Technology. Anti-rabbit and anti-mouse IgG conjugated to horseradish peroxidase were obtained from Santa Cruz Biotechnology. PageRuler Prestained protein ladder was from Fermentas, Thermo Fisher Scientific. All the other chemicals and solvents were of the highest analytical grade.
2.2. Preparation of Large Unilamellar Vesicles
Vesicles were prepared by adding in a round-bottom tube the appropriate amount of phosphatidylcholine. The solvent was carefully removed with a stream of nitrogen to obtain a thin film, then 0.6 mL of phosphate-buffered saline (PBS), pH 7.2, containing 1 mM Na2EDTA were added. The film was vortex-stirred for 7 min and the milky suspension obtained was transferred into LiposoFast (produced by Avestin, Ottawa, Canada) and extruded 21 times back and forth through two polycarbonate filters (100 nm pore size, Nucleopore Corp., Pleasenton, CA) to obtain large unilamellar vesicles (LUVET). The total volume was then adjusted to give a final concentration of 15 mM PC. Ethanol solutions of phenolic acids were added to LUVET to obtain a final concentration between 1.3 and 10 μM of the acid.
2.3. Vesicle Autoxidation
Autoxidation experiments in the presence or absence of antioxidants were carried out by monitoring the oxygen concentration with a Clark-type electrode (Yellow Springs Instruments Co., OH). After thermal equilibration of LUVET at 37°C, the appropriate amount of AAPH was added to the suspension in order to obtain a final AAPH concentration of 17 mM, suitable for LUVET peroxidation initiated by water soluble azo compounds [41]. The reaction cell was always protected from room light to avoid initiator photodecomposition.
2.4. Cell Culture
Human erythromegakaryocytic leukaemia cell line (HEL) established from peripheral blood obtained from a patient with acute myelogenous leukaemia was grown in RPMI supplemented with 10% (v/v) heat-inactivate foetal calf serum supplemented with 100 U/mL penicillin and 100 g/mL streptomycin sulphate at 37°C in a humidified atmosphere maintained at 5% CO2. The cells were treated with different concentrations (5, 10, 20, 50 or 100 μM) of CAF, SYR, PRO, Trolox, and α-Tocopherol, dissolved in ethanol or in ultrapure water, for 20 hours.
2.5. Measurement of Intracellular ROS
Cells (1 × 106/mL) were washed twice in PBS and incubated with 5 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) for 20 min at 37°C. H2DCFDA is a small nonpolar, nonfluorescent molecule that diffuses into the cells, where it is enzymatically deacetylated by intracellular esterases to a polar nonfluorescent compound, that is oxidised to the highly green fluorescent 2′,7′-dichlorofluorescein (DCF). The fluorescence of oxidized probe was measured using a multiwell plate reader (Wallac Victor2, PerkinElmer). Excitation wavelength was 485 nm and emission wavelength was 535 nm [42, 43].
2.6. Cell Viability and Proliferation
Viable cells were evaluated by the Trypan Blue exclusion test. Cell viability was also assayed by the MTT assay, since the reduction of tetrazolium salts is widely accepted as a reliable way to examine cell viability/proliferation [44]. Cells (2 × 104) were incubated in 96-well flat-bottomed plates with 0.5 mg/mL MTT for 4 h at 37°C. At the end of the incubation, blue-violet formazan salt crystals were formed and dissolved by adding the solubilization solution (10% SDS, 0.01 M HCl), then the plates were incubated overnight in humidified atmosphere (37°C, 5% CO2) to ensure complete lysis. The absorbance at 570 nm was measured using a multiwell plate reader (Wallac Victor2, PerkinElmer). In addition, cell viability was assessed by using the ATPlite 1step luminescence kit, an ATP monitoring system based on firefly (Photinus pyralis) luciferase. The emitted light, produced by the reaction of ATP with added luciferase and D-luciferin, is proportional to the concentration of ATP, present in all metabolically active cells. In a 96-well black clear-bottom plate, according to the supplier's instructions, 100 μL of the reconstituted reagent were added to each well containing 2 × 104 cells (100 μL), equilibrated at room temperature. The plate was shaken for 2 minutes at 700 rpm using an orbital microplate shaker, then the luminescence was measured using a multiwell plate reader (Wallac Victor2, PerkinElmer).
2.7. Caspase Activity Assay
Ac-DEVD-AMC was used as fluorogenic substrate for caspase 3, Ac-IETD-AMC for caspase 8, and Ac-LEHD-AFC for caspase 9. After different treatments, cell lysates were incubated with specific substrates at 37°C for 15 min. The activity of caspase 3 and caspase 8 was measured following the hydrolysis of fluorogenic substrates resulting in the release of the fluorescent AMC; excitation wavelength was 370 nm, emission wavelength was 455 nm. The caspase 9 activity was determined by measuring the fluorescence of AFC; the excitation and emission wavelengths of AFC were 400 and 505 nm, respectively. Measurements were performed by a Jasco FP-777 spectrofluorometer.
2.8. Immunoblotting and Densitometric Analysis
Western blot analysis was done to detect procaspase 3, cleaved caspase 3, Bax, Bcl-2, p-Akt, and tubulin, by using the corresponding antibodies. Cells were lysed with a lysis buffer (1% Igepal, 150 mM NaCl, 50 mM Tris-HCl, 5 mM EDTA, 0.1 mM PMSF, 0.1 mM TLCK, 0.1 mM TPCK, 1 mM sodium orthovanadate and protease inhibitor cocktail, pH 8.0) in ice for 15 min. Samples were added to Laemmli sample buffer containing 2-mercaptoethanol and kept at 95°C for 5 min to solubilise the proteins. Then they were separated on 10% SDS-polyacrylamide gel using a Mini-Protean III apparatus (Bio-Rad Laboratories). Proteins were transferred electrophoretically to nitrocellulose membrane at 100 V for 60 min. Nonspecific binding was blocked by incubating with Tris-buffered saline (TBS)/Tween, pH 8.0, containing 5% nonfat, dry milk for 1 h at room temperature. Nitrocellulose membranes were incubated overnight at 4°C with primary antibodies, washed 3 times with TBS/Tween, and incubated for 60 min at room temperature with secondary antibodies in TBS/Tween containing 5% nonfat dry milk. Membranes were washed and developed using Amersham ECL Plus Western Blotting Detection Reagents. Images of the blots were obtained using a CCD imager (Fluor-S Max MultiImager System, Bio-Rad Laboratories) and bands were acquired and analyzed by using Bio-Rad Quantity One analysis software. Cleaved caspase 3 immunoreactive bands were quantitated and expressed as the ratio of each band density to control band density. Bax and Bcl-2 immunoreactive bands were quantitated and expressed as the ratio of Bax band density to Bcl-2 band density for each sample.
2.9. Statistical Analysis
Results are expressed as means of at least three independent experiments with standard deviation. Differences between the means were determined by two-tailed Student's t test or by Newman-Keuls multiple comparison test following one-way ANOVA.
3. Results and Discussion
At first, the antioxidant activity of CAF, SYR, and PRO (see Figure 1) was investigated in large unilamellar vesicles of phosphatidylcholine, model membranes in which interfacial interactions, molecular packing, and dynamics of the lipid phase can be envisaged similar to those of natural membranes.
Figure 1.
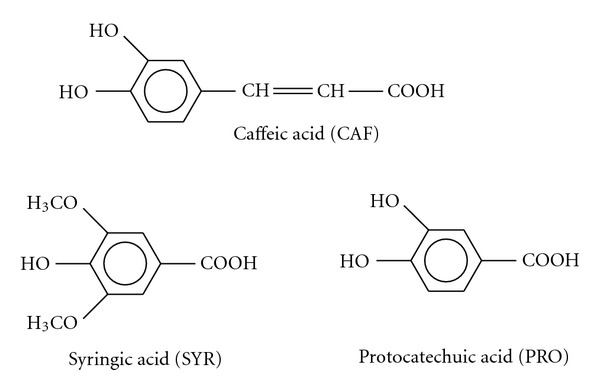
Chemical structures of studied phenolic acids.
Autoxidation experiments were performed by monitoring the oxygen consumption with a miniaturized Clark-type electrode and by using the water soluble azo compound AAPH as initiator of radical peroxidation and Trolox as reference antioxidant. AAPH, owing to its hydrophilicity, gives rise to radical chain initiation at a constant rate in the aqueous phase according to a well-defined pathway [45], being a useful tool for understanding the effectiveness of hydrophilic and lipophilic peroxidation inhibitors against the attack of oxygen radicals to biomembranes from the external water environment.
Antioxidants were tested at several concentrations in the range 1–10 μM, and Figure 2 shows representative oxygen uptake traces acquired at 5 μM, optimum concentration to identify the inhibition period of good antioxidants in our experimental conditions, that is, 15 mM PC unilamellar vesicles and 17 mM AAPH at 37°C [46, 47]. The oxygen consumption traces reported in Figure 2, obtained at 37°C and pH 7.2, indicated that CAF behaves as a very effective antioxidant, showing both a longer inhibition time and a higher rate constant for the reaction with peroxyl radicals than those measured with the same amount of Trolox. Moreover, a linear relationship (r 2 = 0.996) was found between CAF concentrations and the inhibition periods in the concentration range here reported (see inset in Figure 2).
Figure 2.
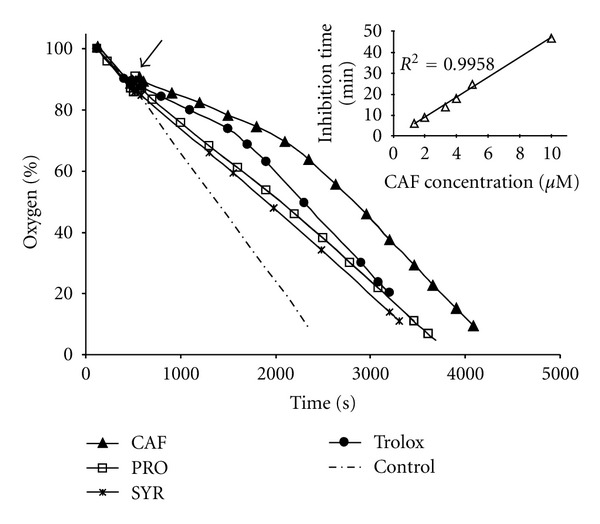
Oxygen uptake traces during AAPH (17 mM) induced peroxidation of PC (15 mM) unilamellar vesicles at 37°C and pH 7.2 in the absence of inhibitor (control) and in the presence of one of the various phenolic antioxidants, each at the same concentration (5 μM). The arrow shows antioxidant injection. Inset: relationship between CAF concentrations (1.3 to 10 μM) and inhibition periods measured.
In the presence of similar amounts of SYR and PRO, the rate of oxygen consumption was somewhat reduced with respect to control experiments, but no evident inhibition time occurred. This reduction was much smaller than that observed when the same amounts of CAF or Trolox were added to the assay (Figure 2). Thus, SYR and PRO did not behave as effective antioxidants, since in their presence no evident inhibition time occurred. According to Pryor et al. [48] “compounds of this type are generally classed as retardants rather than as antioxidants,” since they react with peroxyl radicals slowing the initiation and propagation steps of lipid oxidation, but do not completely stop it.
Many factors are responsible for the free radical scavenging activity of compounds in vitro. Structural, thermodynamic, and kinetic aspects have to be taken into account [47, 49–54]. The presence of a catechol moiety in the molecule is one of the most deeply studied features. A second aspect is the effect of ring substituents. For example, the acrylic group CH=CH−COOH in para position with respect to a phenolic OH is of some relevance in determining the good antioxidant properties of cinnamic acids such as caffeic acid; instead, the lower reactivity of protocatechuic acid is due to the presence of the electron withdrawing COOH ring substituent; electron donating OH groups have stronger effects then OCH3 ones on antioxidant ability. A less frequently considered characteristic is the pK a value of the hydroxyl group(s), that indeed lead to a very strong dependence of antioxidant activity on the pH of the buffer solution where compounds are tested. Finally, but not exhaustively, it is essential to contemplate the different hydrophilicity of molecules, resulting in a different partition of the inhibitors between the water and the lipid phase.
Consequently, we evaluated the ability of CAF, SYR, and PRO to reduce intracellular ROS content after oxidative stress generated by low nonlethal level of exogenous H2O2 in the human hematopoietic cell line HEL (human erythroleukemia) and in primary-cultured HUVEC (Human Umbilical Vein Endothelial Cells).
HUVEC and hematopoietic cells, such as HEL, have a common progenitor, known as hemangioblast, multipotent cell that is able to differentiate to both endothelial and hematopoietic cells. Although many cellular pathways are still unclear, the nearly centenarian hemangioblast hypothesis has now been confirmed [55], since several cellular events and pathways have been detected. Moreover, a strict relationship between angiogenesis and development of cancer is widely documented: it is now well established that VEGF and its receptors are involved in promoting both solid and liquid tumour growth and proliferation [56–61]. Thus, HUVEC are “normal” cells frequently chosen to be compared with tumour cells [62, 63], in particular with HEL cell line in this study.
ROS levels were measured with the cell-permeant probe H2DCFDA, commonly used to detect free radical/ROS production in cells, owing to the intracellular conversion to the highly green fluorescent DCF [64]. In general, dihydrofluorescein does not discriminate between the various reactive oxygen/nitrogen species, but it remains the most straightforward and versatile indicator of cellular oxidative stress.
As shown in Figure 3, the phenolic acids, preincubated for 20 hours, behaved as good antioxidant since they were able to decrease ROS levels after 30-min exposition to 50 μM H2O2 in both cellular systems; this reduction was comparable to that provoked by α-tocopherol, the most effective lipid-soluble antioxidant nutrient, and by its water soluble analogue, Trolox.
Figure 3.
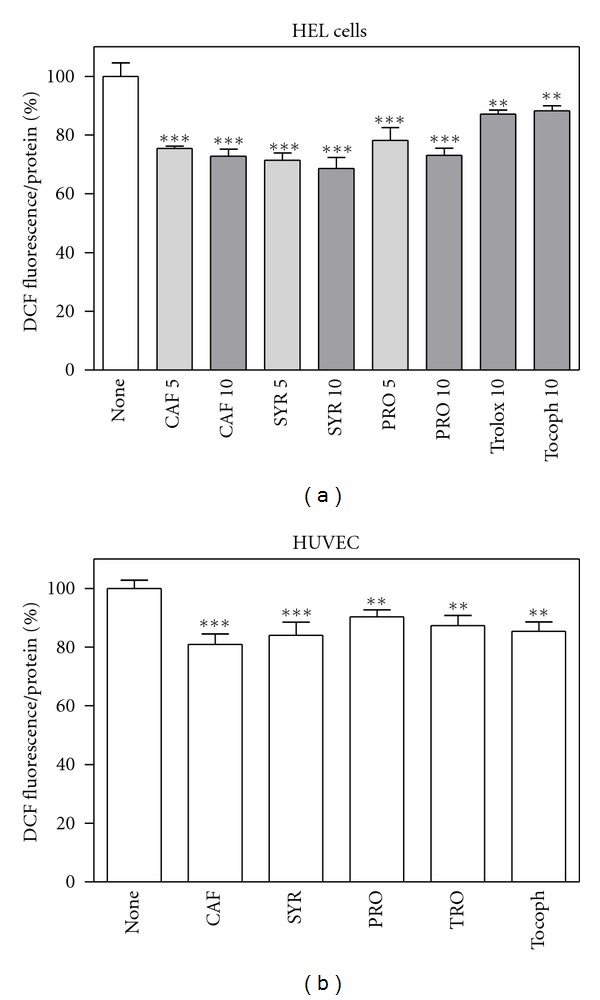
Antioxidant effect of phenolic compounds in HEL cells and HUVEC exposed to oxidative stress generated by 50 μM H2O2. Cells were preincubated with different compounds (5 or 10 μM for HEL cells, (a); 10 μM for HUVEC, (b)) for 20 hours, treated with H2O2 for 30 min, then ROS levels were measured by means of H2DCFDA assay as described in Section 2. Results are expressed as means ± SD of three independent experiments, each performed in octuplicate. **P < 0.005, significantly different from control cells; ***P < 0.0005, significantly different from control cells.
Many tumour cells are characterised by increased ROS generation compared with their noncancerous counterparts. Basal intracellular ROS levels were measured in HEL cells and compared with those exhibited by HUVEC. Figure 4 confirms the notion that cancer cells exhibit constitutively high levels of ROS, showing that ROS content in HEL cells is about eight times as high as in normal cells, HUVEC.
Figure 4.
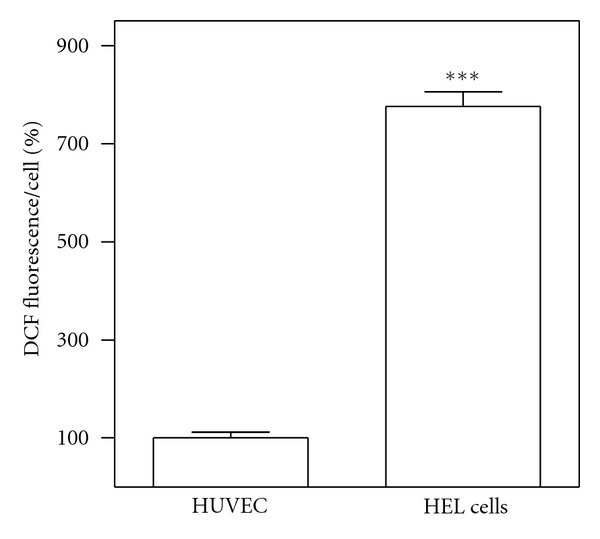
Comparison between basal ROS levels in HEL cells and in HUVEC. ROS levels were measured by means of H2DCFDA assay as described in Section 2. Results are expressed as means ± SD of three independent experiments, each performed in octuplicate. Significant difference ***P < 0.0001.
Subsequently, we investigated whether phenolic acids, at low concentrations, likely comparable to those present in human plasma [12, 65–67], were able to decrease basal reactive species. Graphs reported in Figure 5 show that in HEL cells the reduction was of about 20% after 20-h treatment, whereas no effect was observed in HUVEC even at higher concentrations.
Figure 5.
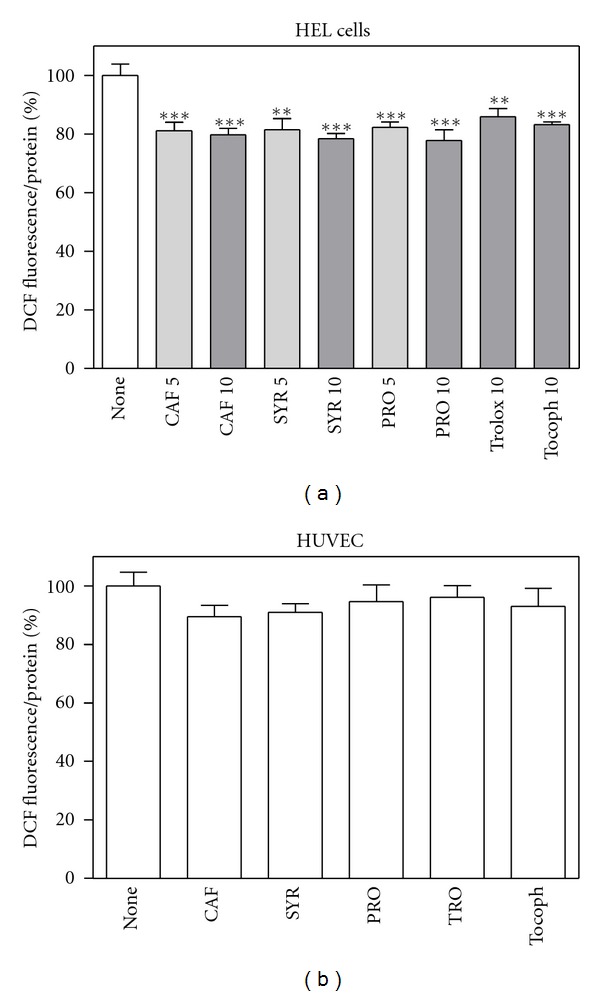
Effect of phenolic compounds on basal ROS levels in HEL cells and HUVEC. Cells were treated with different compounds (5 or 10 μM for HEL cells, (a); 20 μM for HUVEC, (b)) for 20 hours, then ROS levels were measured by means of H2DCFDA assay as described in Section 2. Results are expressed as means ± SD of four independent experiments, each performed in octuplicate. **P < 0.005, significantly different from control cells; ***P < 0.0005, significantly different from control cells.
A large body of literature reports that ROS may promote cellular proliferation and contribute to cancer development. In the last decade, we demonstrated that ROS are essential for cell survival in two different (erythro)megakaryocytic leukaemia cell lines, similar to HEL; Nox family is a major source of ROS (Nox4 and/or Nox2); ROS production can act as prosurvival factor that protects leukaemia cells from apoptosis, effect counteracted by antioxidants [37–40, 68–72].
In order to verify the role of ROS in tumour cell proliferation, we examined the effect of CAF, SYR, and PRO on cell viability, evaluated by Trypan blue exclusion test and by MTT assay (Figure 6), and compared it with Trolox and α-tocopherol effect. All the antioxidants slightly decreased leukaemia cell viability/proliferation (Figures 6(a) and 6(b)), whereas they did not affect HUVEC viability even at higher concentration (Figure 6(c)).
Figure 6.
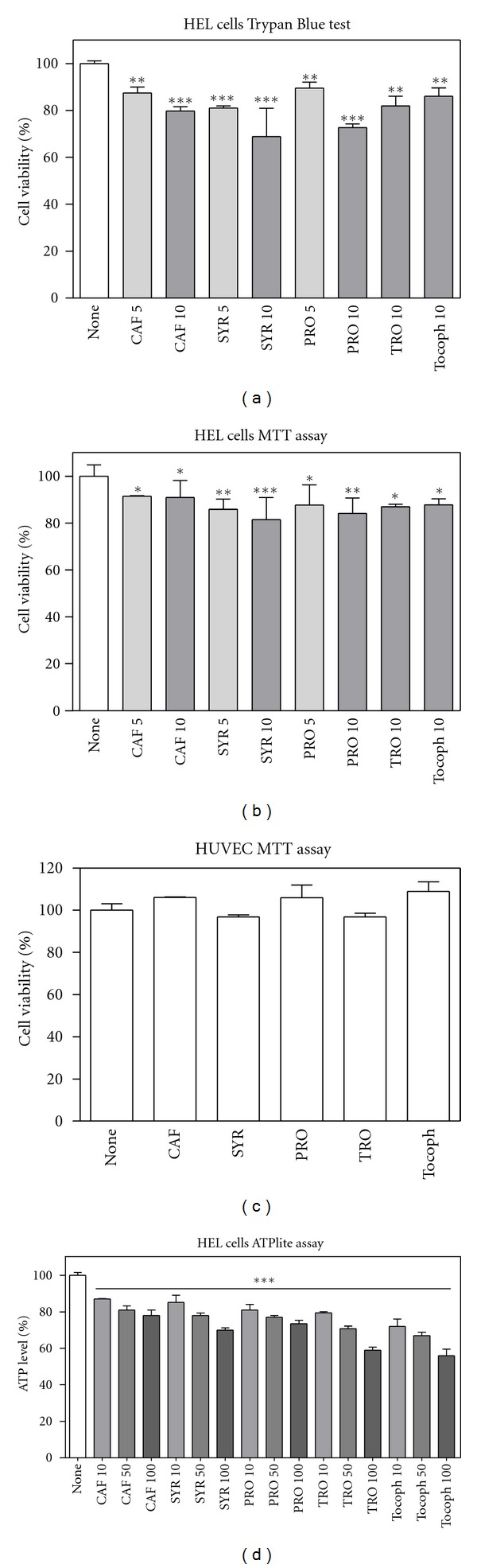
Effect of phenolic compounds on cell viability/proliferation. Cells were treated with different compounds (5 to 100 μM for HEL cells, (a), (b) and (d); 20 μM for HUVEC, (c)) for 20 hours. (a): Viability was estimated by Trypan Blue exclusion test. (b) and (c): Viability/proliferation was evaluated by MTT assay as described in Section 2. Results are expressed as means ± SD of three independent experiments, each performed in quadruplicate. *P < 0.05, significantly different from control cells; **P < 0.01, significantly different from control cells; ***P < 0.001, significantly different from control cells. (d): HEL cells viability, after 20-h treatment with 10, 50, or 100 μM compounds, was determined by the ATPlite 1step luminescence kit as described in Section 2. Results are expressed as means ± SD of three independent experiments, each performed in triplicate. All values are significantly different from control (***P < 0.001).
To exclude artifacts or interferences in MTT assay [73–76], although no direct reactivity toward the tetrazolium salt was detected in vitro, we performed complementary viability experiments by measuring intracellular ATP levels in HEL cells, also at higher phenolic acid concentrations. Measurements reported in Figure 6(d) confirmed the results obtained with the other methods.
Considering the lack of effect of tested compounds on HUVEC viability, further studies were conducted to characterise cell death induced by phenolic acids in leukaemia HEL cells, focusing on typical apoptotic features. Caspases play a central role in mediating various apoptotic responses and are activated in a sequential cascade of cleavages. The activation of an effector caspase, such as caspase 3, is executed by initiator caspases, such as caspases 8 and 9, through proteolytic cleavage after a specific internal aspartate residue, to separate the large and small subunits of the mature caspase. To detect the enzymatic activity of caspases, three fluorogenic substrates were used: Ac-DEVD-AMC was employed as substrate for caspase 3; Ac-IETD-AMC for caspase 8; Ac-LEHD-AFC for caspase 9. Treatments of HEL cells with antioxidants stimulated the activity of all the tested caspases in a dose-dependent manner, as shown in Figure 7, where the fluorescence of AMC or AFC is reported.
Figure 7.
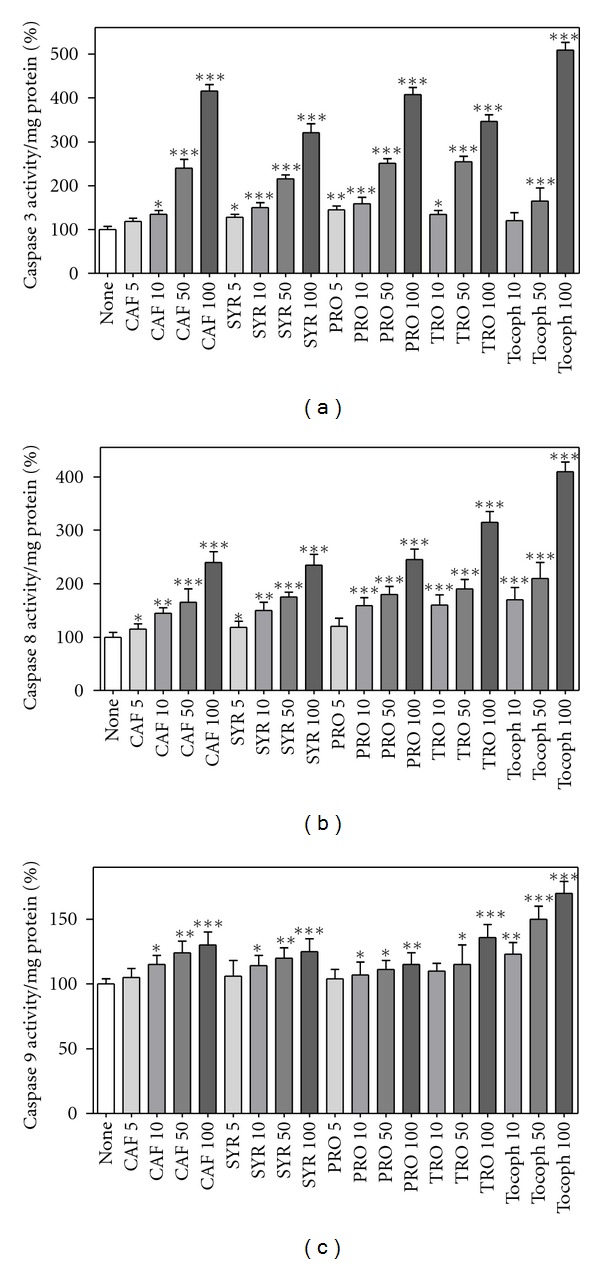
Caspase activity in HEL cells after phenolic compound treatment. HEL cells were incubated with different compounds (5, 10, 50, or 100 μM) for 20 hours, then cell lysates were incubated with three different substrates at 37°C for 15 min, that is Ac-DEVD-AMC as specific fluorogenic substrate for caspase 3 (a), Ac-IETD-AMC for caspase 8 (b), and Ac-LEHD-AFC for caspase 9 (c), as described in Section 2. Results are expressed as means ± SD of four independent experiments, each performed in triplicate. *P < 0.05, significantly different from control cells; **P < 0.01, significantly different from control cells; ***P < 0.001, significantly different from control cells.
Subsequently, we investigated the proapoptotic effect of phenolic compounds in HEL cells, performing SDS-PAGE followed by Western blot on cell lysates for detection of caspase 3, Bax, and Bcl-2 proteins (Figure 8). Western blot results on cleaved caspase 3 were in good agreement with outcomes obtained by fluorimetric assay, as shown in densitometric analysis of Figure 8(c). Moreover, phenolic acids were able to raise the expression of the proapoptotic Bax; in concert, a decrease of prosurvival Bcl-2 was produced. Bax and Bcl-2 are proteins involved in apoptosis acting in opposite way; thus, the Bax/Bcl-2 ratio is a useful index of apoptosis. Antioxidant compounds, after 20-hour incubation, caused a dose-dependent increase of this ratio (Figure 8(d)).
Figure 8.
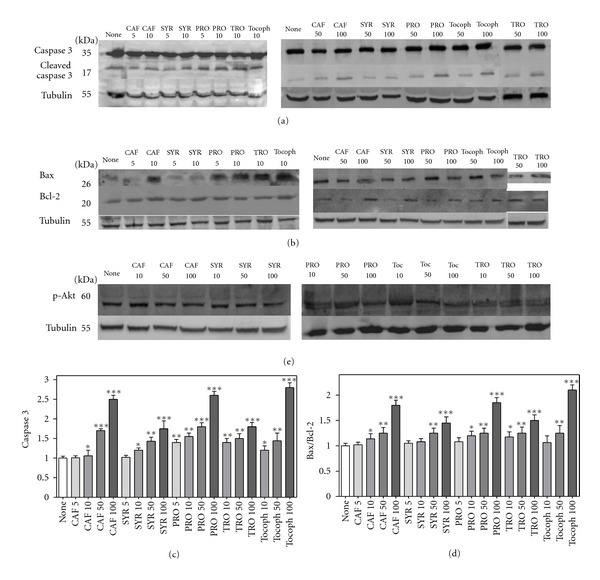
Effect of phenolic compounds on apoptosis. HEL cells were incubated with different compounds (5, 10, 50, or 100 μM) for 20 hours, then cell lysates were subjected to SDS-PAGE and Western blotting with the indicated antibodies as described in Section 2. Tubulin detection was used as a control. Representative immunoblots are shown. (a): anti-caspase 3; (b): anti-Bax and anti-Bcl-2; (e): anti-p-Akt. (c): Densitometric analysis of three independent Western blot assays for cleaved caspase 3 (17 kDa fragment). (d): Bax/Bcl-2 ratio from densitometric analysis of three independent experiments. *P < 0.05, significantly different from control cells; **P < 0.01, significantly different from control cells; ***P < 0.001, significantly different from control cells.
In mammals, one of the most efficient antiapoptotic survival pathway is represented by PI3K/Akt [77, 78]. Activated Akt is the principal mediator of prosurvival signalling regulated by PI3K [79]. The active phopshorylated form of Akt (p-Akt) promotes cell proliferation and survival by phosphorylating downstream molecules that regulate cell cycle and apoptosis [80]. Figure 8(e) shows that the treatment with phenolic molecules under study slightly decreased phosphorylation levels of Akt, confirming once again their proapoptotic role.
4. Conclusions
In this study, we evaluated the antioxidant activity of some phenolic acids, deriving both by direct absorption from food consumption and as a result of the cleavage of flavonoids by gut microflora. These compounds acted as chain-breaking antioxidants, with different effectiveness, in membrane models and were able to contrast intracellular ROS raise due to exogenous oxidative stress in both leukaemia and normal cells. Moreover, we observed that phenolic acids were able to scavenge reactive oxygen species in HEL cells, characterised by very high intracellular ROS levels. They exhibited no toxicity to normal cells, whereas they decreased proliferation in leukaemia cells, inducing apoptosis. Indeed, they rose caspase 3, 8, and 9 activity, increased the ratio of the apoptotic-related protein Bax/Bcl-2, and reduced Akt activation.
In the debate on optimal ROS-manipulating strategies in cancer therapy, our work in leukaemia cells supports the antioxidant ROS-depleting approach.
Authors' Contribution
L. Zambonin and C. Caliceti: contributed equally to this work.
Acknowledgments
The authors are grateful to Dr. Chiara Gamberini and Dr. Francesca Bonafè who kindly provided cultured HUVEC used in this study, and to Prof. Gabriele Hakim for scientific suggestions and guidance. They thank CIRB (Centro Interdipartimentale di Ricerche Biotecnologiche, University of Bologna, Italy) for the use of Bio-Rad Fluor-S Max MultiImager System. This work was supported by grants from MIUR and Fondazione del Monte di Bologna e Ravenna, Italy. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Abbreviations
- CAF:
Caffeic acid
- SYR:
Syringic acid
- PRO:
Protocatechuic acid
- TRO:
Trolox
- Tocoph:
α-Tocopherol
- ROS:
Reactive oxygen species
- AML:
Acute myeloid leukaemia
- Nox:
NAD(P)H oxidase
- VEGF:
Vascular endothelial cell growth factor
- AAPH:
2,2′-azobis(2-methylpropionamidine) dihydrochloride
- PC:
Phosphatidylcholine
- HEL:
Human erythromegakaryocytic leukaemia cell line
- H2DCFDA:
2′,7′-dichlorodihydrofluorescein diacetate (also known as dichlorofluorescin diacetate)
- DCF:
Dichlorofluorescein
- MTT:
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
- HUVEC:
Human Umbilical Vein Endothelial Cells
- Ac-DEVD-AMC:
N-acetyl Asp-Glu-Val-Asp-7-amido-4-methylcoumarin
- Ac-IETD-AMC:
N-Acetyl-Ile-Glu-Thr-Asp-7-amido-4-methylcoumarin
- Ac-LEHD-AFC:
N-Acetyl-Leu-Glu-His-Asp-7-amido-4-trifluoromethylcoumarin
- AMC:
7-amido-4-methylcoumarin
- AFC:
7-amido-4-trifluoromethylcoumarin.
References
- 1.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angewandte Chemie. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 2.Lafay S, Gil-Izquierdo A. Bioavailability of phenolic acids. Phytochemistry Reviews. 2008;7(2):301–311. [Google Scholar]
- 3.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 4.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition. 2005;81(1, supplement):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 5.D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Annali dell’Istituto Superiore di Sanita. 2007;43(4):348–361. [PubMed] [Google Scholar]
- 6.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Natural Product Reports. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 7.D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. International Journal of Molecular Sciences. 2010;11(4):1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forester SC, Waterhouse AL. Metabolites are key to understanding health effects of wine polyphenolics. Journal of Nutrition. 2009;139(9):1824S–1831S. doi: 10.3945/jn.109.107664. [DOI] [PubMed] [Google Scholar]
- 9.Kroon PA, Clifford MN, Crozier A, et al. How should we assess the effects of exposure to dietary polyphenols in vitro? The American Journal of Clinical Nutrition. 2004;80(1):15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Neveu V, Perez-Jiménez J, Vos F. Phenol-explorer: an online comprehensive database on polyphenol contents in foods. doi: 10.1093/database/bap024. Database. http://www.phenol-explorer.eu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. British Journal of Nutrition. 2010;104(supplement 3):S67–S90. doi: 10.1017/S0007114510003958. [DOI] [PubMed] [Google Scholar]
- 12.Nardini M, Cirillo E, Natella F, Scaccini C. Absorption of phenolic acids in humans after coffee consumption. Journal of Agricultural and Food Chemistry. 2002;50(20):5735–5741. doi: 10.1021/jf0257547. [DOI] [PubMed] [Google Scholar]
- 13.Nardini M, Natella F, Scaccini C, Ghiselli A. Phenolic acids from beer are absorbed and extensively metabolized in humans. Journal of Nutritional Biochemistry. 2006;17(1):14–22. doi: 10.1016/j.jnutbio.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Gil MI, Ferreres F, Tomás-Barberán FA. Effect of modified atmosphere packaging on the flavonoids and vitamin C content of minimally processed swiss chard (beta vulgaris subspecies cycla) Journal of Agricultural and Food Chemistry. 1998;46(5):2007–2012. [Google Scholar]
- 15.Vitaglione P, Donnarumma G, Napolitano A, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. Journal of Nutrition. 2007;137(9):2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 16.Kamata H, Hirata H. Redox regulation of cellular signalling. Cellular Signalling. 1999;11(1):1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 17.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biological Chemistry. 2009;390(3):191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fruehauf JP, Meyskens FL., Jr. Reactive oxygen species: a breath of life or death? Clinical Cancer Research. 2007;13(3):789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 19.Battisti V, Maders LD, Bagatini MD, et al. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clinical Biochemistry. 2008;41(7-8):511–518. doi: 10.1016/j.clinbiochem.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar MJ, Bowen DT. Oxidative stress and the myelodysplastic syndromes. International Journal of Hematology. 2003;77(4):342–350. doi: 10.1007/BF02982641. [DOI] [PubMed] [Google Scholar]
- 21.Sallmyr A, Fan J, Rassool FV. Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Letters. 2008;270(1):1–9. doi: 10.1016/j.canlet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25(2):281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochemical Journal. 2007;401(1):1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 24.Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117(22):5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 25.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radical Research. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs. 2011;71(11):1385–1396. doi: 10.2165/11592590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews Drug Discovery. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 28.Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxidative Medicine and Cellular Longevity. 2010;3(1):23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clerkin JS, Naughton R, Quiney C, Cotter TG. Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Letters. 2008;266(1):30–36. doi: 10.1016/j.canlet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxidants and Redox Signaling. 2009;11(12):3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is a question. Cancer Biology and Therapy. 2008;7(12):1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 32.Martelli AM, Tabellini G, Bortul R, et al. Involvement of the phosphoinositide 3-kinase/Akt signaling pathway in the resistance to therapeutic treatments of human leukemias. Histology and Histopathology. 2005;20(1):239–252. doi: 10.14670/HH-20.239. [DOI] [PubMed] [Google Scholar]
- 33.Dong-Yun S, Yu-Ru D, Shan-Lin L, Ya-Dong Z, Lian W. Redox stress regulates cell proliferation and apoptosis of human hepatoma through Akt protein phosphorylation. FEBS Letters. 2003;542(1–3):60–64. doi: 10.1016/s0014-5793(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 34.Cao J, Xu D, Wang D, et al. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radical Biology and Medicine. 2009;47(5):536–547. doi: 10.1016/j.freeradbiomed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Dozio E, Ruscica M, Passafaro L, et al. The natural antioxidant alpha-lipoic acid induces p27Kip1-dependent cell cycle arrest and apoptosis in MCF-7 human breast cancer cells. European Journal of Pharmacology. 2010;641(1):29–34. doi: 10.1016/j.ejphar.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Martelli AM, Nyåkern M, Tabellini G, et al. Phosphoinositide 3-kinase/ Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20(6):911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 37.Maraldi T, Prata C, Vieceli Dalla Sega F, et al. NAD(P)H oxidase isoform Nox2 plays a prosurvival role in human leukaemia cells. Free Radical Research. 2009;43(11):1111–1121. doi: 10.1080/10715760903186132. [DOI] [PubMed] [Google Scholar]
- 38.Maraldi T, Prata C, Fiorentini D, Zambonin L, Landi L, Hakim G. Induction of apoptosis in a human leukemic cell line via reactive oxygen species modulation by antioxidants. Free Radical Biology and Medicine. 2009;46(2):244–252. doi: 10.1016/j.freeradbiomed.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Prata C, Maraldi T, Fiorentini D, Zambonin L, Hakim G, Landi L. Nox-generated ROS modulate glucose uptake in a leukaemic cell line. Free Radical Research. 2008;42(5):405–414. doi: 10.1080/10715760802047344. [DOI] [PubMed] [Google Scholar]
- 40.Maraldi T, Prata C, Caliceti C, et al. VEGF-induced ROS generation from NAD(P)H oxidases protects human leukemic cells from apoptosis. International Journal of Oncology. 2010;36(6):1581–1589. doi: 10.3892/ijo_00000645. [DOI] [PubMed] [Google Scholar]
- 41.Fiorentini D, Cipollone M, Galli MC, Pugnaloni A, Biagini G, Landi L. Characterization of large unilamellar vesicles as models for studies of lipid peroxidation initiated by azocompounds. Free Radical Research. 1994;21(5):329–339. doi: 10.3109/10715769409056585. [DOI] [PubMed] [Google Scholar]
- 42.Lepoivre M, Roche AC, Tenu JP, Petit JF, Nolibe D, Monsigny M. Identification of two macrophage populations by flow cytometry monitoring of oxidative burst and phagocytic functions. Biology of the Cell. 1986;57(2):143–146. doi: 10.1111/j.1768-322x.1986.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 43.Vowells SJ, Sekhsaria S, Malech HL, Shalit M, Fleisher TA. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. Journal of Immunological Methods. 1995;178(1):89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 44.Cole SP. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemotherapy and Pharmacology. 1986;17(3):259–263. doi: 10.1007/BF00256695. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto Y, Niki E, Kamiya Y, Shimasaki H. Oxidation of lipids. 7. Oxidation of phosphatidylcholines in homogeneous solution and in water dispersion. Biochimica et Biophysica Acta. 1984;795(2):332–340. [PubMed] [Google Scholar]
- 46.Pedulli GF, Lucarini M, Marchesi E, et al. Medium effects on the antioxidant activity of dipyridamole. Free Radical Biology and Medicine. 1999;26(3-4):295–302. doi: 10.1016/s0891-5849(98)00191-9. [DOI] [PubMed] [Google Scholar]
- 47.Amorati R, Pedulli GF, Cabrini L, Zambonin L, Landi L. Solvent and pH effects on the antioxidant activity of caffeic and other phenolic acids. Journal of Agricultural and Food Chemistry. 2006;54(8):2932–2937. doi: 10.1021/jf053159+. [DOI] [PubMed] [Google Scholar]
- 48.Pryor WA, Cornicelli JA, Devall LJ, et al. A rapid screening test to determine the antioxidant potencies of natural and synthetic antioxidants. Journal of Organic Chemistry. 1993;58(13):3521–3522. [Google Scholar]
- 49.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Research. 1995;22(4):375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 50.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 51.Bors W, Michel C, Stettmaier K. Antioxidant effects of flavonoids. BioFactors. 1997;6(4):399–402. doi: 10.1002/biof.5520060405. [DOI] [PubMed] [Google Scholar]
- 52.Bors W, Michel C, Stettmaier K. Structure-activity relationships governing antioxidant capacities of plant polyphenols. Methods in Enzymology. 2001;335:166–180. doi: 10.1016/s0076-6879(01)35241-2. [DOI] [PubMed] [Google Scholar]
- 53.Pedrielli P, Pedulli GF, Skibsted LH. Antioxidant mechanism of flavonoids. Solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate. Journal of Agricultural and Food Chemistry. 2001;49(6):3034–3040. doi: 10.1021/jf010017g. [DOI] [PubMed] [Google Scholar]
- 54.Brigati G, Lucarini M, Mugnaini V, Pedulli GF. Determination of the substituent effect on the O-H bond dissociation enthalpies of phenolic antioxidants by the EPR radical equilibration technique. Journal of Organic Chemistry. 2002;67(14):4828–4832. doi: 10.1021/jo025755y. [DOI] [PubMed] [Google Scholar]
- 55.Cao N, Yao ZX. The hemangioblast: from concept to authentication. Anatomical Record. 2011;294(4):580–588. doi: 10.1002/ar.21360. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z, Hattori K, Zhang H, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17(3):604–611. doi: 10.1038/sj.leu.2402831. [DOI] [PubMed] [Google Scholar]
- 57.Aguayo A, Kantarjian H, Manshouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96(6):2240–2245. [PubMed] [Google Scholar]
- 58.McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5(supplement 1):3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 59.Shinkaruk S, Bayle M, Laïn G, Déléris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Current Medicinal Chemistry—Anti-Cancer Agents. 2003;3(2):95–117. doi: 10.2174/1568011033353452. [DOI] [PubMed] [Google Scholar]
- 60.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. Journal of Biochemistry and Molecular Biology. 2006;39(5):469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 61.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature Medicine. 2011;17(11):1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 62.Rajagopalan V, Essex DW, Shapiro SS, Konkle BA. Tumor necrosis factor-α modulation of glycoprotein Ibα expression in human endothelial and erythroleukemia cells. Blood. 1992;80(1):153–161. [PubMed] [Google Scholar]
- 63.Weigel-Kelley KA, Yoder MC, Srivastava A. Recombinant human parvovirus B19 vectors: erythrocyte P antigen is necessary but not sufficient for successful transduction of human hematopoietic cells. Journal of Virology. 2001;75(9):4110–4116. doi: 10.1128/JVI.75.9.4110-4116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods in Molecular Biology. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 65.Farah A, Monteiro M, Donangelo CM, Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. Journal of Nutrition. 2008;138(12):2309–2315. doi: 10.3945/jn.108.095554. [DOI] [PubMed] [Google Scholar]
- 66.Valls RM, Soler A, Girona J, et al. Effect of the long-term regular intake of virgin olive oil on the phenolic metabolites in human fasting plasma. Journal of Pharmaceutical and Biomedical Analysis. 2010;53(1):68–74. doi: 10.1016/j.jpba.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Del Rio D, Stalmach A, Calani L, Crozier A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients. 2010;2(8):820–833. doi: 10.3390/nu2080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maraldi T, Prata C, Fiorentini D, Zambonin L, Landi L, Hakim G. Signal processes and ROS production in glucose transport regulation by thrombopoietin and granulocyte macrophage-colony stimulation factor in a human leukaemic cell line. Free Radical Research. 2007;41(12):1348–1357. doi: 10.1080/10715760701730347. [DOI] [PubMed] [Google Scholar]
- 69.Maraldi T, Fiorentini D, Prata C, Landi L, Hakim G. Glucose-transport regulation in leukemic cells: how can H2O2 mimic stem cell factor effects? Antioxidants and Redox Signaling. 2007;9(2):271–279. doi: 10.1089/ars.2007.9.271. [DOI] [PubMed] [Google Scholar]
- 70.Prata C, Maraldi T, Zambonin L, Fiorentini D, Hakim G, Landi L. ROS production and Glut1 activity in two human megakaryocytic cell lines. BioFactors. 2004;20(4):223–233. doi: 10.1002/biof.5520200406. [DOI] [PubMed] [Google Scholar]
- 71.Fiorentini D, Prata C, Maraldi T, et al. Contribution of reactive oxygen species to the regulation of Glut1 in two hemopoietic cell lines differing in cytokine sensitivity. Free Radical Biology and Medicine. 2004;37(9):1402–1411. doi: 10.1016/j.freeradbiomed.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 72.Maraldi T, Fiorentini D, Prata C, Landi L, Hakim G. Stem cell factor and H2O2 induce GLUT1 translocation in M07e cells. BioFactors. 2004;20(2):97–108. doi: 10.1002/biof.5520200204. [DOI] [PubMed] [Google Scholar]
- 73.Sims JT, Plattner R. MTT assays cannot be utilized to study the effects of STI571/Gleevec on the viability of solid tumor cell lines. Cancer Chemotherapy and Pharmacology. 2009;64(3):629–633. doi: 10.1007/s00280-009-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruggisser R, von Daeniken K, Jundt G, Schaffner W, Tullberg-Reinert H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Medica. 2002;68(5):445–448. doi: 10.1055/s-2002-32073. [DOI] [PubMed] [Google Scholar]
- 75.Peng L, Wang B, Ren P. Reduction of MTT by flavonoids in the absence of cells. Colloids and Surfaces B. 2005;45(2):108–111. doi: 10.1016/j.colsurfb.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 76.Wisman KN, Perkins AA, Jeffers MD, Hagerman AE. Accurate assessment of the bioactivities of redox-active polyphenolic in cell culture. Journal of Agricultural and Food Chemistry. 2008;56(17):7831–7837. doi: 10.1021/jf8011954. [DOI] [PubMed] [Google Scholar]
- 77.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/pbotein kinace B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Research. 2001;61(10):3986–3997. [PubMed] [Google Scholar]
- 78.Dutton A, Reynolds GM, Dawson CW, Young LS, Murray PG. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin’s lymphoma cells through a mechanism involving Akt kinase and mTOR. Journal of Pathology. 2005;205(4):498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- 79.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nature Reviews Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]


