Abstract
Objective
To evaluate the safety and outcomes of mitral isthmus (MI) linear ablation with temporary spot occlusion of the coronary sinus (CS).
Background
CS blood flow cools local tissue precluding transmurality and bidirectional block across MI lesion.
Methods
In a randomized, controlled trial (CS-occlusion = 20, Control = 22), MI ablation was performed during continuous CS pacing to monitor the moment of block. CS was occluded at the ablation site using 1 cm spherical balloon, Swan–Ganz catheter with angiographic confirmation. Ablation was started at posterior mitral annulus and continued up to left inferior pulmonary vein (LIPV) ostium using an irrigated-tip catheter. If block was achieved, balloon was deflated and linear block confirmed. If not, additional ablation was performed epicardially (power ≤25 W). Ablation was abandoned after ~30 minutes, if block was not achieved.
Results
CS occlusion (mean duration –27 ± 9 minutes) was achieved in all cases. Complete MI block was achieved in 13/20 (65%) and 15/22 (68%) patients in the CS-occlusion and control arms, respectively, P = 0.76. Block was achieved with significantly small number (0.5 ± 0.8 vs 1.9 ± 1.1, P = 0.0008) and duration (1.2 ± 1.7 vs 4.2 ± 3.5 minutes, P = 0.009) of epicardial radiofrequency (RF) applications and significantly lower amount of epicardial energy (1.3 ± 2.4 vs 6.3 ± 5.7 kJ, P = 0.006) in the CS-occlusion versus control arm, respectively. There was no difference in total RF (22 ± 9 vs 23 ± 11 minutes, P = 0.76), procedural (36 ± 16 vs 39 ± 20 minutes, P = 0.57), and fluoroscopic (13 ± 7 vs 15 ± 10 minutes, P = 0.46) durations for MI ablation between the 2 arms. Clinically uneventful CS dissection occurred in 1 patient
Conclusions
Temporary spot occlusion of CS is safe and significantly reduces the requirement of epicardial ablation to achieve MI block. It does not improve overall procedural success rate and procedural duration. Tissue cooling by CS blood flow is just one of the several challenges in MI ablation.
Keywords: ablation, balloon, coronary sinus, mitral isthmus, occlusion
Introduction
In addition to pulmonary vein isolation (PVI), left atrial (LA) linear lesions are performed to modify the substrate for atrial fibrillation (AF) and they have been recommended to improve the clinical outcome.1-6 However, discontinuities within linear lesion can be pro-arrhythmic and should be avoided.1,7 Achievement of bidirectional block at mitral isthmus (MI) continues to remain a major challenge. Variable success rates ranging from 65% to 92% have been reported after a combination of endocardial- and epicardial-coronary sinus (CS) radiofrequency (RF) ablation.2-10 Combined endo-epicardial approach has been reported in 50–75% cases of MI linear ablation. The difficulty in obtaining bidirectional block is multifactorial and results from poor electrode tissue contact, anatomic irregularities, heat sink effect from coronary vascular flow, and variable thickness of the local tissue.11-13 Besides, the potential “heat-sink” effect of the venous blood flow in the CS overlying the site of RF delivery is hypothesized to cool the local tissue by convective heat loss and limit the lesion size.14,15 Therefore, we performed a randomized controlled trial to evaluate the feasibility, safety, acute success rate, and other procedural parameters of MI linear ablation using a 1-cm spherical balloon for temporary spot occlusion of the CS.
Methods
Study Population
This study was performed according to the institutional guidelines. We studied 42 patients (age 57 ± 11 years) referred for the index ablation of symptomatic, drug refractory, paroxysmal (n = 16) or persistent (n = 26) AF, who provided written informed consent for participation in the study.
Electrophysiological Study
Antiarrhythmic medications were discontinued ≥5 half-lives prior to ablation with the exception of amiodarone. For at least 1 month prior to the procedure, patients took oral anticoagulants and achieved target INR of 2–3. Transesophageal echocardiography was performed within 5 days to exclude atrial thrombus. Oral anticoagulation was stopped 48 hours before the procedure and resumed on the day following the ablation. After transseptal access, an intravenous bolus of heparin (0.5 mg/kg of body weight) was administered and repeated later, if the activated clotting time (ACT) was <250 seconds.
Femoral venous access was established under local anesthesia and conscious sedation. A steerable quadripolar or decapolar catheter (5 mm electrode spacing, Xtrem, ELA Medical, Montrouge, France) was placed within the CS. Surface and bipolar endocardial electrocardiograms (ECGs) were continuously monitored at a sweep speed of 100 mm/s and recorded (Labsystem Pro, Bard, Tewksbury, MA, USA). ECG and intracardiac electrograms were filtered from 0.05 to 100 Hz and 30 to 500 Hz, respectively.
Study Protocol
PVI was performed at the start of the procedure in all patients. For MI ablation, the patients were randomized to CS-occlusion (n = 20) or no CS-occlusion (control) (n = 22) arms. MI ablation was performed in sinus rhythm (CS pacing) to monitor the occurrence of block. In addition, most patients underwent cavotricuspid isthmus ablation with an endpoint of bidirectional isthmus block based on conventional assessment technique.
Ablation Procedure
PVI
PVI was performed as previously described in sinus rhythm (n = 7) or in AF (n = 35).16 Ablation was performed using a 3.5 mm externally irrigated-tip catheter (Thermocool, Biosense Webster, Diamond Bar, CA, USA). Ablation energy was delivered to PV-LA junction with a power of 30–35 W using irrigation rates of 5–60 mL/min (0.9% normal saline via a Cool Flow® pump, Biosense Webster) to achieve the desired power. The endpoint was complete electrical isolation of all PVs.1 Following PVI, cardioversion was required in 15 patients before starting MI ablation in sinus rhythm (CS pacing).
Coronary sinus occlusion
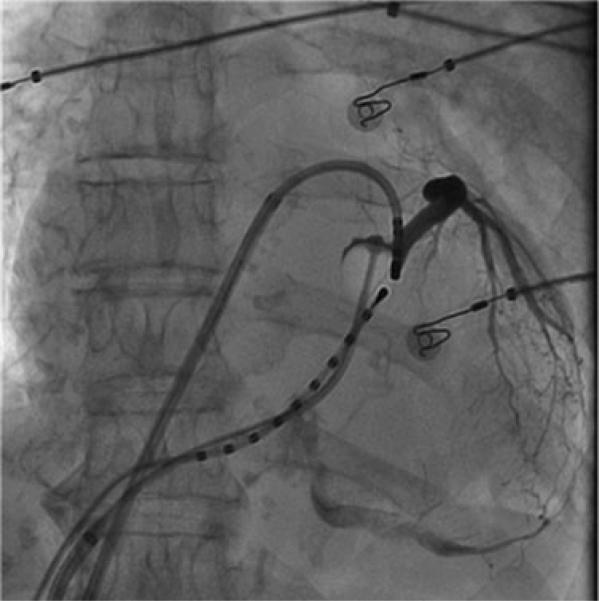
A 7F Swan-Ganz balloon catheter (Edwards Lifesciences, Irvine, CA, USA) was used for temporarily occluding the CS. CS cannulation was facilitated by the use of a long sheath (Preface, Biosense Webster or SL0 St. Jude Medical Inc., St. Paul, MN, USA). The distal tip of the catheter was positioned at 2–3 o'clock on the annulus. A spherical balloon (1 cm diameter) located just proximal to the catheter tip, was inflated with 1 cc air and complete obstruction of blood flow across the inflated balloon in the CS confirmed with contrast injection (Fig. 1). There is a thermocouple on the distal end of the catheter that was used to measure the epicardial temperature data throughout the endocardial MI ablation.
Figure 1.
Venogram shows complete distal occlusion of the coronary sinus using 1 cc balloon catheter. Ablation catheter is located on the endocardial mitral isthmus and decapolar catheter is positioned inside the coronary sinus.
Mitral Isthmus Ablation
Control arm
MI ablation was performed during distal CS pacing by creating a linear lesion joining the infero-lateral mitral annulus to the left inferior PV as previously described.17 Briefly, the ablation catheter was introduced through the long sheath that would impact stability during ablation. The catheter was curved 90–180° and ablation commenced at around 3–4 o'clock on the ventricular edge of the lateral mitral annulus. The catheter was dragged gradually back into the sheath to extend the lesion along the isthmus in a linear fashion up to the left inferior PV ostium lying at 2–3 o'clock position at the upper end of the line. After initial ablation, endocardial and epicardial mapping was performed to identify and ablate the gaps. Endocardial ablation was performed with a flow rate of 17–60 mL/min, and target temperature of 45°C (usually below 42°C) with power up to 35 W. Epicardial ablation was performed in the CS with a power limited to 20–25 W.
CS occlusion arm
The balloon was inflated with approximately 1 mL of air and occlusion of the CS-great cardiac vein junction confirmed by contrast injection through distal tip of the balloon catheter. Ablation was started with the catheter positioned at the annular (ventricular) end of the isthmus at the site facing the occlusive CS balloon (Fig. 1). If linear block was achieved, the balloon was deflated and linear block confirmed. If not, additional ablation was performed epicardially in the CS after deflating and removing the balloon.
Ablation Endpoint
The endpoint of ablation was the achievement of bidirectional MI conduction block demonstrated using previously described technique and criteria.17,18 In CS occlusion and control arms, ablation was abandoned after about 30 minutes of RF application, if the block was not achieved.
Ablation and Procedural Parameters
The ablation parameters (average power, temperature [endo ± epi] and impedance, duration of RF application) and the conduction delay were recorded continuously on the electrophysiology recording system during each RF application. The periprocedural parameters (total duration of RF application, procedural time, fluoroscopic time, preand postprocedural delay across the line) were also noted separately.
Electrogram Amplitude
During sinus rhythm, the amplitude of bipolar electrograms was measured at 3 different sites along the MI line before local ablation: at the level of the mitral annulus, in the middle of the MI, and at the top of the MI line. A possible relationship between the preablation local electrogram amplitude and the acute success rate of MI linear ablation was investigated parallelly.
Monitoring Complications
Acute periprocedural complications related to temporary CS balloon occlusion and MI ablation were systematically noted. Acute onset of ischemic symptoms and ECG changes as markers of acute procedure-related ischemia were also monitored
Study Endpoints
The primary endpoint of the study was the feasibility and safety of spot balloon occlusion of CS and acute procedural success demonstrated by achievement of bidirectional and complete (transmural) MI block. The secondary endpoints were the RF duration, number of endocardial and epicardial lesions, amount of RF energy and procedural and fluroscopic durations for MI ablation.
Follow-Up
All patients were monitored in hospital for at least 5 days postprocedure. Following ablation, all antiarrhythmic drugs were ceased unless otherwise indicated. Patients were reevaluated at 1, 3, 6, 9, and 12 months, and in the absence of AF or symptoms, followed up with their referring physician. At each visit, exercise testing and ambulatory 48 hours monitoring were performed to detect asymptomatic arrhythmias and provocable ischemia. In the event of symptomatic or asymptomatic arrhythmia recurrence, patients were offered additional ablation after a trial of drug therapy. Cessation of anticoagulant therapy was considered 6–9 months after the last procedure in the absence of recurrence.
The follow-up pattern described is general and was not different for the patients enrolled in the study. The study was not aimed at evaluating the parameters beyond the acute procedural outcomes.
Statistical Analysis
Continuous variables are expressed as mean ± SD except where stated as median and interquartile (IQ) range. Statistical significance was assessed using the unpaired Student's t-test or Mann–Whitney test, if necessary. Categorical variables, expressed as numbers or percentages, were analyzed with the χ2 test or Fisher's exact test. All tests were 2-tailed and a P-value <0.05 was considered statistically significant.
Results
Clinical Characteristics
Among 42 patients involved in the study, 20 patients were randomized to CS occlusion arm and the remaining 22 formed the control arm. The comparison of all the baseline clinical characteristics among the 2 arms is provided in Table 1. There was no significant difference in the age, gender, type of AF, presence of structural heart disease, duration of uninterrupted persistent AF, use of antiarrhythmic drugs prior to ablation, and echocardiographic left heart dimensions between the 2 arms.
TABLE 1.
Baseline Characteristics
| Clinical Characteristic | Cases (n = 20) | Controls (n = 22) | P-Value |
|---|---|---|---|
| Age (years) | 57 ± 10 | 57 ± 11 | 1.0 |
| Male | 15 (75%) | 17 (77%) | 1.0 |
| Hypertension | 10 (50%) | 8 (36%) | 0.53 |
| Structural heart disease | 5 (25%) | 8 (36%) | 0.51 |
| Persistent AF | 11 (55%) | 16 (73%) | 0.33 |
| Uninterrupted persistent AF (months) | 18 ± 13 (n = 9) | 31 ± 23 (n = 14) | 0.14 |
| Antiarrhythmic drugs | 2 ± 0.9 | 1.7 ± 0.7 | 0.23 |
| Amiodarone usage | 12 (60%) | 12 (55%) | 0.57 |
| LVEDD (mm) | 54 ± 8 | 55 ± 9 | 0.71 |
| LVESD (mm) | 36 ± 8 | 36 ± 7 | 1.0 |
| LVEF(%) | 59 ± 13 | 55 ± 16 | 0.38 |
| LA diameter (mm) | 43 ± 7 | 47 ± 9 | 0.12 |
AF = atrial fibrillation; EDD = end diastolic dimension; EF = ejection fraction; ESD = end systolic dimension; LA = left atrium; LV = left ventricular.
PV Isolation
PVI was achieved in all patients after 50 ± 22 minutes versus 45 ± 20 minutes of RF delivery in the case versus control groups (P = 0.49). Sinus rhythm was achieved during ablation in 27 patients and the remaining 15 patients required cardioversion for MI ablation.
MI Ablation Parameters
Primary endpoints
Feasibility and safety of spot balloon occlusion of CS
The spherical balloon could be inflated and steadied in the CS in all the 20 cases. Angiographically confirmed CS occlusion was also achieved in all of them. Mean CS occlusion time was 27 ± 9 minutes. One patient developed dissection from balloon occlusion of the CS. There was no clinical event ensuing from this complication during postprocedural hospital stay and at >1 year of follow-up despite continued anticoagulation during the initial period of follow-up. There was no tamponade, stroke, acute ischemia or other embolic event in the periprocedural period.
Acute procedural success
At the end of the procedure, 13 (65%) patients in the CS occlusion arm and 15 (68%) in the control arm attained complete, bidirectional MI block (P = 0.76). In the CS occlusion arm, balloon occlusion time was not significantly different (25 ± 11 minutes vs 30 ± 5 minutes, respectively; P = 0.27) between the patients with and without MI block.
Secondary endpoints
CS occlusion arm versus control arm: all patients
The comparison of the secondary endpoints between the patients in 2 study-arms is provided in Table 2. Total (endocardial + epicardial) RF application time at the MI (22 ± 9 minutes vs 23 ± 11 minutes, P = 0.75), the amount of RF energy applied on the MI (39.6 ± 19.9 kJ vs 47.2 ± 28.1 kJ, P = 0.32), the MI ablation procedural (36 ± 16 minutes vs 39 ± 20 minutes, P = 0.57), and the MI ablation fluoroscopic (13 ± 7 minutes vs. 15 ± 10 minutes) durations did not differ between the occlusion and control arms. The RF application, procedural, and fluoroscopic durations for the entire AF ablation procedure were also similar between the 2 arms (Table 2).
TABLE 2.
Procedural Parameters
| Procedural Parameter | Cases (n = 20) | Controls (n = 22) | P-Value |
|---|---|---|---|
| MI RF duration—total (minutes) | 22 ± 9 | 23 ± 11 | 0.75 |
| MI RF energy—total (kJ) | 39.6 ± 19.9 | 47.2 ± 28.1 | 0.32 |
| MI RF lesions—endo (N) | 7 ± 4 | 7 ± 4 | 1.0 |
| MI RF duration—endo (minutes) | 20 ± 8 | 19 ± 8 | 0.69 |
| MI RF energy—endo (kJ) | 37.1 ± 18 | 39.4 ± 23.5 | 0.72 |
| MI RF lesions—epi (N) | 0.9 ± 0.9 | 2.1 ± 1.2 | 0.001 |
| MI RF duration—epi (minutes) | 2 ± 2 | 5 ± 4 | 0.004 |
| MI RF energy—epi (kJ) | 2.5 ± 2.7 | 7.8 ± 6.3 | 0.001 |
| MI fluoroscopy time (minutes) | 13 ± 7 | 15 ± 10 | 0.46 |
| MI procedure time (minutes) | 36 ± 16 | 39 ± 20 | 0.57 |
| PV isolation rf duration (minutes) | 50 ± 22 | 45 ± 20(n = 16) | 0.49 |
| Total RF duration (minutes) | 84 ± 26 | 81 ± 32 | 0.74 |
| Total fluoroscopy time (minutes) | 67 ± 20 | 65 ± 26 | 0.78 |
| Total procedure time (minutes) | 262 ± 67 | 239 ± 86 | 0.34 |
endo = endocardial; epi = epicardial; kJ = kilo Joules; MI = mitral isthmus; PV = pulmonary vein; RF = radiofrequency.
When the RF application parameters were analyzed separately for endocardial and epicardial applications on MI line (Table 2), the number of epicardial lesions (0.9 ± 0.9 vs 2.1 ± 1.2, P = 0.001), duration of RF (2 ± 2 minutes vs 5 ± 4 minutes, P = 0.004), and amount of RF energy applied epicardially on MI line (2.5 ± 2.7 kJ vs 7.8 ± 6.3 kJ, P = 0.001) were significantly fewer in the CS occlusion arm in comparison with the control arm. However, there were no significant differences among these parameters for endocardial RF applications on MI line (Table 2).
CS occlusion arm versus control arm: MI block patients only
As stated earlier, MI block could be achieved in statistically similar number of patients (65% vs 68%, P = 0.76) without significant difference in time to acute block from the onset of RF application (18 ± 8 minutes vs 18 ± 11 minutes, respectively; P = 1.0) in the 2 arms. Also, the perimitral conduction time from distal CS to the site across the line of block was not different (160 ± 30 milliseconds vs 174 ± 36 milliseconds, respectively; P = 0.27) between the CS occlusion and control arms. Nevertheless, in the occlusion arm, 7/13 (54%) patients with MI block developed rapid-onset conduction delay across the lesion after mean 3.6 ± 0.8 minutes of endocardial RF application including 1 patient with epicardial conduction block (Figs. 2 and 3). Such rapid-onset delay in conduction across the linear lesion was observed in 1/15 (7%) patients of the control arm (54% vs 7%; P = 0.01).
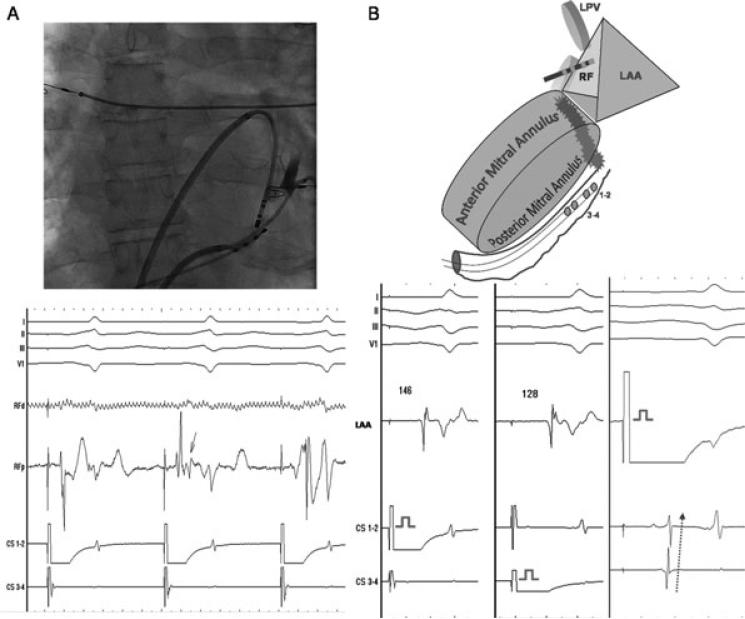
Figure 2.
A: Ablation with balloon occluded coronary sinus (shown in the fluoroscopic image) results in rapid-onset conduction delay (on RFp: from 72 milliseconds to 146 milliseconds) at the mitral isthmus after 4 minutes and 25 seconds of endocardial RF application. The arrow denotes the moment of split in the potential, which was followed by sudden prolongation of delay. B: Schema and electrograms demonstrating bidirectional block across the mitral isthmus during differential CS pacing, and pacing from the LAA at the end of the procedure. CS = coronary sinus; LAA = left atrial appendage; LPV = left pulmonary veins = RF = radiofrequency.
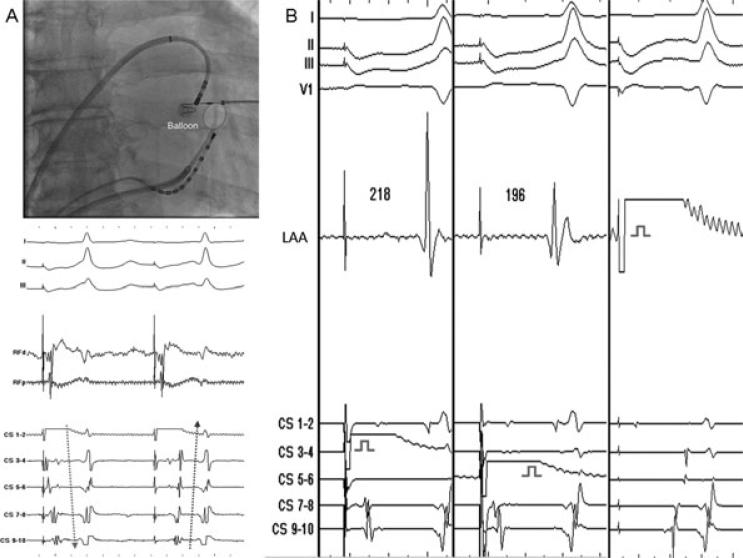
Figure 3.
A: Endocardial ablation with balloon occluded coronary sinus results in acute onset (marked by red star) of epicardial block (compare the arrows) at the mistral isthmus after 3 minutes of application. There is no influence on endocardial conduction across the mitral isthmus at this stage as evident by the absence of alteration in the activation of the contiguous left atrium determined from the low-amplitude, slushy, far-field left atrial potentials recorded on CS bipoles. B: Demonstration of bidirectional block across the mitral isthmus during differential CS pacing, and pacing from the LAA at the end of the procedure. CS = coronary sinus; LAA = left atrial appendage; RF = radiofrequency.
The comparison of the secondary endpoints between the patients with bidirectional MI block in the 2 arms is provided in Table 3. The combined (endocardial + epicardial) MI RF application time (18 ± 8 minutes vs 19 ± 10 minutes, P = 0.77), amount of RF energy applied on MI line (29.6 ± 17.3 kJ vs 40.5 ± 30.7, P = 0.26), the MI ablation procedural (30 ± 14 minutes vs 33 ± 19 minutes, P = 0.64), and fluoroscopic 12 ± 8 minutes vs 13 ± 9 minutes, P = 0.76) durations were similar between the 2 arms.
TABLE 3.
Blocked Mitral Isthmus Parameters
| MI Block Parameters | Cases (n = 13) | Controls (n = 15) | P-Value |
|---|---|---|---|
| MI blocked (%) | 13/20 (65) | 15/22 (68) | 0.76 |
| Time to block (minutes) | 18 ± 8 | 18 ± 11 | 1.0 |
| Delay on MI during distal CS pacing (milliseconds) | 160 ± 30 | 174 ± 36 | 0.27 |
| MI ablation time (minutes) | 30 ± 14 | 33 ± 19 | 0.64 |
| Fluoroscopy for MI (minutes) | 12 ± 8 | 13 ± 9 | 0.76 |
| MI RF duration—total (minutes) | 18 ± 8 | 19 ± 10 | 0.77 |
| MI RF energy—total (kJ) | 29.6 ± 17.3 | 40.5 ± 30.7 | 0.26 |
| MI RF lesions—endo (N) | 6 ± 4 | 6 ± 3 | 1.0 |
| MI RF duration—endo (minutes) | 16 ± 8 | 15 ± 7 | 0.72 |
| MI RF energy—endo (kJ) | 28.3 ± 16.1 | 34.2 ± 25.8 | 0.48 |
| Patients with epicardial ablation (%) | 5113 (38) | 14115 (93) | 0.004 |
| MI RF lesions–epi (N) | 0.5 ± 0.8 | 1.9 ± 1.1 | 0.0008 |
| MI RF duration—epi (minutes) | 1.2 ± 1.7 (0,0–1.5) | 4.2 ± 3.5 | 0.009 |
| MI RF energy—epi (kJ) | 1.3 ± 2.4 | 6.3 ± 5.7 | 0.006 |
| Rapid-onset conduction delay—N (%) | 7 (54) | 1 (7) | 0.01 |
CS = coronary sinus; endo = endocardial; epi = epicardial; kJ = kilo Joules; MI = mitral isthmus; RF = radiofrequency.
On analyzing the secondary parameters separately between the epicardial and endocardial RF applications (Table 3), 5/13 (38%) patients required epicardial ablation in the CS occlusion arm versus 14/15 (93%) in the control arm (P = 0.004) to achieve MI block. Block was achieved with significantly small number (0.9 ± 0.9 vs 2.1 ± 1.2, P = 0.001) and duration 2 ± 2 minutes vs 5 ± 4 minutes, P = 0.004) of epicardial RF applications and significantly lower amount of epicardial RF energy (1.3 ± 2.4 kJ vs 6.3 ± 5.7 kJ, P = 0.006) in the occlusion arm as against the control arm, respectively. No such difference was found when the endocardial RF applications were compared between the 2 arms (Table 3).
Electrogram Amplitude
Table 4A and B shows the electrogram amplitude at high, mid, and low points on the MI line during sinus rhythm. There was no significant difference in the amplitude of local electrograms anywhere on MI line between the 2 arms (Table 4A).
TABLE 4A.
Amplitude of Electrograms
| Amplitude | Cases | Controls | P-Value |
|---|---|---|---|
| MI block + (13:15) | |||
| Mitral annulus | 1.2 ± 0.7 mV | 1.3 ± 0.7 mV | 0.71 |
| Mid mitral line | 1.4 ± 0.2 mV | 1.4 ± 0.6 mV | 1.0 |
| High mitral line | 1.2 ± 0.4 mV | 1.2 ± 0.6 mV | 1.0 |
| CS distal | 1.5±0.6mV | 1.1 ± 0.8 mV | 0.15 |
| MI block – (7:7) | |||
| Mitral annulus | 1.3 ± 0.9 mV | 0.9 ± 0.7 mV | 0.37 |
| Mid mitral line | 1.6 ± 0.4 mV | 1.9 ± 0.1 mV | 0.08 |
| High mitral line | 1.7 ± 0.3 mV | 1.4 ± 0.8 mV | 0.37 |
| CS distal | 1.9 ± 0.9 mV | 1.8 ± 1.6 mV | 0.89 |
CS = coronary sinus; mV = millivolt.
TABLE 4B.
Amplitude of Electrograms
| Amplitude | MI block + | MI block – | P-Value |
|---|---|---|---|
| Cases (13:7) | |||
| Mitral annulus | 1.2 ± 0.7 mV | 1.3 ± 0.9 mV | 0.78 |
| Mid mitral line | 1.4 ± 0.2 mV | 1.6 ± 0.4 mV | 0.14 |
| High mitral line | 1.2 ± 0.4 mV | 1.7 ± 0.3 mV | 0.009 |
| CS distal | 1.5 ± 0.6 mV | 1.9 ± 0.9 mV | 0.24 |
| Controls (15:7) | |||
| Mitral annulus | 1.3 ± 0.7 mV | 0.9 ± 0.7 mV | 0.22 |
| Mid mitral line | 1.4 ± 0.6 mV | 1.9 ± 0.1 mV | 0.04 |
| High mitral line | 1.2 ± 0.6 mV | 1.4 ± 0.8 mV | 0.52 |
| CS distal | 1.1 ± 0.8 mV | 1.8 ± 1.6 mV | 0.18 |
CS = coronary sinus; mV = millivolt.
Among the patients in the CS occlusion arm, the amplitude of electrograms recorded on the top of the MI line was significantly higher (1.7 ± 0.3 mV vs 1.2 ± 0.4 mV, respectively; P = 0.009) in patients who did not achieve MI block when compared with those who achieved block. The electrogram amplitudes elsewhere on the MI line were not significantly different (Table 4B). Similarly, among the patients in the control arm, the amplitude of electrograms recorded in the middle of the MI line was significantly higher (1.9 ± 0.1 mV vs 1.4 ± 0.6 mV, respectively; P = 0.04) in the patients who failed to achieve MI block than those who succeeded. Elsewhere on the mitral line, electrogram amplitudes were similar (Table 4B).
Discussion
Main Findings
The main findings of this study are as follows: (i) temporary balloon occlusion of CS during MI line ablation is feasible and safe; (ii) it obviates or curtails epicardial ablation to achieve MI block without altering the total amount of RF energy and the procedural and fluoroscopic durations for MI ablation; and (iii) it does not improve acute success rate of MI ablation.
Clinical Safety versus Clinical Benefit of the Novel Technique
Linear lesions improve the outcome of AF ablation procedures but their major drawback is proarrhythmia from incomplete block or recovery of conduction across the line of previous block.1,7 Using current ablation tools, it is not only difficult to achieve permanent block across the MI, but attaining 100% acute success rate as for PVI is not possible without compromising the patient safety.
In general, tamponade is the most commonly reported complication of AF ablation procedure with an incidence of 1.31%.19 High target temperatures (>45°C) and power delivery (>40 W) facilitated by higher irrigation rates, which led to acute success rates above 90% in a previous study, were associated with more than 3 times higher rate (4%) of cardiac tamponade than that observed in general.8 Consequently, the temperature and power were limited to <45°C (usually <42°C) and 35 W, respectively, for MI ablation protocol in the current study. These explain lower acute procedural success rates of 65–70%; however, notably, there were no adverse events. One patient who developed CS dissection was 1 of the first few cases enrolled in the study.
Collateral injury to left circumflex circulation has been reported during endo-epicardial MI ablation, independent of balloon occlusion of CS due to its proximity to the MI.8,20,21,22 High power (>40 W) and high target temperature have been held responsible for the damage.8,20 When low power and temperature targets were used as in the current study, no angiographic (with or without clinical) evidence of injury to the left circumflex circulation was observed.8 Stress tests undertaken at each follow-up visit were also negative for provocable ischemia.
Epicardial Ablation in MI Linear Lesion vis-à-vis CS Occlusion
During MI ablation, epicardial ablation from the CS is undertaken in 50–75% of cases to achieve transmural lesion.2-9 With CS overlying the lower (annular) end of the MI line, blood flow acts as a “heat-sink” for the endocardially delivered lesions in this area necessitating ablation from within the CS in the majority of cases. This hypothesis has been revalidated in the current study wherein CS occlusion curtailed the need for epicardial ablation. Besides, rapid onset of transmitral conduction delay during endocardial RF application was observed in the majority of the patients in the CS occlusion arm, but in only 1 patient in the control arm of the current study. Reduction in the need for epicardial ablation (48% vs. 83%, P = 0.01) was also the major finding in the CS occlusion arm of a recent report.20
Histologically, CS and inferior LA myocardium have multiple connections that play a vital role in sustaining mitral-isthmus-dependent arrhythmias.23,24 Thus, complete block across MI line is not possible without successful ablation of these epicardial connections. Due to variable anatomy and thickness of the myocardium in the region and frequently suboptimal tissue contact from the endocardium, ablating the epicardial component of MI line is a formidable task from the endocardium in up to 75% cases.2-9 All these factors and the “heat-sink” effect of CS blood flow are obviated by ablating directly from within the CS. Due to spot occlusion of CS by the inflated balloon facing the MI line, the “heat-sink” was voided regionally, thereby allowing endocardially deployed lesion attain a transmural depth in a large number of patients in this study. Only a minority of patients required additional ablation from the CS to achieve MI block and, of note, its duration was substantially reduced.
Lack of Improvement in Overall Outcome of MI Ablation with CS Occlusion
MI ablation is a challenging procedure due to multiple factors. Routine epicardial ablation necessarily resolves one but not all of these factors. Using CS occlusion obviated the need for epicardial ablation of the lower end of the MI line but could not improve the rates of acute MI block. Similar results were reported from another group of single-center investigators.20
MI is a long structure of variable length.25 The “heat-sink” effect of CS blood flow is limited to its lower end. The thickness of the tissue is not uniform all along its length.11-13 MI is located most distally in the left atrium when the latter is accessed via the transseptal route. Also, the catheter gets oriented parallel to the tissue and not perpendicular lowering the ablation–current density at the site of lesion. Although LA access via foramen ovale or transseptal hole does not influence the outcome of linear ablation at MI,10 it certainly does not provide good leverage for the application of uniform contact force all along the MI line. Also, the catheter stability in the region where the ablation should be uniformly undertaken over a long length of tissue is difficult. Not surprisingly, overall success rate, procedural duration, fluoroscopic time, and the amount of energy deposited endocardially and combined endo-epicardially were not significantly influenced by CS occlusion.
Role of Balloon Size and Shape in Temporary CS Occlusion for MI Ablation
Experimental14 and clinical20 studies undertaken hitherto have used long rectangular balloons (2–4 cm) to occlude CS along its entire length. Importantly, in humans, CS does not run all along the length of MI but only abuts its lower end. Inflating 1 cm spherical balloon in the distal CS against the lower spot on MI line could sufficiently replace the flowing blood and also occlude blood flow into its proximal portion. MI line runs perpendicular to the long axis of rectangular balloons positioned in the CS. Using long balloons may not extend the advantage higher up on the MI line where there is no epicardial “heat-sink.”
If balloon length could have impacted MI line ablation, Wong et al. should have observed higher rates of MI block in the occlusion arm than the control.20 But, there was no significant difference in the overall MI block rates (85% vs 93%, P = 0.43)20 between the 2 arms, which concurs with the outcome of the current study. Although direct comparison between 2 types of balloons would be ideal, similar observations made by 2 separate groups of investigators using different balloons indicate toward absence of any larger influence of long and big balloons on MI ablation than that currently observed with spherical balloon.
Local Electrogram Amplitude and MI Block
Local electrogram amplitude prior to any ablation (i.e., without local edema) in the region could represent the MI tissue mass (transmural thickness) and may impact its ablation. Although many factors like the propagation of wavefront, the orientation of bipole, and the degree of proximity to the tissue (contact) determine the amplitude of a bipolar electrogram, the amplitudes were uniformly measured in all the patients in sinus rhythm before undertaking MI ablation. We did not find significant difference in the amplitude of local electrograms between the 2 arms in the study. However, lower amplitude of electrograms was observed either on the top or middle of MI line in patients with MI block when compared with those without MI block. A possible influence of tissue mass in determining the acute success rates of MI ablation cannot be ruled out. Prospective evaluation of the impact of amplitude in patients matched for their ablation strategies could appropriately address their role in MI ablation.
Study Limitations
Specially designed balloons conforming to the shape of CS are not available. “One size balloon fits all” concept may not accurately reflect the influence of CS occlusion on MI ablation. The use of steerable sheath could have facilitated the procedure by improving the catheter–tissue contact force during MI ablation.26 However, we have uniformly used non-steerable sheaths in both the arms of the study.
Conclusions
Temporary spot occlusion of CS is feasible and safe using a 1 cm spherical balloon. It significantly reduces the requirement of epicardial ablation that is necessarily undertaken when the endocardial approach fails to achieve MI block. However, the overall success rate, the amount of RF energy applied endo-epicardially and the total fluoroscopic and procedural durations for MI ablation do not improve. CS blood flow cools the local MI tissue epicardially but it is not the only challenge in MI ablation. The others include the tissue thickness and length, the catheter orientation, and its contact with the tissue and the coronary arterial blood flow.
Footnotes
No disclosures.
References
- 1.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJJ, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, Mccarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert consensus statement on catheter surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Fassini G, Riva S, Chiodelli R, Trevisi N, Berti M, Carbucicchio C, Maccabelli G, Giraldi F, Bella PD. Left mitral isthmus ablation associated with PV isolation: Long-term results of a prospective randomized study. J Cardiovasc Electrophysiol. 2005;16:1150–1156. doi: 10.1111/j.1540-8167.2005.50192.x. [DOI] [PubMed] [Google Scholar]
- 3.Willems S, Klemm H, Rostock T, Brandstrup B, Ventura R, Steven D, Risius T, Lutomsky B, Meinertz T. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: A prospective randomized comparison. Eur Heart J. 2006;27:2871–2878. doi: 10.1093/eurheartj/ehl093. [DOI] [PubMed] [Google Scholar]
- 4.Yao Y, Zheng L, Zhang S, He DS, Zhang K, Tang M, Chen K, Pu J, Wang F, Chen X. Stepwise linear approach to catheter ablation of atrial fibrillation. Heart Rhythm. 2007;4:1497–1504. doi: 10.1016/j.hrthm.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Gaita F, Caponi D, Scaglione M, Montefusco A, Corleto A, Di Monte F, Coin D, Di Donna P, Giustetto C. Long-term clinical results of 2 different ablation strategies in patients with paroxysmal and persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2008;1:269–275. doi: 10.1161/CIRCEP.108.774885. [DOI] [PubMed] [Google Scholar]
- 6.Knecht S, Hocini M, Wright M, Lellouche N, O'Neill MD, Matsuo S, Nault I, Chauhan VS, Makati KJ, Bevilacqua M, Lim KT, Sacher F, Deplagne A, Derval N, Bordachar P, Jaïs P, Clémenty J, Haïssaguerre M. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008;29:2359–2366. doi: 10.1093/eurheartj/ehn302. [DOI] [PubMed] [Google Scholar]
- 7.Chae S, Oral H, Good E, Dey S, Wimmer A, Crawford T, Wells D, Sarrazin JF, Chalfoun N, Kuhne M, Fortino J, Huether E, Lemerand T, Pelosi F, Bogun F, Morady F, Chugh A. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: Mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007;50:1781–1787. doi: 10.1016/j.jacc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 8.Jaïs P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC, Le Metayer P, Clémenty J, Haïssaguerre M. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo S, Wright M, Knecht S, Nault I, Lellouche N, Lim KT, Arantes L, O'Neill MD, Hocini M, Jaïs P, Haïssaguerre M. Peri-mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm. 2010;7:2–8. doi: 10.1016/j.hrthm.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki S, Shah AJ, Nault I, Wright M, Jadidi AS, Forclaz A, Liu X, Linton N, Xhaët O, Rivard L, Derval N, Sacher F, Hocini M, Jaïs P, Haïssaguerre M. Impact of patent foramen ovale on left atrial linear lesions in the context of atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2011;22:846–850. doi: 10.1111/j.1540-8167.2010.02007.x. [DOI] [PubMed] [Google Scholar]
- 11.Wittkampf FH, Van Oosterhout MF, Loh P, Derksen R, Vonken EJ, Slootweg PJ, Ho SY. Where to draw the mitral isthmus line in catheter ablation of atrial fibrillation: Histological analysis. Eur Heart J. 2005;26:689–695. doi: 10.1093/eurheartj/ehi095. [DOI] [PubMed] [Google Scholar]
- 12.Becker AE. Left atrial isthmus: Anatomic aspects relevant for linear catheter ablation procedures in humans. J Cardiovasc Electrophysiol. 2004;15:809–812. doi: 10.1046/j.1540-8167.2004.03651.x. [DOI] [PubMed] [Google Scholar]
- 13.West JJ, Norton PT, Kramer CM, Moorman JR, Mahapatra S, DiMarco JP, Mangrum JM, Mounsey JP, Ferguson JD. Characterization of the mitral isthmus for atrial fibrillation ablation using intracardiac ultra-sound from within the coronary sinus. Heart Rhythm. 2008;5:19–27. doi: 10.1016/j.hrthm.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 14.D'Avila A, Thiagalingam A, Foley L, Fox M, Ruskin JN, Reddy VY. Temporary occlusion of the great cardiac vein and coronary sinus to facilitate radiofrequency catheter ablation of the mitral isthmus. J Cardiovasc Electrophysiol. 2008;19:645–650. doi: 10.1111/j.1540-8167.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 15.Reddy V, Ruskin JN, D'Avila A. Balloon occlusion of the coronary sinus to facilitate mitral isthmus ablation. J Cardiovasc Electrophysiol. 2008;19:651. doi: 10.1111/j.1540-8167.2008.01186.x. [DOI] [PubMed] [Google Scholar]
- 16.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 17.Jaïs P, Hocini M, O'Neill MD, Klein GJ, Knecht S, Sheiiro M, Arentes L, Kodali S, Clémenty J, Haïssaguerre M. How to perform linear lesions. Heart Rhythm. 2007;4:803–809. doi: 10.1016/j.hrthm.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Shah D, Haïssaguerre M, Takahashi A, Jaïs P, Hocini M, Clémenty J. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation. 2000;102:1517–1522. doi: 10.1161/01.cir.102.13.1517. [DOI] [PubMed] [Google Scholar]
- 19.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 20.Wong KC, Jones M, Qureshi N, Sadarminutes PP, De Bono J, Rajappan K, Bashir Y, Betts TR. Balloon occlusion of the distal coronary sinus facilitates mitral isthmus ablation. Heart Rhythm. 2011;8:833–839. doi: 10.1016/j.hrthm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Jais P, Hocini M, Sanders P, Rotter M, Rostock T, Sacher F, Jaïs C, Clémenty J, Haïssaguerre M. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J Cardiovasc Electrophysiol. 2005;16:1104–1107. doi: 10.1111/j.1540-8167.2005.50124.x. [DOI] [PubMed] [Google Scholar]
- 22.Yokokawa M, Sundaram B, Garg A, Stojanovska J, Oral H, Morady F, Chugh A. Impact of mitral isthmus anatomy on the likelihood of achieving linear block in patients undergoing catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8:1404–1410. doi: 10.1016/j.hrthm.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Chauvin M, Shah DC, Haïssaguerre M, Marcellin L, Brechenmacher C. The anatomic basis of connections between the coronary sinus musculature and the left atrium in humans. Circulation. 2000;101:647–652. doi: 10.1161/01.cir.101.6.647. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki S, Shah AJ, Haissaguerre M. Recurrent peri-mitral tachycardia using epicardial coronary sinus connection to bypass endocar-dial conduction block at mitral isthmus. Circ Arrhythm Electrophysiol. 2011;4:e39–241. doi: 10.1161/CIRCEP.111.963157. [DOI] [PubMed] [Google Scholar]
- 25.Becker AE. Left atrial isthmus: Anatomic aspects relevant for linear catheter ablation procedures in humans. J Cardiovasc Electrophysiol. 2004;15:809–812. doi: 10.1046/j.1540-8167.2004.03651.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo S, Yamane T, Date T, Hioki M, Narui R, Ito K, Tanigawa SI, Nakane T, Yamashita S, Tokuda M, Inada K, Nojiri A, Kawai M, Sugimoto KI, Yoshimura M. Completion of mitral isthmus ablation using a steerable sheath: Prospective randomized comparison with a nonsteerable sheath. J Cardiovasc Electrophysiol. 2011;22:1331–1338. doi: 10.1111/j.1540-8167.2011.02112.x. [DOI] [PubMed] [Google Scholar]