Abstract
Rationale
Transcatheter, intramyocardial injections of bone marrow derived cell therapy produces reverse remodeling in large animal models of ischemic cardiomyopathy.
Objective
We used cardiac magnetic resonance imaging (CMR) in patients with LV dysfunction related to remote myocardial infarction (MI) to test the hypothesis that bone marrow progenitor cell injection cause functional recovery of scarred myocardium and reverse remodeling.
Methods and Results
Eight patients (age 57.2±13.3) received transendocardial, intramyocardial injection of autologous bone marrow progenitor cells (mononuclear or mesenchymal stem cells) in LV scar and border zone. All patients tolerated the procedure with no serious adverse events. CMR at 1-year demonstrated a decrease in end-diastolic volume (208.7±20.4 vs. 167.4±7.32mL; p=0.03), a trend towards decreased end-systolic volume (142.4±16.5 vs. 107.6±7.4mL; p=0.06), decreased infarct size (p<0.05), and improved regional LV function by peak Ecc in the treated infarct zone (-8.1±1.0 vs. -11.4±1.3; p=0.04). Improvements in regional function were evident at 3 months, while the changes in chamber dimensions were not significant until 6 months. Improved regional function in the infarct zone strongly correlated with reduction of EDV (r2=0.69, p=0.04) and ESV (r2=0.83, p=0.01).
Conclusions
These data suggest that transcatheter, intramyocardial injections of autologous bone marrow progenitor cells improve regional contractility of a chronic myocardial scar and these changes predict subsequent reverse remodeling. The findings support the potential clinical benefits of this new treatment strategy and ongoing randomized clinical trials.
Keywords: heart failure, bone marrow, stem cells, reverse remodeling
INTRODUCTION
In a series of detailed preclinical porcine studies, we have demonstrated that transcatheter, intramyocardial injection of bone marrow derived progenitor cells to chronically scarred myocardium is safe and improves left ventricular (LV) regional function, reduces scar size, and creates reverse remodeling1. A therapy that leads to reverse remodeling in chronically scarred human hearts would be precedent setting, and predicted to have favorable clinical benefits for patients. Using various imaging modalities, a growing number of clinical trials of intramyocardial bone marrow cell injections in patients with chronic ischemic heart failure have shown improved regional function; however, these improvements in regional contractility have only translated to modest global functional changes.2,3 Furthermore, the relationship of improved regional contractility and reverse remodeling has not been investigated. Here we tested the hypothesis that intramyocardial injection of bone marrow derived stem cells into humans with chronically scarred myocardium causes functional restoration followed by reverse remodeling. The data here derive from the phase I pilot of the Transendocardial Autologous Cells in Ischemic Heart Failure (TAC-HFT, clinicaltrials.gov identifier NCT00768066) study of catheter-based intramyocardial injections of autologous bone marrow progenitor cells in patients with LV dysfunction secondary to remote myocardial infarction.4
METHODS
Eight patients with a diagnosis of ischemic cardiomyopathy underwent bone marrow aspiration from the iliac crest followed by transendocardial injection (Helical Infusion Catheter; BioCardia, San Carlos, CA) of autologous bone marrow progenitor cells (mononuclear cells, n=4 or mesenchymal stem cells, n=4). Cardiac magnetic resonance imaging (CMR) phenotyping of structure and function was conducted at baseline, 3 and 6 months, and 1 year after cell therapy. Written informed consent was obtained from all patients on this Institutional Review Board approved protocol. Online Supplement contains detailed methods.
RESULTS
Eight patients [age 57.2±13.3 years, all male with remote MI (68.8±21.4 months, range 4 months to 11 years)] underwent transendocardial injection of bone marrow progenitor cells. No patient experienced a serious adverse event (Online Supplement).
Cine CMR demonstrated a decrease in EDV (p=0.033) and a trend towards decreased ESV (p=0.058) (Figure 1, Online Figure II). Reductions in chamber volumes were significant at 6 and 12 months compared to baseline for ESV (p=0.022 and p=0.023, respectively) and EDV (p=0.009 and p=0.014, respectively). EF and LV mass did not change (p=NS). A strong correlation between the changes in EDV and ESV (r2=0.87, p=0.002) demonstrates parallel decreases in chamber volumes. MI size evaluated by delayed myocardial hyper-enhancement decreased by 18.3±8.3% (p<0.05; Fig 1D and Online Figure I).
Figure 1.
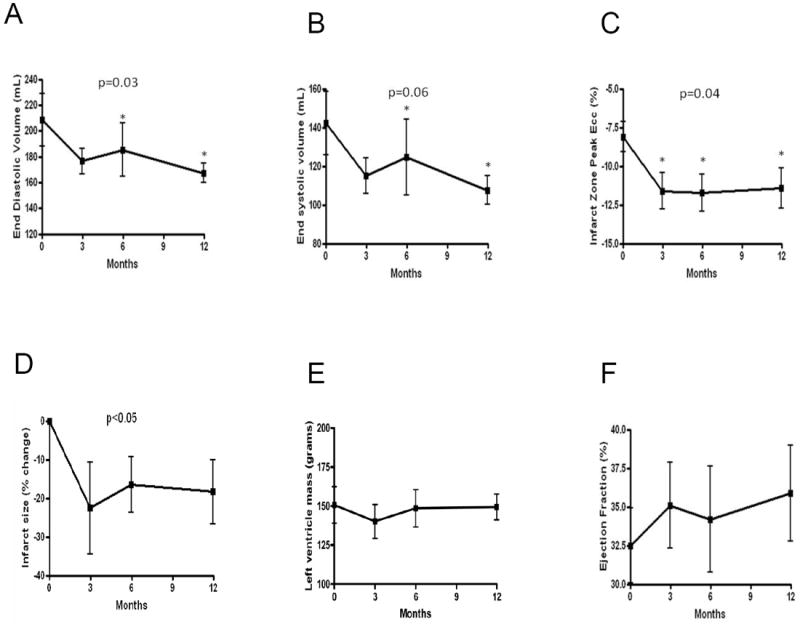
CMR changes after bone marrow progenitor cell injections. Cine changes in (A) EDV and (B) ESV show reverse remodeling occurred at 6 months and continued through 1 year. Peak Ecc of tagged tissue images demonstrates improved regional function of the (C) treated infarct zone (IZ). Improved IZ contractility (more negative Ecc indicates greater circumferential contractility) occured by 3 months following cell injection and persisted at 6 and 12 months. (D) Shows reduced delayed enhancement infarct size as a percentage of LV mass. (E) LV mass or (F) EF are unchanged. *p<0.05 for difference between time post-injection and baseline in the repeated measures ANOVA.
Peak eulerian circumferential strain (Ecc), a measure of regional contractility calculated from tagged CMR images, showed improved regional function of the infarct zone (IZ) (p=0.039) that restored function to the level of the borderzone (Figure 1 & 2). The IZ improvements were evident as early as 3 months and persisted at 6 and 12 months compared to baseline (p=0.02, p=0.013, and p=0.029, respectively), and strongly correlated with the changes in EDV (r2=0.69, p=0.04) and ESV (r2=0.83, p=0.01) (Figure 2). Regional function in border and remote zones did not change (p=NS).
Figure 2.
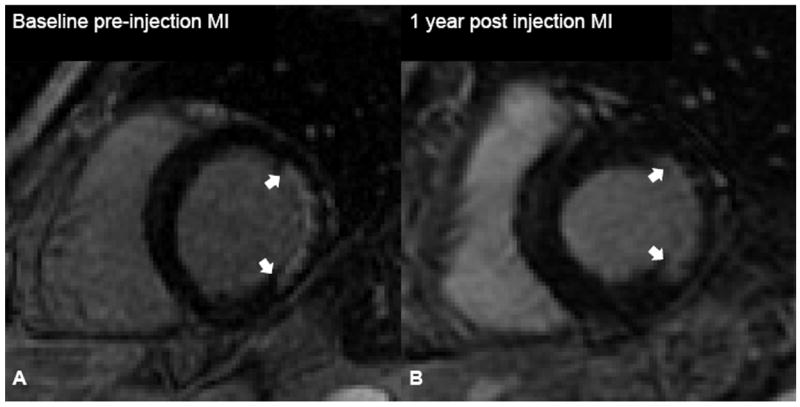
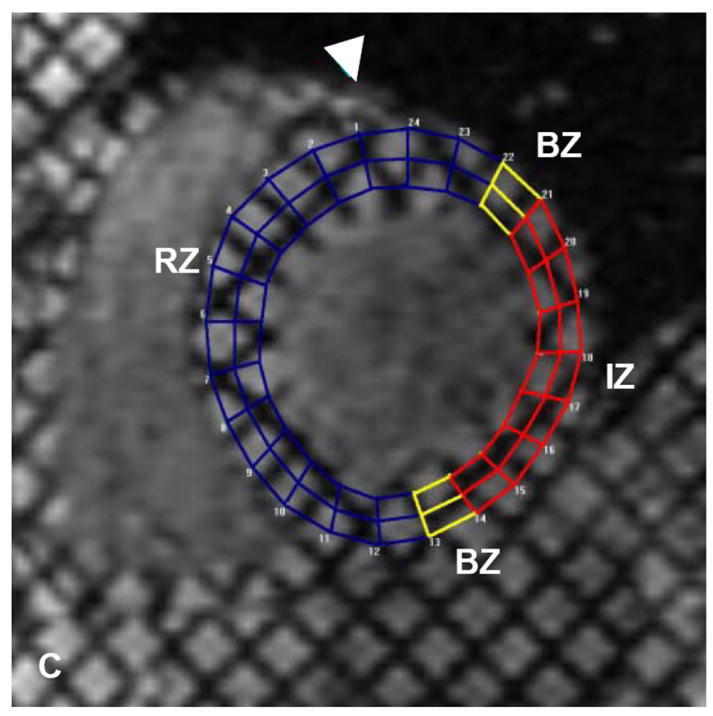
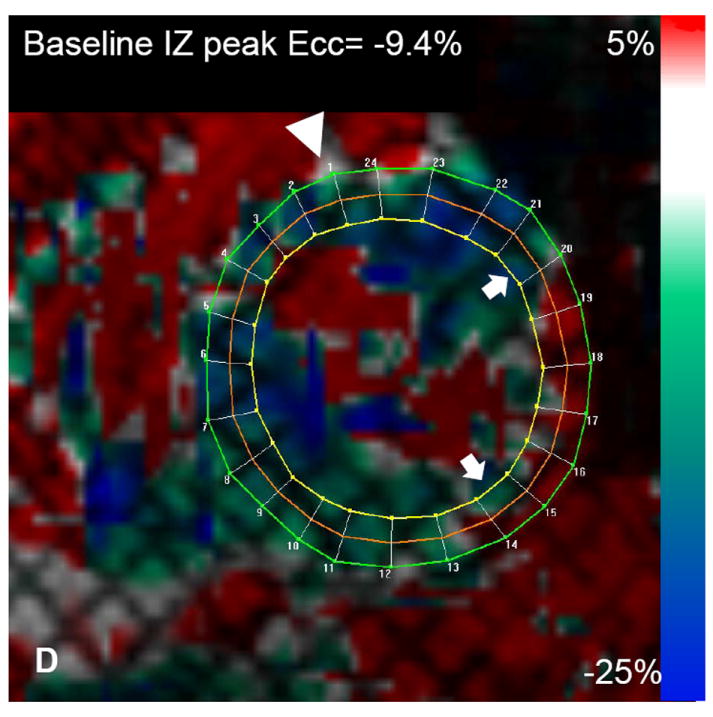
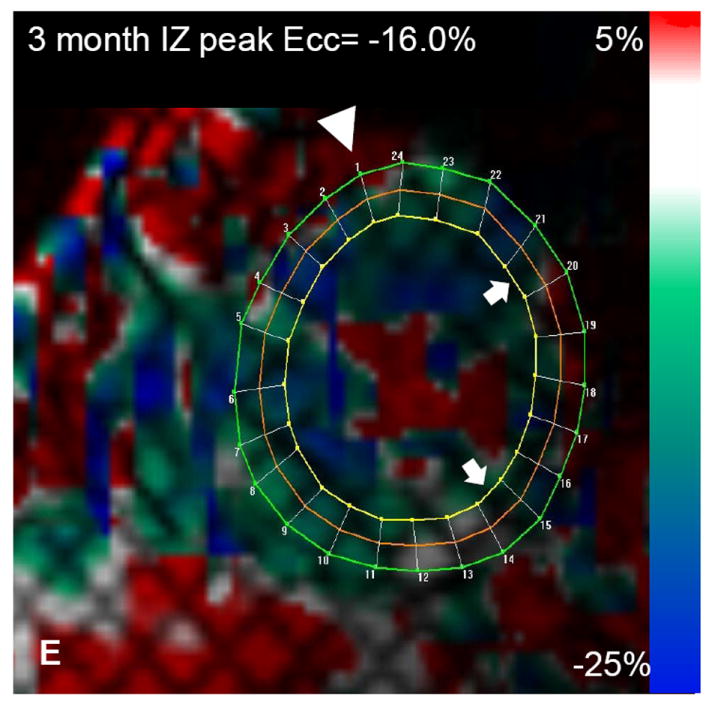
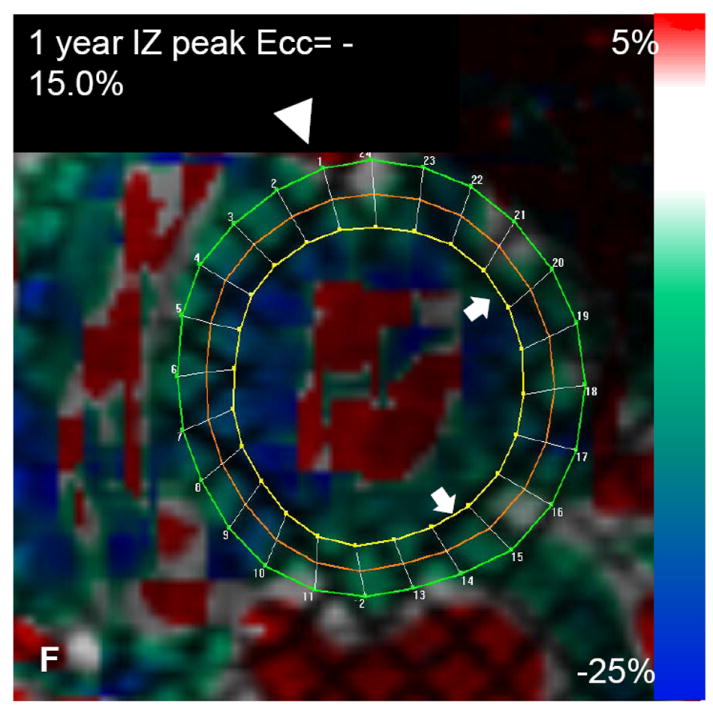
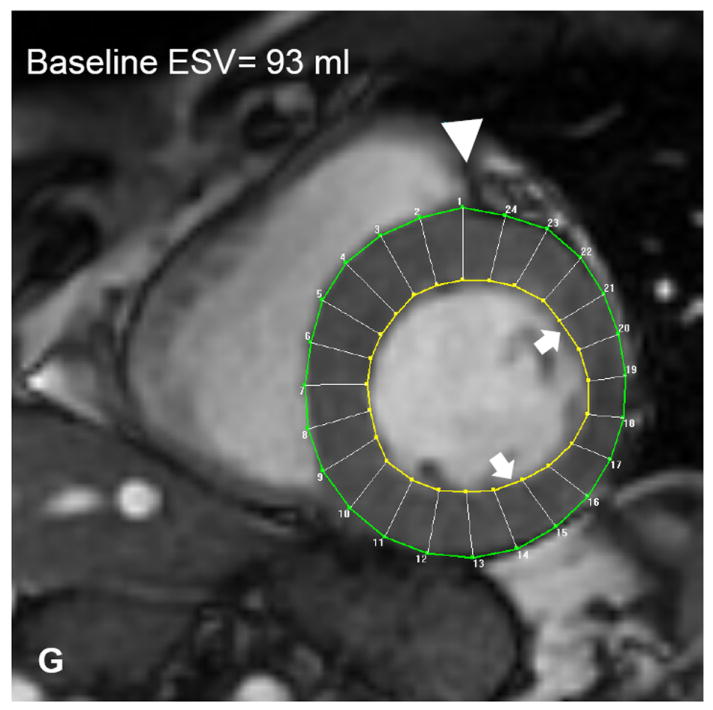
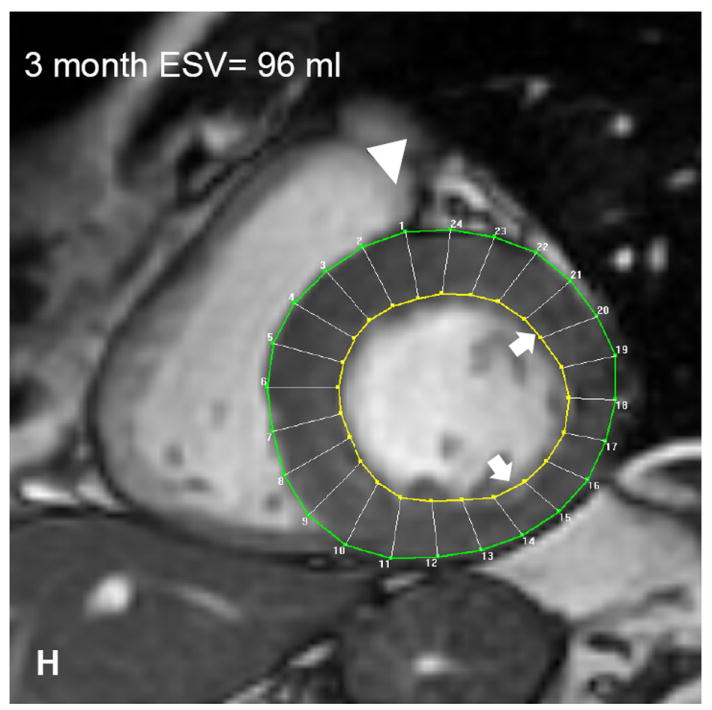
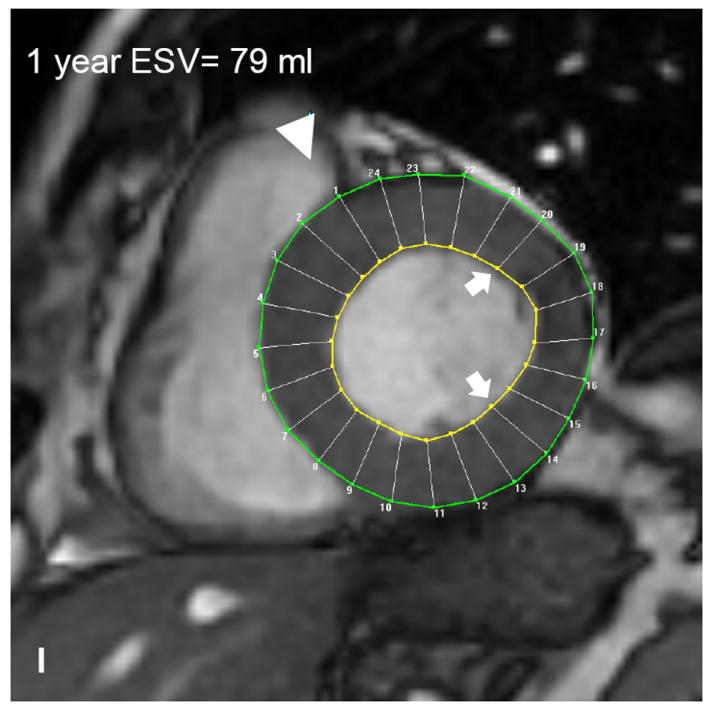
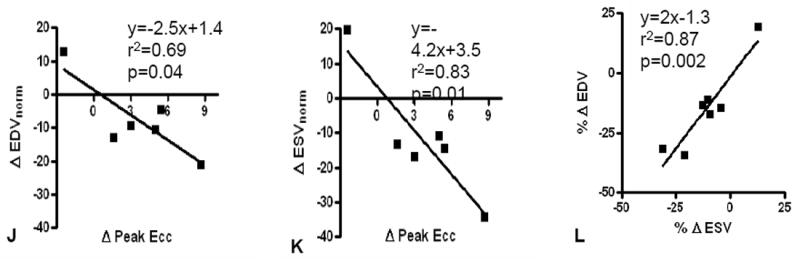
Autologous bone marrow progenitor cell injections increase regional function and precede reverse remodeling. Panels A-I depict a patient injected with bone marrow progenitor cells. (A) Baseline lateral wall infarct in delayed enhancement CMR (white arrows), (B) 1 year post-injection infarct, (C) infarct mapped to corresponding segmented tagged CMR image with IZ defined by the presence of enhancing myocardium, neighboring myocardium as BZ, and normal myocardium as RZ (first segment of each map correlated by right ventricle insertion point, white arrow head). Tagged harmonic phase CMR strain maps show (D) significantly depressed regional function by peak Ecc at baseline in IZ (white arrows). Red/white indicates weak contractility (more positive Ecc) and green/blue indicates vigorous contractility (more negative Ecc) in harmonic phase strain maps. At (E) three months after cell injection the IZ contractility has improved (less red/white, more green/blue) and by (F) 12 months is contracting similar to the BZ (mostly green/blue). ESV remains relatively stable between (G) baseline and (H) 3 months; however, at (I) 1 year reverse remodeling is present. Changes in peak Ecc of the IZ had a strong correlation with the changes in (J) EDV and (K) ESV at 12 months compared to baseline (difference in peak Ecc between baseline and 1 year versus difference in normalized EDV and ESV, respectively). (L) Decreases in EDV and ESV strongly correlate with each other indicating parallel decreases in chamber sizes contributes to unchanging EF after bone marrow stem cell injections.
DISCUSSION
Here we show that transcatheter, intramyocardial injection of autologous bone marrow preparations in patients with chronic ischemic cardiomyopathy is well tolerated and produces functional recovery in scarred myocardium and reverse remodeling of the LV chamber. Importantly, the functional recovery is evident at 3 months following injection, and precedes reverse remodeling. As all patients had documented MI and were previously revascularized, these findings support myocardial regeneration and mirror the phenotype observed in porcine models of chronic ischemic cardiomyopathy.1,5
The present findings have several major implications. First, we have established that bone marrow derived cell therapy clearly can enable reverse remodeling of dilated hearts, a phenotypic change that would be expected to have positive clinical implications. Second, the reverse remodeling caused substantial parallel declines in systolic and diastolic volumes, so that an EF increase was obscured; accordingly, this strongly suggests that EF is not a good primary endpoint for assessing responses to cell therapy in dilated hearts and that chamber size, MI size, or regional function are more likely to reflect a favorable outcome. Finally, these data strongly indicate that a high-resolution imaging tool such as MRI, capable of detecting the latter outcomes, is necessary for trials of cardiac regeneration.
While there is enormous enthusiasm for the possibility of repair of ischemic cardiomyopathy with cell-based therapy, mechanisms and manifestations of this strategy remain highly controversial. To address this, we phenotyped patients using cardiac MRI, which is recognized as the “reference standard” for quantitative assessment of LV function and has been shown to be the most accurate and reproducible imaging modality to assess global and regional LV function.6,7 Regional functional improvements (peak Ecc) of the infarct territory were calculated by harmonic phase MRI, arguably the most accurate non-invasive analytical imaging modality to measure regional strain8,9
The early improvements in regional contractility of a treated infarct predicted later reverse remodeling. Regional strain of the infarct territory improved as early as 3 months following cell injection, which persisted at 6 months and 1 year (Figure 1). The reduction in chamber volumes was not evident until 6 months and the correlation with improved regional strain was not apparent until 6 months and continued at 1 year (Figure 2). This suggests that the first manifestation of bone marrow cell therapy on the heart is improved regional function that later contributes to reverse remodeling. As a corollary to this finding, infarct size was reduced by 3 months after treatment.
Preclinical studies of bone marrow cell therapy for ischemic heart disease have demonstrated improved EF, but this has not translated to early phase studies in humans. We show a strong parallel decrease in both EDV and ESV (r2=0.87, p=0.002) 12 months after treatment. Indeed this finding strongly suggests that EF may not be a good outcome measure for studies of cell therapy for remodeled ventricles. Importantly, both LV size and infarct size are important clinical indicators of outcome in patients with ischemic cardiomyopathy,10 and the results here highlight a highly adaptive response to cell therapy, even in the absence of a net increase in EF.
This study lacks the power to determine superiority between MSCs or whole bone marrow, and does not have a placebo comparison group. Although, only 4 patients had longitudinal assessment of scar size (due to artifact distortion from delayed enhancement imaging from ICD leads), MI size was reduced in each of these subjects. Each of these limitations will be definitively addressed in the ongoing TAC-HFT and POSEIDON trials, which together will treat 90 patients in placebo-controlled, blinded fashion.
In conclusion, MRI provides a unique opportunity to image the in-vivo structural and functional changes after bone marrow stem cell therapy for the heart. Our data suggest human autologous bone marrow progenitor cells increase regional contractility of injected myocardial scar tissue within 3 months of treatment, and these functional changes are associated with later reverse remodeling. These findings strongly support ongoing clinical investigation of cell-based therapy for cardiomyopathy, and support the use of regional function and chamber size as important endpoints.
Supplementary Material
Novelty and Significance.
What is known?
Catheter-based bone marrow progenitor cell injections improve cardiac function and reduce scar burden in large animal models of ischemic cardiomyopathy.
Using various imaging modalities, early human studies of bone marrow progenitor cell therapy for ischemic cardiomyopathy have shown improved regional function.
What new information does this article contribute?
Durable reverse remodeling results from cell-based therapy for healed myocardial infarction / chronic ischemic cardiomyopathy
Intramyocardial injections of bone marrow progenitor cells to infarct and border zones of humans with ischemic cardiomyopathy improve regional function of the scar within 3 months of therapy, and this improved regional function predicts subsequent reductions in LV chamber volumes
Endpoints for cell based therapy should focus on chamber dimensions and regional function using reliable and reproducible imaging modalities, such as cardiac MRI
Early clinical trials have shown the capacity of intramyocardial injections of bone marrow derived progenitor cells to improve regional function with modest improvements in ejection fraction (EF). We used cardiac MRI to phenotype the in-vivo changes of the left ventricle following catheter-based intramyocardial injections of bone marrow progenitor cells. We show improved regional function of scarred myocardium within 3 months of therapy and this predicts later reverse remodeling. For the first time, we show that this therapy can improve diastolic volumes and parallel decreases in both end-systolic and end-diastolic volume contribute to the lack of overall improvements in EF.
Acknowledgments
FUNDING SOURCES
This study was funded by the University of Miami Interdisciplinary Stem Cell Institute and BioCardia, Inc. Dr. Hare is also funded by NIH grant U54-HL081028.
Non-standard Abbreviations and Acronyms
- CMR
cardiac magnetic resonance imaging
- Ecc
eulerian circumferential strain
- MSC
mesenchymal stem cell
- LV
left ventricle
- MI
myocardial infarction
- IZ
infarct zone
- BZ
border zone
- RZ
remote zone
- ESV
end systolic volume
- EDV
end diastolic volume
- ICD
implantable cardioverter defibrillator
Footnotes
DISCLOSURES
Authors who are employees of BioCardia, Inc are identified as such. All authors reviewed and approved the analysis and manuscript. The University of Miami supervised the management of the study and EMMES Corporation was responsible for data collection, site monitoring and analyses.
Reference List
- 1.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I101–I107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 3.Beeres SL, Bax JJ, bbets-Schneider P, Stokkel MP, Fibbe WE, van der Wall EE, Schalij MJ, Atsma DE. Intramyocardial injection of autologous bone marrow mononuclear cells in patients with chronic myocardial infarction and severe left ventricular dysfunction. Am J Cardiol. 2007;100:1094–1098. doi: 10.1016/j.amjcard.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 4.Trachtenberg B, Velazquez D, Williams A, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman A, Hare J. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trail: a randomized, double-blinded, placebo controlled study of safety and efficacy. Amer Heart J. doi: 10.1016/j.ahj.2010.11.024. In press. [DOI] [PubMed] [Google Scholar]
- 5.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Cohen MC, Garcia MJ, Hodgson JM, Hundley WG, Lima JA, Manning WJ, Pohost GM, Raggi PM, Rodgers GP, Rumberger JA, Taylor AJ, Creager MA, Hirshfeld JW, Jr, Lorell BH, Merli G, Rodgers GP, Tracy CM, Weitz HH. ACCF/AHA clinical competence statement on cardiac imaging with computed tomography and magnetic resonance: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2005;46:383–402. doi: 10.1016/j.jacc.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Garot J, Bluemke DA, Osman NF, Rochitte CE, McVeigh ER, Zerhouni EA, Prince JL, Lima JA. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101:981–988. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 9.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 10.Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, Carr JC, Holly TA, Lloyd-Jones D, Klocke FJ, Bonow RO. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730–736. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


