Abstract
Objectives
The aim of this study was to determine whether onset sites of human atrial fibrillation (AF) exhibit conduction slowing, reduced amplitude, and/or prolonged duration of signals (i.e., fractionation) immediately before AF onset.
Background
Few studies have identified functional determinants of AF initiation. Because conduction slowing is required for reentry, we hypothesized that AF from pulmonary vein triggers might initiate at sites exhibiting rate-dependent slowing in conduction velocity (CV restitution) or local slowing evidenced by signal fractionation.
Methods
In 28 patients with AF (left atrial size 43 ± 5 mm; n = 13 persistent) and 3 control subjects (no AF) at electrophysiological study, we measured bi-atrial conduction time (CT) electrogram fractionation at 64 or 128 electrodes with baskets in left (n = 17) or both (n = 14) atria during superior pulmonary vein pacing at cycle lengths (CL) accelerating from 500 ms (120 beats/min) to AF onset.
Results
Atrial fibrillation initiated in 19 of 28 AF patients and no control subjects. During rate acceleration, conduction slowed in 23 of 28 AF patients (vs. no control subjects, p = 0.01) at the site of AF initiation (15 of 19) or latest activated site (20 of 28). The CT lengthened from 79 ± 23 ms to 107 ± 39 ms (p < 0.001) on acceleration, in a spectrum from persistent AF (greatest slowing) to control subjects (least slowing; p < 0.05). Three patterns of CV restitution were observed: 1) broad (gradual CT prolongation, 37% patients); 2) steep (abrupt prolongation, at CL 266 ± 62 ms, 42%); and 3) flat (no prolongation, 21% AF patients, all control subjects). The AF initiation was more prevalent in patients with CV restitution (17 of 23 vs. 2 of 8; p = 0.03) and immediately followed abrupt reorientation of the activation vector in patients with broad but not steep CV restitution (p < 0.01). Patients with broad CV restitution had larger atria (p = 0.03) and were more likely to have persistent AF (p = 0.04). Notably, neither amplitude nor duration (fractionation) of the atrial signal at the AF initiation site were rate-dependent (both p = NS).
Conclusions
Acceleration-dependent slowing of atrial conduction (CV restitution) precedes AF initiation, whereas absence of CV restitution identifies inability to induce AF. Conduction restitution, but not fractionated electrograms, may thus track the functional milieu enabling AF initiation and has implications for guiding AF ablation and pharmacological therapy.
Keywords: activation time, atrial conduction restitution, atrial fibrillation, conduction slowing, conduction velocity
Premature atrial complexes or rapid tachycardias might trigger human atrial fibrillation (AF) (1,2) yet likely interact with dynamic mechanisms (“substrates”) to do so, because most triggers do not cause AF. Plausible mechanisms include conduction velocity (CV) and repolarization (3,4) dynamics. Slowing of atrial CV might result from atrial fibrosis on magnetic resonance imaging (5) (with its surrogate of low voltage) (6), yet structural elements are constant and thus do not per se explain why AF initiation is dynamic and typically rate-related.
We hypothesized that AF initiation requires the emergence of slow conduction at fast rates that might not be evident at slow baseline rates and that this might arise dynamically at the precise onset site of AF. Although prior studies have shown slow atrial CV at slow rates in AF patients (7,8), even those with “lone” AF (9), regionally slow baseline CV might also occur in patients without AF (10,11). Notably, prior work has examined CV at limited rates without defining its rate response (restitution), has rarely studied CV in the context of a spectrum of AF vulnerability (i.e., persistent AF, paroxysmal AF, and control subjects without AF), and has not studied the very rapid rates most relevant to AF initiation.
Prolongation of the electrocardiographic (ECG) P-wave, an indirect index of slow atrial conduction, is also an actuarial predictor of AF (12,13). However, ECG studies cannot localize sites of slowing within the atrium and also have not studied very rapid rates relevant to AF. As a corollary, the biophysics of charge conservation dictates that prolonged atrial electrograms from CV slowing should also exhibit reduced amplitude, comprising both “fractionated” electrograms observed during sinus rhythm (14) and AF (15). However, the relationship of fractionated electrograms to CV slowing before AF onset has also not been studied.
We tested our hypotheses by examining bi-atrial conduction time (CT) and atrial signal diminution and prolongation during incremental pacing from the superior pulmonary veins to AF initiation, at the site of AF onset defined from contact 64-128 electrode maps of left or both atria in patients with persistent AF, paroxysmal AF, and control subjects before ablation.
Methods
Patient flow
We prospectively enrolled 31 consecutive patients referred for ablation to the Veterans Affairs and University of California Medical Centers in San Diego—28 for AF (15 paroxysmal, 6 persistent, 7 longstanding persistent), and 3 control subjects (1 with left-sided accessory pathway, 1 with atrial tachycardia, and 1 with left ventricular tachycardia) without AF. In AF patients, left atrial (LA) thrombus was excluded by transesophageal echocardiography. In control subjects, pre-procedural AF was excluded by Holter monitoring and several ECGs. The study protocol was approved by our joint Institutional Review Board, and all patients provided written informed consent. Some patients were included in recent reports that action potential duration (APD) restitution slope >1 explains the initiation of paroxysmal AF from ectopic beats (3) and that APD alternans precedes AF onset (4).
Placement of recording electrodes
Electrophysiology study was performed >5 half-lives after discontinuing anti-arrhythmic medications (3 patients had discontinued amiodarone >30 days prior) (Table 1). A decapolar catheter was placed in the coronary sinus via femoral venous access, and after trans-septal puncture, a deflectable 4-mm tip catheter was used to record and pace at the right or left superior pulmonary vein antra, common sites of AF-triggering ectopy or tachycardia (Fig. 1). A 64-pole basket catheter (Constellation, Boston Scientific, Natick, Massachusetts) was advanced trans-septally to the LA. Right atrial recordings were made either from a quadipolar catheter or, in 14 patients, an additional basket for a total of 138 (128 basket plus 10 coronary sinus) electrodes (Fig. 1). Baskets were manipulated to maintain optimum electrode contact with the atrial wall with fluoroscopy, electrograms, and intra-cardiac echocardiography.
Table 1.
Clinical Characteristics
| Characteristic | Persistent AF (n = 13) | Paroxysmal AF (n = 15) | Control Subjects (n = 3) | p Value |
|---|---|---|---|---|
| Age, yrs | 64 ± 11* | 62 ± 6* | 48 ± 22 | 0.43 |
| History of AF, months | 74 ± 67 | 31 ± 30 | – | 0.02 |
| Left atrial diameter, mm | 46 ± 4*† | 41 ± 5 | 37± 2 | <0.01 |
| LV ejection fraction, % | 55 ± 11 | 59 ± 7 | 54± 18 | 0.58 |
| CHF, n (%) | 5(38) | 2(13) | 1 (33) | 0.38 |
| Coronary disease, n (%) | 5(23) | 3 (33) | 1 (33) | 0.26 |
| Medications, n | ||||
| ACEI/ARB | 6 | 8 | 0 | 0.28 |
| Statins | 7 | 10 | 2 | 0.84 |
| Beta-blockers | 9 | 7 | 2 | 0.58 |
| Class I agents | 1 | 1 | 0 | 0.52 |
| Amiodarone | 2 | 0 | 1 | 0.21 |
| Sotalol | 1 | 5 | 1 | 0.49 |
| Dofetilide | 0 | 2 | 0 | 0.27 |
p < 0.05 versus control subjects
p < 0.05 versus paroxysmal atrial fibrillation (AF).
ACEI = angiotensin-converting enzyme inhibitors; ARB = aldosterone receptor blockers; CHF = congestive heart failure; LV = left ventricular; NYHA = New York Heart Association.
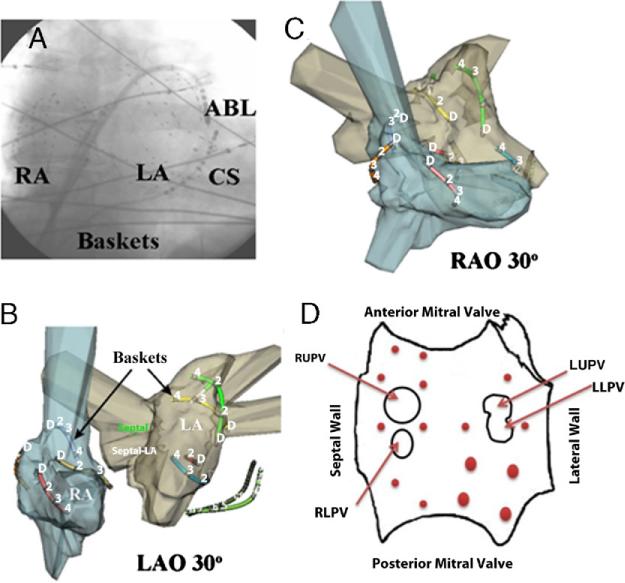
Figure 1. Bi-Atrial Electrode Recordings to Record Sites of AF Initiation.
(A) Fluoroscopy showing 64-pole catheters in each atrium. Selected splines are shown in patient-specific electro-anatomic models (NavX, St. Jude Medical, St. Paul, Minnesota) in (B) left anterior oblique (LAO) and (C) right anterior oblique (RAO) views. (D) Left atrial (LA) schematic shows sites of atrial fibrillation (AF) initiation in each patient (red dots, size representing number of patients). The LA is opened transversely at the mitral annulus, with the anterior annulus opened upward. ABL = ablation; CS = coronary sinus; LLPV = left lower pulmonary vein; LUPV = left upper pulmonary vein; RA = right atrium; RLSP = right lower pulmonary vein; RUPV = right upper pulmonary vein.
Pacing protocol
Patients in AF were electrically cardioverted to sinus rhythm, and the protocol started after 15 min, prior to ablation. We delivered 74 paced beats at cycle lengths (CL) of 500 ms, 450 ms, 400 ms, 350 ms, and 300 ms, then in 10-ms steps to AF (sustained if >1 min), capture failure (n = 6), or patient intolerance, whichever came first.
Signal filtering was 30 to 500 Hz for intracardiac signals and 0.05 to 100 Hz for ECGs, then digitized at 1 kHz to 16-bit resolution (Bard Pro, Billerica, Massachusetts) and exported for analysis with software written by S.M.N. in Labview (National Instruments, Austin, Texas). Only noise-free beats were analyzed.
Measurement of CT and site of AF initiation
Because rate-response ranges for atrial conduction, atrial signal amplitude, and duration have not previously been defined, we compared these metrics between AF patients and control subjects.
Local CTs were assigned at each electrode throughout both atria in sinus rhythm and each pacing rate, referenced to the P-wave onset in sinus rhythm or stimulus artifact in pacing. At each rate, intra-atrial CT range was defined as the difference in CT between latest and earliest activated sites.
The AF initiation was assigned when activation dissociated from pacing and the earliest site of activation for the first AF cycle was defined. The CT rate-response was determined for all rates at this site and at the latest activated site during slow baseline pacing. All measurements were made at 100- to 200-mm/s scale and confirmed by 3 observers (G.L., A.S., S.M.N.).
Defining CT slowing and its dynamics (restitution)
Control patients showed no CT prolongation at any site with rate acceleration, whereas AF patients showed CT slowing of 48 ± 50%. We thus defined CT of ≥15% from baseline as “CV slowing.”
We defined 3 rate-response patterns for atrial CT, as described in ventricle (16,17). Steep restitution was defined when CT prolonged only at fast rates (i.e., minimal CT prolongation at slow rates). From our preliminary data, this was CT prolongation ≥15% with the slope of the best-fit line of CT to pacing CL (with R2 >0.50) ≤–0.5. Steep restitution was summarized by the onset CL of CT prolongation (i.e., its intersection with the near-horizontal line of baseline CT) (Fig. 2). Broad restitution describes CT prolongation over a wide rate range. From our preliminary data, broad CV restitution was defined when CT prolongation was ≥15% with the slope of the best-fit line of CT to pacing CL >–0.5 (Fig. 3). Flat restitution was assigned when no significant rate-related change in CT (<15%) was observed (Fig. 4).
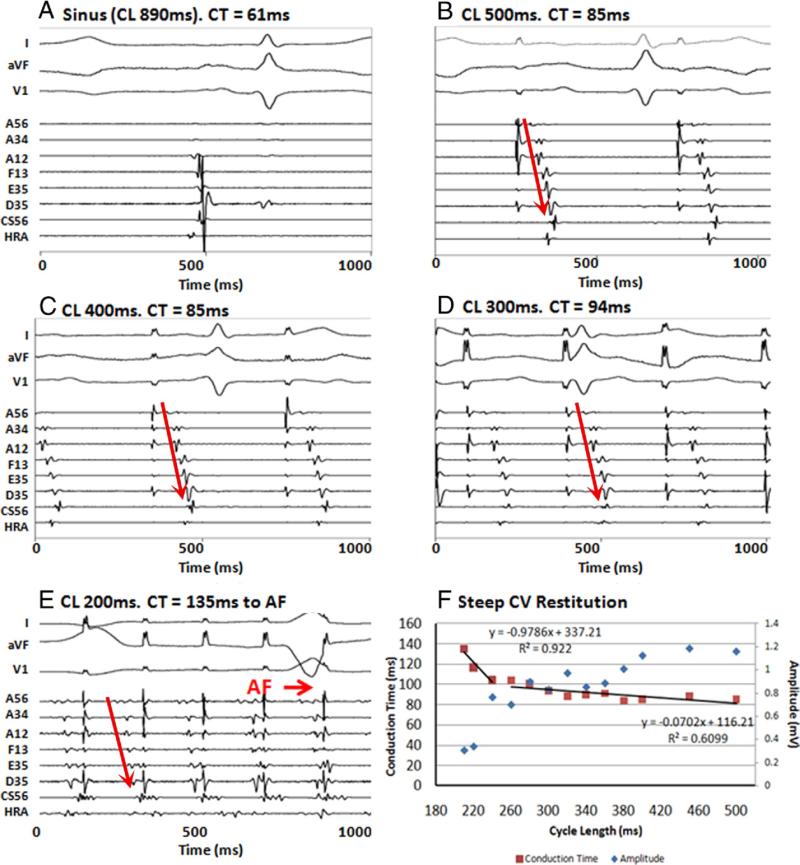
Figure 2. Steep Conduction Restitution in a Patient With Persistent AF.
(A) Intra-atrial activation delay (61 ms) in sinus rhythm. During pacing, conduction time (CT) to latest electrode (D35) shows minimal prolongation from 85 to 94 ms at (B) cycle length (CL) 500 ms, (C) CL 400 ms, and (D) CL 300 ms. (E) At CL 200 ms, just before atrial fibrillation (AF) initiation, CT prolongs dramatically to 135 ms. (F) Steep conduction velocity (CV) restitution. Note the reciprocal decrease in amplitude with CT prolongation (blue diamonds). A12, A34, A56 = anterior roof; CS56 = mid coronary sinus; D35 = inferior posterior wall; E35 = posterior mitral valve annulus; F13 = anterior mitral valve annulus; HRA = high right atrium.
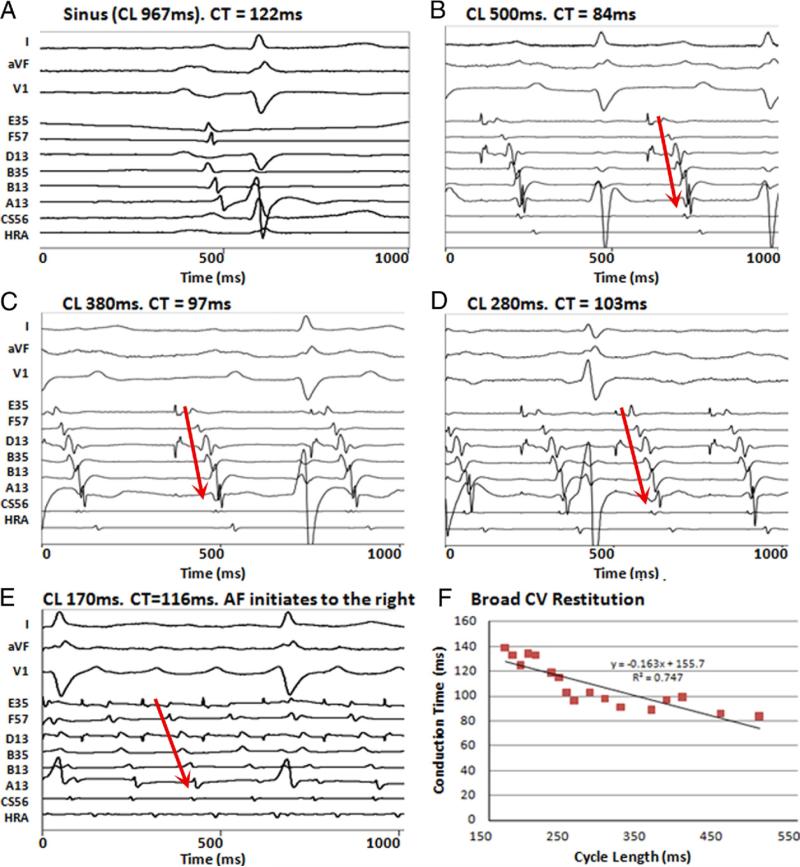
Figure 3. Broad Conduction Restitution in a Patient With Persistent AF.
(A) Intra-atrial activation delay (122 ms) in sinus rhythm. During pacing, CT to latest electrode (A13) prolongs progressively from 84 to 139 ms at (B) CL 500 ms, (C) CL 380 ms, (D) CL 280 ms, and (E) CL 170 ms, before AF initiation. (F) Broad CV restitution. A13 = anterior mitral valve annulus; B13, B35 = anterior roof; D13 = superior posterior wall; E35 = inferior posterior wall; F57 = posterior mitral valve annulus; other abbreviations as in Figure 2.
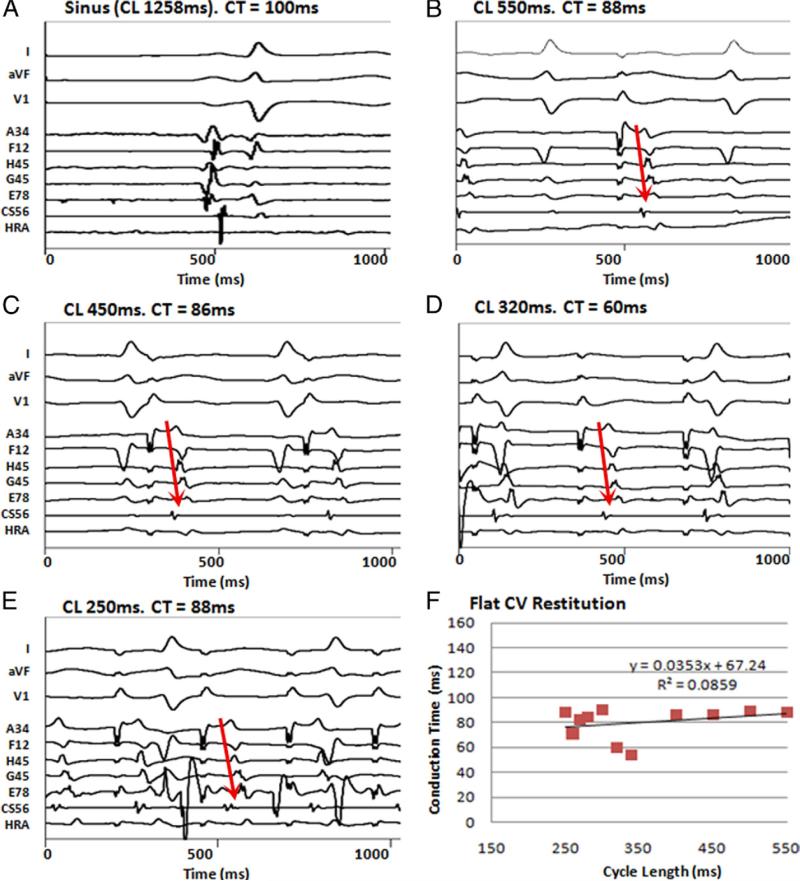
Figure 4. Flat Conduction Restitution in a Patient Without AF.
(A) Intra-atrial activation delay (100 ms) in sinus rhythm. During pacing, CT to latest electrode (G56) shows no slowing from (B) CL 550 ms, (C) CL 450 ms, and (D) CL 320 ms to (E) CL 250 ms. Atrial fibrillation did not initiate. (F) Flat CV restitution is seen. A34 = anterior roof; E78 = posterior mitral valve annulus; F12 = inferior mitral valve; G45 = superior mitral valve; H45 = anterior mitral valve; other abbreviations as in Figure 2.
Measurement of rate-dependent atrial signal duration and amplitude
We measured prolongation of signal duration prolongation (ms) and peak-to-peak atrial amplitude attenuation (mV), at each electrode and rate.
In control subjects, atrial electrograms did not prolong with rate at any site (–7 ± 5 ms, p = NS). Thus, we defined abnormal rate-response of duration as a net prolongation and a negative best-fit line slope. Similarly, in control subjects, amplitude did not diminish with rate (+0.18 ± 0.18 mV, p = NS); thus we defined abnormal amplitude rate-response as a net diminution and a positive best-fit line slope.
Spatial patterns of intra-atrial activation
Conduction times were defined throughout the LA at each basket electrode location registered within patient-specific electroanatomic shells (NavX, St. Jude Medical, St. Paul, Minnesota) (Figs. 1B and 1C), in relation to the posterior wall, pulmonary vein antra, and other locations.
We calculated the vector of atrial activation from the earliest to latest activated site in the LA and its angular deviation from the slowest to fastest rate before AF onset or capture failure. Vector calculations assumed equidistant adjacent electrodes, which introduces minimal error between splines and, because splines move little over time, will not impact angular deviation with rate for any given patient.
Statistical analysis
Continuous data are represented as mean ± SD. The Kruskal-Wallis test was used to compare variables between 3 patient groups, such onset CL of CT prolongation, with post-hoc Mann-Whitney U test to identify differences between group pairs. The Mann-Whitney U test was used to compare variables between 2 groups, such as extent of CT prolongation, electrogram duration prolongation, and amplitude attenuation. Paired continuous variables, such as LA diameter and CV restitution slope, were compared with linear regression and the Wilcoxon signed rank test. The Fisher exact test was applied to contingency tables. Spearman's rank correlation was used to measure association. A probability of <5% was considered statistically significant.
Results
The demographic data of our patients are summarized in Table 1. Patients with persistent AF had larger left atria and a longer duration of AF than those with paroxysmal AF.
Sites of AF initiation
Sustained AF was initiated by pacing in 19 patients at CL 219 ± 35 ms. Notably, the first AF beat arose in the LA in all cases (before any right atrial site), as shown in Figure 1D.
Group differences in bi-atrial CT at baseline
In sinus rhythm, there was no difference in P-wave duration (p = 0.35) or intracardiac CT range (p = 0.51) between patients with persistent AF, paroxysmal AF, and control subjects (Table 2). At the slowest pacing rate (CL = 444 ± 94 ms), CT range was longer in patients with persistent than paroxysmal AF (Table 2) (p = 0.03). Baseline CT is illustrated for 1 patient in each group in Figures 2B, 3B, and 4B.
Table 2.
Conduction Dynamic Status
| Characteristic | Persistent AF | Paroxysmal AF | Control Subjects | p Value |
|---|---|---|---|---|
| Baseline | ||||
| Sinus CL | 940 ± 130* | 1,048 ± 192 | 974 ± 338 | 0.06 |
| P-wave duration | 147 ± 27 | 136 ± 11 | 158 ± 39 | 0.35 |
| Bi-atrial CT range in sinus rhythm | 131 ± 36 | 120 ± 22 | 129 ± 49 | 0.51 |
| CL of AF initiation | 206 ± 38 | 230 ± 32 | – | 0.14 |
| Incremental pacing | ||||
| CT range, slowest CL | ||||
| Site of AF initiation | 59 ± 14† | 46 ± 23† | – | 0.87 |
| Latest activated site | 90 ± 29* | 68 ± 16† | 84 ± 13 | 0.03 |
| CT range, fastest CL | ||||
| Site of AF initiation | 99 ± 39 | 79 ± 34 | – | 0.27 |
| Latest activated site | 137 ± 39*† | 101 ± 27 | 66 ± 17 | 0.01 |
| ΔCT range (fastest-slowest CL) | ||||
| Site of AF initiation | 42 ± 30 | 32 ± 26 | – | 0.47 |
| Latest activated site | 46 ± 32† | 33 ± 25† | –14 ± 27 | 0.06 |
| CT dynamics (slowing/no slowing) | ||||
| Site of AF initiation | 6/2 | 9/2 | – | 1.00 |
| Latest activated site | 10/3 | 10/5 | 0/3 | 0.04 |
Values are mean ± SD. Values given in milliseconds, unless otherwise indicated.
p < 0.05 versus paroxysmal atrial fibrillation (AF).
p < 0.05 versus control subjects.
CL = cycle length (of pacing); CT = conduction time.
CT prolonged in differing patterns between groups on transitions to AF
Rate acceleration prolonged bi-atrial CT in 23 of 28 (79%) AF patients but no control subjects (p = 0.01). Atrial fibrillation initiated in most patients with rate-dependent CV slowing, whereas patients without CV restitution were rarely inducible for AF (17 of 23 vs. 2 of 8; p = 0.03). Three patterns of CV restitution were observed at the site of AF initiation (Table 2).
Figures 2B to 2E show abrupt and marked LA CT prolongation in an 82-year-old man with persistent AF and LA diameter 4.4 cm. Right superior pulmonary vein pacing produced CT to the mid-inferior posterior LA of 85 ms at CL 500 ms that increased minimally as CL shortened to 240 ms then abruptly prolonged to 135 ms at CL 210 ms (just before AF onset at this location). This steep activation restitution is summarized in Figure 2F. Steep CV restitution was engaged in 8 patients (1 persistent AF, 7 paroxysmal AF, no control subjects) at the AF initiating site. Electrogram amplitudes diminished with rate in this patient (blue markers in Fig. 2F) but not in the group as a whole (p = 0.38) (Table 2).
Figures 3B to 3E show gradual conduction slowing (CT prolongation) with rate acceleration in a 60-year-old patient with persistent AF and LA diameter 5.0 cm, leading to AF. This broad activation restitution is shown in Figure 3F. Broad CV restitution was engaged in 7 patients (5 persistent AF, 2 paroxysmal AF, no control subjects) at the site of AF initiation.
Figures 4B to 4E illustrate activation dynamics in a 45-year-old woman with atrial tachycardia (no AF) and LA diameter 3.9 cm. During pacing, CT did not significantly prolong in 128-pole bi-atrial recordings at any CL. Figure 4F shows this pattern of flat (absent) restitution, which was observed in 4 AF patients and all control subjects.
Conduction dynamics at the latest activated site trended similarly to dynamics at the AF initiation site (p = 0.090) (Table 2). Right atrial conduction dynamics (n = 14) was similar to that of the LA, and the global pattern in each patient (steep, broad, flat) showed a trend toward concordance with the LA (p = 0.06).
Rate-dependent changes in atrial signal duration and amplitude
Atrial electrograms did not prolong with rate for the group at the AF initiation site (68 ± 20 ms vs. 66 ± 29 ms, p = NS) or the latest activated site (66 ± 19 ms vs. 58 ± 17 ms, p = NS). In addition, there was no correlation between atrial electrogram duration and conduction slowing (p = NS).
Atrial electrogram amplitude trended to diminish at the AF initiation site (0.58 ± 0.42 mV vs. 0.39 ± 0.31 mV; p = 0.09) (Fig. 2F) but not at the latest activated site (0.64 ± 0.66 mV vs. 0.44 ± 0.35 mV; p = NS) (Table 3). There was no correlation between atrial electrogram amplitude diminution and rate-dependent conduction slowing (p = NS) or between rate-dependent atrial electrogram duration and amplitude (p = NS).
Table 3.
Atrial Electogram Duration and Amplitude Dynamic Status
| Characteristic | Persistent AF | Paroxysmal AF | Control Subjects | p Value |
|---|---|---|---|---|
| Atrial signal duration, ms | ||||
| Longest CL | ||||
| Site of AF initiation | 82 ± 20* | 58 ± 15 | – | 0.01 |
| Latest activated site | 73 ± 22 | 59 ± 13 | 66 ± 18 | 0.09 |
| Shortest CL | ||||
| Site of AF initiation | 63 ± 29 | 69 ± 31 | – | 0.68 |
| Latest activated site | 56 ± 13 | 60 ± 22 | 56 ± 14 | 0.57 |
| Atrial signal amplitude, mV | ||||
| Slowest CL | ||||
| Site of AF initiation | 0.69 ± 0.47 | 0.51 ± 0.41 | – | 0.41 |
| Latest activated site | 0.55 ± 0.45 | 0.74 ± 0.83 | 0.34 ± 0.31 | 0.22 |
| Fastest CL | ||||
| Site of AF initiation | 0.46 ± 0.35 | 0.28 ± 0.22 | – | 0.22 |
| Latest activated site | 0.51 ± 0.32 | 0.37 ± 0.37 | 0.52 ± 0.25 | 0.34 |
p < 0.05 versus paroxysmal atrial fibrillation (AF).
CL = cycle length (of pacing).
Reorientation in the activation vector in patients with conduction slowing
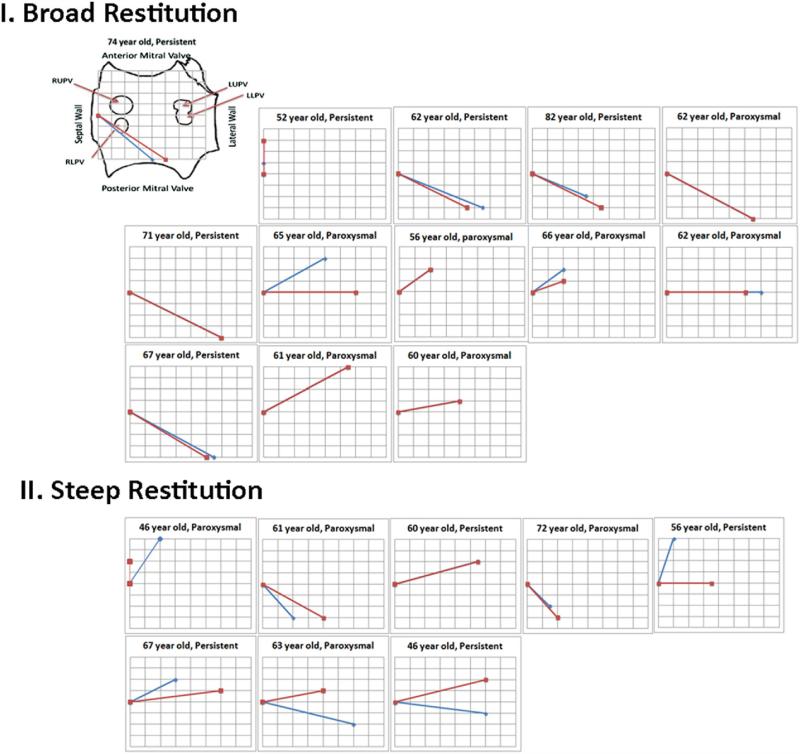
Patients with broad CV restitution showed an abrupt angular shift of 29 ± 22° in activation vector between slowest and fastest rates (angle between red and blue lines in Fig. 5). Conversely, patients with steep activation restitution showed minimal change in the activation vector with rate acceleration (6 ± 11°; p < 0.01 vs. broad).
Figure 5. Rate-Dependent Reorientation of Conduction Time Vectors in Patients With Broad Restitution.
In each patient, vectors are shown between slowest (CL 500 ms, red) and fastest (blue) rate pacing, for: I) Steep CV restitution; and II) Broad CV restitution. Note the greater vectorial shift in activation between slow/fast rates for patients with broad compared with steep restitution. First panel shows orientation of electrodes in left atrium, and pacing site superimposed on schematic left atrium. Nodes on the grid represent electrodes on the basket. Abbreviations as in Figures 1 and 2.
Relationship of conduction slowing to structural disease
Of patients in whom pacing initiated AF, those with broad CV restitution had larger LA diameters than patients with steep restitution (47 ± 6 mm vs. 40 ± 5 mm, p = 0.03). Patients with broad CV restitution at the AF initiation site were more likely to have persistent AF, whereas patients with steep restitution were more likely to have paroxysmal AF (p = 0.04). Patients with persistent AF had longer LA CT than those with paroxysmal AF at baseline (p = 0.04) and the fastest pacing rate (p < 0.01) (at the site of latest activation at baseline).
Notably, 8 patients had clinical heart failure (2 preserved EF) (Table 1) yet did not differ in the presence or absence of CV restitution from those without heart failure (p = 1.00). We observed no relationship between the type or extent of CV restitution and patient age for the entire population or within persistent and paroxysmal AF groups.
Discussion
This study demonstrates that the initiation of human AF is preceded by rate-dependent conduction slowing (CV restitution) at the site of onset in patients with clinical AF but not control subjects without clinical AF. Although bi-atrial CT at baseline was similar between AF patients and control subjects, 74-138-pole contact mapping unmasked CV restitution in AF patients. Conduction velocity restitution exhibited steep and broad patterns, the latter associated with an abrupt vector shift in atrial activation at AF onset that indicates conduction block that might enable re-entrant AF. These results suggest that the atrial site where AF initiates might be identified in individual patients from propagation dynamics during pacing and opens the possibility of modifying them to treat AF.
Sites of AF initiation
The AF initiated in the LA in all instances but not consistently at the pulmonary veins, sites of pacing, or other identifiable anatomic landmarks. Therefore, we set out to identify functional determinants of AF onset. Although we have reported that atrial repolarization alternans (4,18) and/or steep repolarization restitution (3) arise before AF onset, they might arise away from AF initiation sites, such as in the right atrium, as well as near the pulmonary veins. Thus, the mechanistic contribution of APD oscillations to AF initiation is likely a complex interplay between alternans, complex non-alternating periodicity (as we have recently described), and resulting spatial gradients in repolarization that likely cause functional conduction block and re-entry that might not only initiate AF but also possibly anchor re-entrant rotors (19,20) of ongoing AF.
Dynamic conduction slowing as an initiating mechanism for AF
Because atrial CV slowed immediately before AF onset in AF patients and flat (absent) CV restitution was associated with AF noninducibility, these data support the hypothesis that CV restitution is mechanistically related to AF initiation. Our use of 74-138 contact electrodes in the left or both atria represents the highest-resolution in situ bi-atrial study of human conduction dynamics to date.
One plausible hypothesis for AF initiation is that triggering ectopy or tachycardias (1,2) interact with slowly conducting tissue to initiate reentrant AF (21), because conduction delay might lead to wave break and enable rotor formation or anchoring (22). Notably, early published reports on focally triggered AF remarked that ectopy within critical ranges of prematurity (“P on T” ectopy) (2) was most successful in initiating AF. These findings are consistent with our findings of steep CV restitution in which a critical rate threshold is required to elicit CV slowing imminently before AF onset.
Stiles et al. (9) recently observed that patients with lone AF exhibit conduction slowing, despite lack of detectable structural remodeling, and previously reported bi-atrial conduction slowing in patients with more advanced AF (23). However, these and other prior human studies examined relatively slow rates and did not study transitions to AF. The current study mimicked pulmonary vein tachycardias with burst pacing to directly examine spatial patterns of conduction dynamics to AF onset.
Our results show clear differences in CV dynamics across a wide range of AF phenotypes, including paroxysmal AF, persistent AF, and longstanding persistent AF (which would be classified as permanent AF had we elected not to perform catheter ablation). It is likely that additional mechanistic differences might be revealed with future studies in realistic animal models of AF or computational models (as we have described in the ventricle) (17).
Mechanisms of conduction restitution and AF initiation
Precise mechanisms linking each of the 3 patterns of CV restitution vis-à-vis atrial remodeling or AF substrates remain unclear. However, in the ventricle, steep CV restitution has been linked with the initiation of fibrillation due to multi-wavelet re-entry, whereas broad restitution has been linked to localized sources such as rotors (21).
Steep CV restitution was more frequent in patients with paroxysmal AF and less enlarged atria than persistent AF. It is possible that steep CV restitution interacts with steep APD restitution (3) in paroxysmal AF patients to reduce re-entrant wavelength, enabling rapid tachycardias or early focal ectopic triggers to initiate AF. Mechanistically, differences in CV restitution between persistent and paroxysmal AF suggest that fibrosis and scar slow conduction at slower rates in more remodeled atria. Although 1 potential confounder is that longer CT in larger atria increased the sensitivity for detecting CT prolongation, atrial dimensions differed by only approximately 10%, and sites of AF initiation were rarely on opposite sides of the atria from pacing (Fig. 1D).
Patients with persistent AF and broad CV restitution exhibited AF initiation with less dramatic heart rate acceleration (24,25). Notably, in ventricular models of heart failure, areas of greater fibrosis might be regions of greater propensity to wave break (26). Thus, CV slowing for a wide range of atrial rates in our study might have facilitated wave break and fibrillatory conduction (15) from slower organized tachycardias, creating a suitable environment for AF perpetuation by mechanisms, including rotors (19,20) or meandering reentrant waves (27).
Several tissue and cellular factors might explain broad CV restitution and arrhythmic susceptibility. In remodeled atria, conduction slowing might mechanistically reflect fibrosis, border zone of atrial scar (6), altered gap-junctional coupling or decreased excitability (24,25). Cardiac fibroblasts are activated in response to atrial stretch and other factors and lead to remodeling and fibrosis. The increased deposition of extracellular matrix proteins decreases local tissue excitability and might create a barrier to longitudinal conduction (28). Moreover, in ventricular myocytes, paracrine factors secreted by cardiac fibroblasts lead to downregulation of sodium current, which results in decrease in upstroke velocity, and potassium rapid delayed-rectifier current, prolonging APD (29). Computational models have shown that fibroblast-myocyte coupling alters myocyte resting potential and conduction (30).
Regionally, conduction slowing might arise at the septopulmonary bundle of Papez and elsewhere in the LA that exhibit abrupt changes in muscle thickness and fiber direction (19,31). Alterations in gap-junctions and redistribution of connexins have been implicated in AF vulnerability, but their overall role in maintaining AF remains unclear, because patients with AF exhibit varying connexin distribution and degree of expression (32).
In summary, steep CV restitution might be primarily dependent on electrical remodeling, and broad restitution might be primarily dependent on structural remodeling. Nevertheless, other influences might be operative (1,33), and further studies should determine whether patterns of CV restitution predominantly reflect structural or electrical remodeling (21). Notably, however, flat CV restitution in control subjects indicates that marked rate-dependent CV slowing is likely not a property of normal atrial electrophysiology.
Conduction restitution and spatial complexity of activation
Abrupt reorientation in the activation vector in patients with broad CV restitution might reflect the development of rate-dependent conduction block, as suggested at slower rates by Markides et al. (7), and reentry leading to AF. Notably, vector reorientation was less commonly observed in patients with steep CV restitution (mostly paroxysmal AF), suggesting a different mechanism of AF initiation in that population.
Because tissue heterogeneity likely plays a role in rate-dependent atrial activation in AF patients, the location of initiating tachycardias or pacing might affect CV dynamics and fibrillatory conduction. We paced from near the pulmonary veins to mimic clinical AF triggers (1), and future studies should examine CV dynamics and AF initiation for other tachycardia/pacing locations.
Conduction slowing and fractionated electrograms
Notably, we observed no consistent rate-dependent prolongation in atrial electrogram duration or diminution in amplitude, both of which are components of complex fractionated atrial electrograms (CFAE) (15). These results suggest that CFAE in sinus rhythm (14) and pacing do not indicate the central mechanisms of AF initiation. Thus, although fractionated signals arise widely within the atria, they were nonspecific for AF initiation sites. This conclusion agrees with recent reports that CFAE during AF also represent multiple etiologies (34).
Clinical implications
These results suggest a method whereby dynamic atrial CV slowing, revealed for instance by ECG P-wave prolongation with rate, might predict imminent AF onset. Conversely, atrial pacing to minimize P-wave duration at fast rates might attenuate AF initiation. Because areas of conduction slowing might represent critical myocardium for AF initiation, it might be possible to prevent AF onset by ablating these sites to achieve block. Conduction dynamics might also explain differing responses to anti-arrhythmic medications. Class I medications slow conduction and might exaggerate broad CV restitution, potentially explaining their relative lack of efficacy in patients with persistent AF. Conversely, increased reentry wavelength by APD prolongation from class III agents might explain the reduced propensity for AF initiation and perpetuation in persistent AF patients with broad CV restitution.
Study limitations
One major limitation is our limited number of control subjects, which reflects difficulties in enrolling patients without AF or atrial flutter, who required clinical LA access at electrophysiological study and also consented to LA basket recordings. We observed no age dependence of CV restitution, although control subjects were (nonsignificantly) younger than AF patients. We assumed that distances between adjacent electrodes was linear and did not analyze activation contours, because this requires detailed knowledge of surface geometry. Accordingly, we measured CT rather than true CV. Although we did not perform right atrial basket recordings in all patients, AF initiated in the LA in all patients and right atrial data trended to be concordant (p = 0.06) with LA basket recordings obtained in all patients. We did not, given the already prolonged protocol duration, systematically repeat AF inductions or repeat induction from alternate pacing sites. Although amiodarone might have a very prolonged half-life, our results are statistically unchanged if these data are excluded. Lastly, although gender differences in AF are unclear, further studies should include more female patients.
Conclusions
Human AF onset is preceded by dynamic atrial conduction slowing (CV restitution) that was absent in patients in whom AF could not be dynamically induced and control subjects without clinical AF. These data support the hypothesis that conduction restitution is a mechanism enabling a rapid tachycardia to disorganize to AF via “fibrillatory conduction.” These results suggest that the atrial site where AF initiates in individual patients might be identified from conduction dynamics during pacing and open the possibility of modifying them by ablation, pharmacology, or other interventions to treat AF.
Acknowledgments
The authors thank Judith Hildreth, RN, Sherie Janes, RN, Elizabeth Greer, RN, Stephanie Yoakum, NP, Donna Cooper, RN, Anthony Moyeda, CVT, and Kenneth Hopper, CVT, for their great assistance in the clinical study. The authors would like to thank Kathleen Mills, BA, for data coordination.
This work was supported in part by grants from the American Heart Association to Dr. Krummen and from the Doris Duke Charitable Foundation and the National Institutes of Health (HL70529, HL83359) to Dr. Narayan. Dr. Krummen is a consultant for Topera Medical and InsilicoMed; and has received fellowship support from Boston Scientific, Medtronic, St. Jude Medical, and Biotronik. Dr. Narayan has received support from St. Jude Medical, Medtronic, Biotronik, Topera Medical Inc., and the National Institutes of Health.
Abbreviations and Acronyms
- AF
atrial fibrillation
- APD
action potential duration
- CFAE
complex fractionated atrial electrograms
- CL
cycle length (of pacing)
- CT
conduction time
- CV
conduction velocity
- ECG
electrocardiogram/electrocardiographic
- LA
left atrium/atrial
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Chen S-A, Hsieh M-H, Tai C-T, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–86. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 3.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008;52:1222–30. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–30. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus GM, Yang Y, Varosy PD, et al. Regional left atrial voltage in patients with atrial fibrillation. Heart Rhythm. 2007;4:138–44. doi: 10.1016/j.hrthm.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markides V, Schilling RJ, Ho SY, Chow AW, Davies DW, Peters NS. Characterization of left atrial activation in the intact human heart. Circulation. 2003;107:733–9. doi: 10.1161/01.cir.0000048140.31785.02. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Hertervig EJ, Kongstad O, et al. Deterioration of interatrial conduction in patients with paroxysmal atrial fibrillation: electroanatomic mapping of the right atrium and coronary sinus. Heart Rhythm. 2004;1:548–53. doi: 10.1016/j.hrthm.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Stiles MK, John B, Wong CX, et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”. J Am Coll Cardiol. 2009;53:1182–91. doi: 10.1016/j.jacc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 10.Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–16. doi: 10.1016/j.jacc.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Roberts-Thomson KC, Stevenson IH, Kistler PM, et al. Anatomically determined functional conduction delay in the posterior left atrium relationship to structural heart disease. J Am Coll Cardiol. 2008;51:856–62. doi: 10.1016/j.jacc.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Guidera SA, Steinberg JS. The signal-averaged P wave duration: a rapid and noninvasive marker of risk of atrial fibrillation. J Am Coll Cardiol. 1993;21:1645–51. doi: 10.1016/0735-1097(93)90381-a. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Menchavez E. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation. 1993;88:2618–22. doi: 10.1161/01.cir.88.6.2618. [DOI] [PubMed] [Google Scholar]
- 14.Lellouche N, Buch E, Celigoj A, et al. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 2007;50:1324–31. doi: 10.1016/j.jacc.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 15.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 16.Weiss JN, Karma A, Shiferaw Y, Chen P-S, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans (review). Circ Res. 2006;98:1244–53. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 17.Narayan SM, Bayer J, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure: a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008;52:1782–92. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials as a precursor of atrial fibrillation. Circulation. 2002;106:1968–73. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 19.Atienza F, Calvo D, Almendral J, et al. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 57:1081–92. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayan SM, Shivkumar K, Mittal S, Briggs CR, Sehra R, Miller JM, Krummen DE. Conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation: the CONFIRM trial (abstr). Heart Rhythm. 2011;8(5S):LB–04. [Google Scholar]
- 21.Weiss JN, Qu Z, Chen P-S, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–40. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- 22.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: from ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–9. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CX, Stiles MK, John B, et al. Direction-dependent conduction in lone atrial fibrillation. Heart Rhythm. 2010;7:1192–9. doi: 10.1016/j.hrthm.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res. 1998;83:781–94. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 25.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–41. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Zlochiver S, Vikstrom KL, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–47. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 27.de Groot N, Houben R, Smeets J, et al. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–82. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 28.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–53. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedrotty DM, Klinger RY, Kirkton RD, Bursac N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res. 2009;83:688–97. doi: 10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y, Garfinkel A, Camelliti P, Kohl P, Weiss JN, Qu Z. Effects of fibroblast-myocyte coupling on cardiac conduction and vulnerability to reentry: a computational study. Heart Rhythm. 2009;6:1641–9. doi: 10.1016/j.hrthm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klos M, Calvo D, Yamazaki M, et al. Atrial septopulmonary bundle of the posterior left atrium provides a substrate for atrial fibrillation initiation in a model of vagally mediated pulmonary vein tachycardia of the structurally normal heart. Circ Arrhythm Electrophysiol. 2008;1:175–83. doi: 10.1161/CIRCEP.107.760447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaldoupi SM, Loh P, Hauer RN, de Bakker JM, van Rijen HV. The role of connexin40 in atrial fibrillation. Cardiovasc Res. 2009;84:15–23. doi: 10.1093/cvr/cvp203. [DOI] [PubMed] [Google Scholar]
- 33.Calkins H, Brugada J, Packer D, et al. for the European Heart Rhythm Association (EHRA) European Cardiac Arrhythmia Society (ECAS) American College of Cardiology (ACC) American Heart Association (AHA) Society of Thoracic Surgeons (STS) HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Narayan SM, Wright M, Derval N, et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011;8:244–53. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]