Abstract
Retinal progenitor cells (RPCs) are programmed early in development to acquire the competence for specifying the seven retinal cell types. Acquiring competence is a complex spatiotemporal process that is still only vaguely understood. Here, our objective was to more fully understand the mechanisms by which RPCs become competent for specifying a retinal ganglion cell (RGC) fate. RGCs are the first retinal cell type to differentiate and their abnormal development leads to apoptosis and optic nerve degeneration. Previous work demonstrated that the paired domain factor Pax6 and the bHLH factor Atoh7 are required for RPCs to specify RGCs. RGC commitment is marked by the expression of the Pou domain factor Pou4f2 and the Lim domain factor Isl1. We show that three RPC subpopulations can specify RGCs: Atoh7-expressing RPCs, Neurod1-expressing RPCs, and Atoh7-Neurod1-expressing RPCs. All three RPC subpopulations were highly interspersed throughout retinal development, although each subpopulation maintained a distinct temporal pattern. Most, but not all, RPCs from each subpopulation were postmitotic. Atoh7-Neurod1 double knockout mice were generated and double mutant retinas revealed an unexpected role for Neurod1 in specifying RGC fate. We conclude that RPCs have a complex regulatory gene expression program in which they acquire competence using highly integrated mechanisms.
Keywords: Retinal development, retinal progenitor cells, retinal ganglion cells, cell cycle, homeobox factor Pax6, proneural bHLH factors Atoh7, Neurod1
1. Introduction
In the past twenty years, an impressive amount of knowledge has been generated on molecular regulatory mechanisms that control retinal development in embryonic and neonatal life (for a recent review, see Agathocleous and Harris, 2009). Many of these mechanisms have been found to be important in the preservation of the adult retina as well (Lamba and Reh, 2008). Despite this substantial knowledge database, we still remain woefully uninformed about many of the fundamental mechanisms that are required to generate a fully functional neural tissue capable of receiving light and sending the information to the visual centers in the brain for visual perception.
Retinal development begins immediately after the presumptive retinal epithelial layer separates from the presumptive retinal pigmented epithelium and retinal progenitor cells (RPCs) arise from pluripotent neural stem cells. RPCs are intrinsically programmed at very early stages in retinal development to follow certain lineage paths but they are not yet committed to any of the seven retinal cell types (Livesey and Cepko, 2001, Agathocleous and Harris, 2009). Rather, RPCs become competent over developmental time to commit to one or more individual cell types. RPC competency changes continuously during retinogenesis as the extrinsic environment of the developing retina changes (Livesey and Cepko, 2001, Agathocleous and Harris, 2009). RPC competency is marked by the expression of transcriptional regulatory factors. Expression of the paired box-homeodomain factor Pax6 is necessary for the specification of all the retinal cell types except amacrine cells (Marquardt et al., 2001; Marquardt and Gruss, 2002; Oron-Karni et al., 2008). Pax6 action is counteracted by the Notch-Delta signaling pathway, which keeps RPCs in the cell cycle by activating the bHLH transcriptional repressors Hes1 and Hes5 (Agathocleous and Harris, 2009; Riesenberg et al., 2009b).
In this present study, we focus on the development of one of the seven retinal cell types, retinal ganglion cells (RGCs). In the mouse, as with other vertebrates, RGCs are the first cells to differentiate from RPCs. For RGC commitment to occur, a population of Pax6-expressing RPCs must downregulate the Notch signaling pathway, exit the cell cycle, and express the proneural bHLH gene Atoh7 (also called Math5) (Mu and Klein, 2004, 2008; Agathocleous and Harris, 2009). These events render Pax6-Atoh7-RPCs competent for specifying the RGC lineage.
Lineage analysis has shown that in addition to RGCs, Atoh7-expressing RPCs can give rise to all the other retinal cell types (Yang et al., 2003). Indeed, most Atoh7-expressing cells are thought not to form RGCs (T. Glaser, unpublished results). However, retinas of Atoh7-knockout mice lack virtually all RGCs yet still form all the other retinal cell types (Brown et al, 2001; Wang et al., 2001). Atoh7 is therefore necessary but not sufficient for commitment to an RGC fate (Yang et al., 2003; Mu and Klein, 2008). In the mouse retina, the earliest signs of overt RGC differentiation are the downregulation of Atoh7 expression and the onset of expression of two key transcription factors that are essential for normal RGC differentiation to occur. These are the Pou domain factor Pou4f2 and the Lim domain factor Isl1 (Gan et al., 1999; Mu et al., 2008; Pan et al., 2008). Pou4f2, Isl1, and most likely other early-expressing transcriptional regulators activate a hierarchical RGC gene regulatory network consisting of more downstream transcription factors as well as secreted signaling molecules that feedback to RPCs to maintain a correct balance of proliferating RPCs and differentiating RGCs (Mao et al., 2008a; Mu and Klein, 2008; Mu et al., 2008).
To fully understand how RPCs give rise to RGCs, more precise analyses are required than those that have been previously reported. Towards this end, we recently generated a mouse line in which Atoh7 is replaced with a hemagglutinin (HA) epitope-tagged knock-in construct (Atoh7-HA) (Fu et al., 2009). Retinal cells expressing Atoh7-HA can be readily recognized by immunohistochemical methods using an anti-HA antibody. The Atoh7-HA-knock-in mice provide the means to examine over spatiotemporal development the relationship of Atoh7-expressing RPCs with other transcriptional regulators and with markers of cell cycle progression. Our results show that distinct populations of Atoh7-expressing RPCs are present in the developing retina and reveal an unexpected role for the proneural bHLH gene Neurod1 in specifying a subpopulation of RGCs. We conclude that RPCs are far more heterogeneous than previously thought and that they exhibit a complex regulatory gene expression program in which they acquire competence using highly integrated mechanisms.
2. Methods
2.1. Genetically engineered mice
Atoh7-knockout mice are described in Wang et al. (200), Neurod1-knockout mice are described in Pennesi et al. (2003), and Atoh7-HA mice are described in Fu et al. (2009). Atoh7-Neurod1-double heterozygous and double homozygous mice were generated by breeding single mutant heterozygous and homozygous mice. Mice were genotyped by Southern blot analysis or PCR using tail DNA as previously described (Fu et al., 2009). Embryos were designated embryonic day (E) 0.5 at noon on the day in which vaginal plugs were observed.
All animal procedures in this study follow the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center.
2.2. Immunohistochemical analysis and X-gal staining
Embryos or eyes were collected and fixed with 4% paraformaldehyde for 30 min, washed three times with PBST (PBS pH7.4, 0.2% Tween 20), embedded in OCT and frozen. Sixteen-micron cryosections were collected, washed 3 times for 10 min with PBST, and blocked with 2% BSA in PBST for 1 hr. For some staining, frozen or paraffin-embedded sections were placed in a microwave oven at 600 W in 10 mM sodium citrate for 18 min to expose the antigen epitopes, and then blocked in 10% normal serum and 0.1% Tween 20 for 1 hr at room temperature. The sections were then incubated with primary antibodies with appropriate dilution in 2% BSA-PBST for 1 hr. X-gal staining was done as described elsewhere (Mao et al., 2008a). Primary antibodies were anti-HA (Santa Cruz, 1:800), anti-Pax6 (DSHB, 1:400), anti-Chx10 (Exalpha, 1:300, Covance, 1:1000), anti-NeuroD (Santa Cruz Biotechnology, 1:400), anti-cyclinD1 (Cell Signaling, 1:300), anti-lacZ (Cell Signaling, 1:400), anti-Chat (Swant, 1:500), anti-calbindin (Swant, 1:500), anti-glutamate synthase (Sigma, 1:300), anti- rhodopsin (Cell Sigma, 1:500), anti-NFL (InVitrogen, 1:200), anti-B-opsin and anti-R-opsin (1:500, Chemicon). Secondary antibodies were conjugates of Alexa Fluor 488 and Alexa Fluor 555 (InVitrogen). Secondary antibodies were conjugates of Alexa Fluor 488 and Alexa Fluor 555 (Invitrogen). DAPI (4′, 6-diamidino-2-phenylindole) or PI (propidium iodide) were used as a nuclear counterstain. Sections were washed with PBST and mounted in Fluoromount G (EMS) and examined under a confocal microscope.
2.3. BrdU labeling
For BrdU labeling, 0.1 mg of BrdU per g body weight was injected intraperitoneally into pregnant mice one hour prior to euthanization. Embryos were fixed and washed for immunohistochemistry, embedded in OCT and frozen. Sixteen-micron of cryosection were washed three times with PBST for 10 min followed by 4N HCl treatment for 1 hr. Sections were washed three times with PBST for 10 min and incubated with anti-BrdU antibody (Upstate, 1:5). Alexa 488-conjugated anti-mouse IgG antibody was used for secondary antibody labeling.
2.4. Retina flat-mount analysis
To detect RGC axons, eyes were removed from postnatal mice and fixed with 4% paraformaldehyde for 30 min. The cornea, ciliary band, and lens were removed using a pair of iris scissors. The remaining retinal tissue and attached pigmented epithelium were fixed for 1 hr and then washed four times in PBS saline and 0.1% Triton X-100 at room temperature. The retinas were then incubated in blocking solution (PBST plus 5% fetal bovine serum) for 1 hr, and incubated with anti-NFL (1:250) for 48 hr at 4°C. Retinas were washed four times with PBST and stained with Alexa488-conjugated goat anti-mouse IgG1 secondary antibody (Invitrogen). After washing thoroughly with PBS, the pigmented epithelium was removed from the retinas and four or five symmetrical cuts were made halfway from the peripheral rim to the central optic disk. Retinas were then flat-mounted onto glass slides and analyzed using an Olympus FluoView1000 confocal microscope.
2.5. Determination of cell number
Cell number was determined by cell counting as described previously (Mu et al., 2005). Sections from three individual retinas were used for each staining. Two confocal images of central retinal region from each section were taken and cells stained in the neuroblast layer were counted. The neuroblast layer was determined based on morphology. Number of HA-expressing cells was represented as a fraction of cells stained with HA antibody per cells stained with PI. For co-immunostaining, numbers of cells were represented as fractions of cells stained by HA and Pax6, Chx10 or NeuroD1 antibodies per cells stained by HA antibody.
3. Results
3.1. Characterization of Atoh7-HA expression with an anti-HA antibody
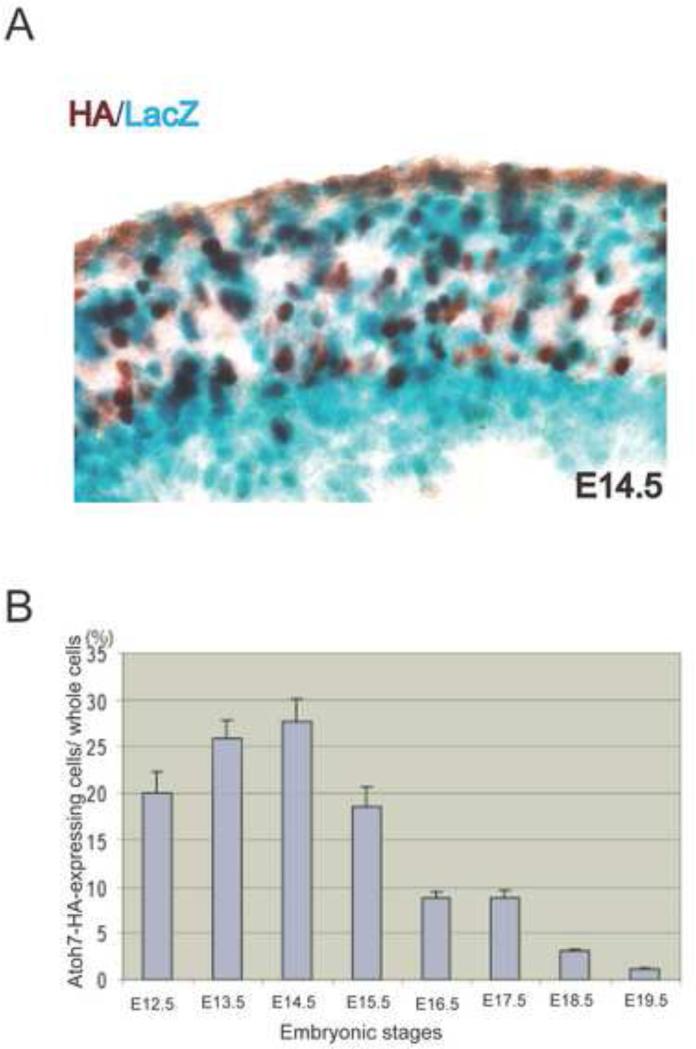
Because a robust anti-Atoh7antibody for immunohistochemistry is not available, we recently generated and characterized Atoh7-HA knock-in mice, which could then be used to detect Atoh7-expression with any number of well-described, commercially available anti-HA antibodies (Fu et al., 2009). To determine whether immunohistochemical labeling with anti-HA antibody accurately reflected endogenous Atoh7 expression in developing retinas, we made use of Atoh7-lacZ knock-in mice that had been previously generated in our laboratory (Wang et al. 2001). E14.5 retinas from Atoh7lacZ/HA heterozygous mice were immunohistochemically labeled with anti-HA antibody and histologically stained for X-gal (Fig. 1A). HA-positive cells were detected in the neuroblast layer but not in the ganglion cell layer, whereas lacZ-stained cells were present in both the neuroblast and ganglion cell layers (Fig. 1A). In the neuroblast layer, approximately 65% of the HA-labeled cells were also labeled with lacZ. However, 35% of the HA-positive cells were not co-labeled with lacZ, and similarly, we observed lacZ-positive cells that were not labeled with HA (Fig. 1A). HA-positive cells were found distributed throughout the neuroblast layer, while lacZ-positive cells accumulated more in the ventricular region than the central region of the neuroblast layer. Because lacZ is known to be a highly stable protein, the differences observed between HA-positive cells and lacZ-positive cells might reflect differences in the stabilities of lacZ and Atoh7-HA proteins. The stability of the Atoh7-HA fusion protein was likely to be more similar to that of Atoh7, which is thought to have a higher turnover rate than lacZ (Wang et al., 2001; Fu et al., 2009). If this was the case, Atoh7-HA labeling would be indicative of cells expressing Atoh7 in a narrower time window than those expressing lacZ, whose expression might reflect the more long-term history of cells that once expressed Atoh7 but were no longer doing so. Despite this caveat, the majority of cells in the neuroblast layer of E14.5 retinas were co-labeled with HA and lacZ, supporting the view that the anti-HA antibody was a suitable proxy for Atoh7-expressing cells.
Figure 1.
Expression of HA and lacZ in Atoh7HA/lacZ retinas. (A) Atoh7HA/LacZ retinas at E14.5 were immunohistochemically labeled with anti-HA antibody (brown) and histological stained for X-gal (blue). The ganglion cell layer is at the bottom on the image. (B) Histogram showing the temporal expression pattern of Atoh7-HA in the neuroblast layer of developing retinas. Atoh7-expressing cells from E12.5 to E19.5 were detected by immunolabeling retinas with anti-HA antibody. To identify cells, their nuclei were stained with propidium iodide (PI). The number of HA-positive cells and nuclei in the neuroblast layer were quantified as described in the Methods section. The HA-positive cells are calculated as a fraction of total cells in the neuroblast layer (n=3).
Atoh7-HA mice provide a means to determine many of the features of Atoh7-expressing RPCs that have been difficult to determine by other methods. For example, neither the time when Atoh7 exerts its activity or the fraction of Atoh7-expressing RPCs in the neuroblast layer of the developing retina has been accurately determined. If Atoh7-HA expression does in fact accurately reflect Atoh7-expression, it is reasonable to assume that all the rates associated with the synthesis, processing, and degradation of Atoh7-HA mRNA and protein are the same as the corresponding rates for Atoh7 mRNA and protein. To obtaine a clearer picture of the temporal expression pattern of Atoh7 and number of Atoh7-expressing RPCs, we immunolabeled retinas at different developmental times with anti-HA antibody. In E12.5 retinas, approximately 20% of cells in the neuroblast layer were HA positive (Fig. 1B). The fraction of cells expressing Atoh7-HA increased until E14.5, when 27% of the cells in the neuroblast layer were expressing Atoh7-HA. The fraction of HA-positive cells then rapidly declined; only 8% of the cells were HA positive at E17.5, and only 2% were positive at E19.5 (Fig. 1B). HA-positive cells were not detected in adult retinas (date not shown). These results were consistent with and considerably extend those reported previously (Brown et al., 1998; Wang et al., 2001; Willardsen et al., 2009).
3.2. Atoh7- HA-expression in proliferating RPCs
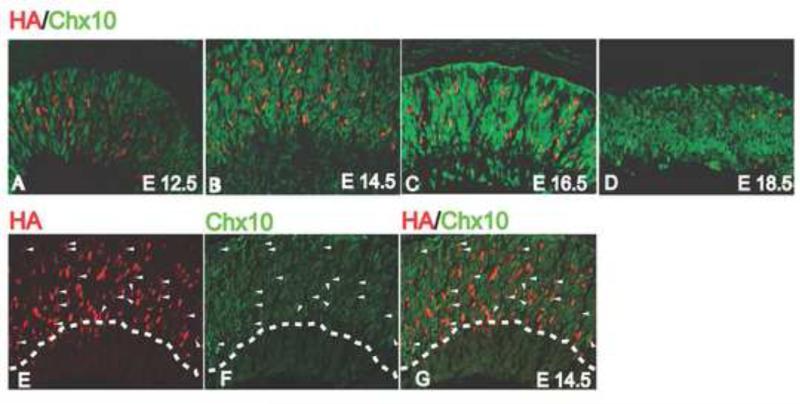
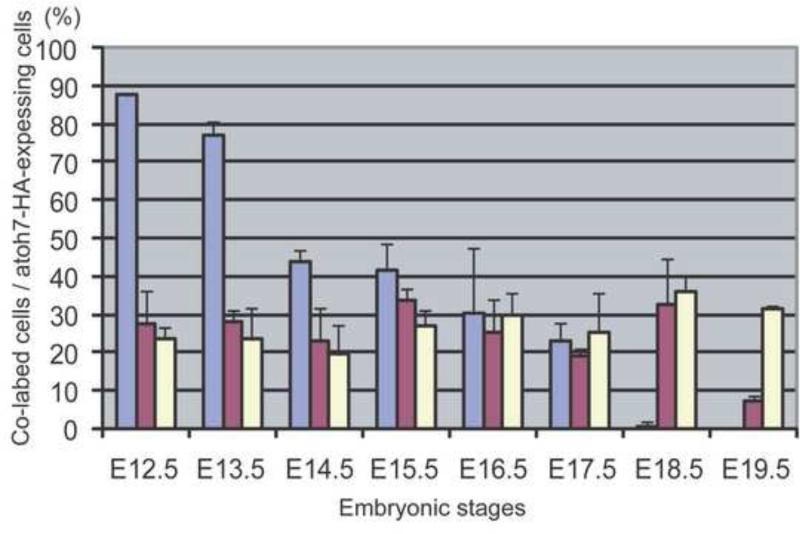
Two previously published reports differed on when in the cell cycle RPCs are expressing Atoh7. One study using Atoh7-Cre and Rosa26-lacZ expression to determine the lineage of Atoh7-expressing RPCs found that all Atoh7-expressing RPCs were postmitotic (Yang et al., 2003). However, a second study using an Atoh7-lacZ knock-in line and antibodies against the cell cycle kinase inhibitor p27/Kip1 found that Atoh7 was expressed in cells exiting the cell cycle, and in fact was required for cell cycle exit to occur normally (Le et al., 2006). In an attempt to resolve these differences and determine more directly whether Atoh7 was expressed in proliferating RPCs, we co-labeled developing retinas with anti-HA and anti-Chx10 antibodies. Chx10 is a homeobox transcription factor that is required for RPC proliferation and for bipolar cell development (Burmeister et al., 1996; Hatakeyama et al., 2001; Dyer, 2003; Liang and Sandell, 2008). From E12.5 to E19.5, Chx10-positive cells were observed uniformly distributed throughout the neuroblast layer (Fig. 2A–2D). During these times, HA-positive cells were detected both in Chx10-positive and Chx10-negative cells (Fig. 2A –2D). The fraction of HA-positive cells co-expressing Chx10 ranged from 20-30% from E12.5 until E18.5, and declined to 8% at E19.5 (Fig. 3). In contrast to the earlier studies of Yang et al. (2003) and Le et al. (2006), our results indicated that many Atoh7-expressing RPCs were actively proliferating. The reason for the discrepancies in the three studies is not clear. Our approach was to use anti-HA and anti-Chx10 antibody co-labeling, which was clearly distinct from the approaches used in the other studies. It is possible that HA labeling overestimates the number of Atoh7-expressing RPCs that are in the cell cycle while the other approaches underestimate this value.
Figure 2.
Co-expression of HA and Chx10 in developing retinas. (A–D) Retinas co-labeled with anti-HA (red) and anti-Chx10 (green) antibodies at the indicated developmental stages. (E-G) Double labeling of Chx10 and HA in E14.5 retina. Stained retinaa are represented as HA-staining (E), Chx10 staining (F) and merge (G). Cells co-labeled with HA and Chax10 antibodies are indicated by an arrow head.
Figure 3.
Histogram showing the fraction of Atoh7 -expressing RPCs in the neuroblast layer of the developing retinas that are co-expressing Pax6 (blue-gray), Chx10 (red) or Neurod1 at the indicated developmental times. Anti-HA, anti-Pax6, and anti-Neurod1 antibodies were used to label the retinas (n=3) and the fraction of cells expressing the indicated transcription factor was determined as described in the Methods section.
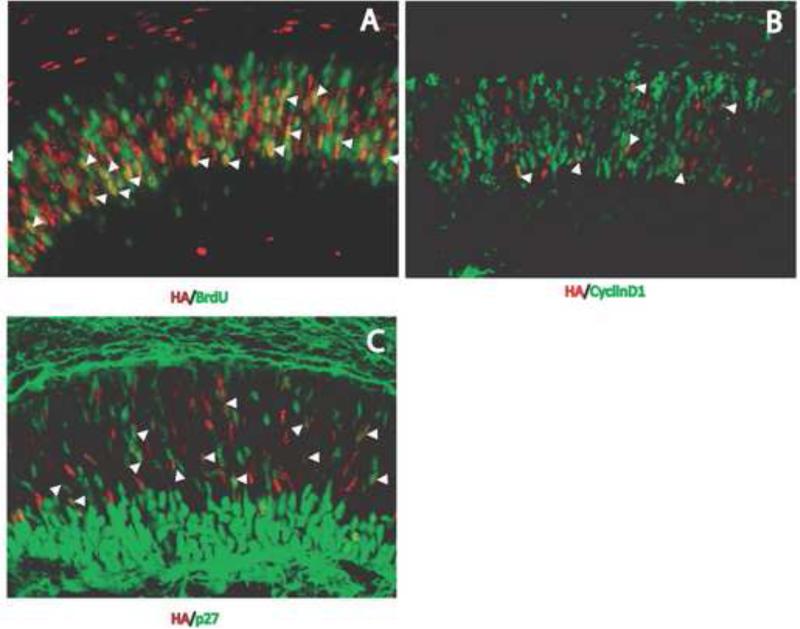
To confirm our results with Chx10, we used other cell cycle indicators in conjunction with anti-HA antibody labeling. BrdU was used to determine the number of Atoh7-expressing RPCs that were in the S-phase of the cell cycle. Cells positive for both HA and BrdU and cells positive for HA but not BrdU were observed in the neuroblast layer (Fig. 4A). Approximately 25% of the HA-positive cells were co-labeled with anti-BrdU antibody. We observed a similar labeling pattern using anti-cyclinD1 antibody, which marks the G-phase of the cell cycle (Fig. 4B). Approximately 25% of the HA-positive cells were co-labeled with anti-cyclinD1. We also used an anti-p27 antibody (Fig. 4C). In this case, approximately half of the HA-positive cells were also labeled with anti-p27 antibody. These results differ substantially from those observed by Lee et al. (2003) using an anti-p27 antibody and lacZ expression. In contrast to Yang et al. (2003) and Lee et al. (2006), our experiments suggested that although the majority of Atoh7-expressing RPCs in the developing retina were postmitotic, many were also in RPCs that were actively dividing.
Figure 4.
Co-expression of Atoh7-HA with cell cycle markers. Retinas at E14.5 were immunolabeled with anti-HA antibody (red) and antibodies against BrdU (A), cyclinD1 (B), or p27 (C) (green). Cells co-labeled with anti-HA and anti-BrdU, anti-CyclinD1 or anti-p27 antibodies are indicated by the arrowheads.
3.3. Co-expression of Atoh7 with Pax6
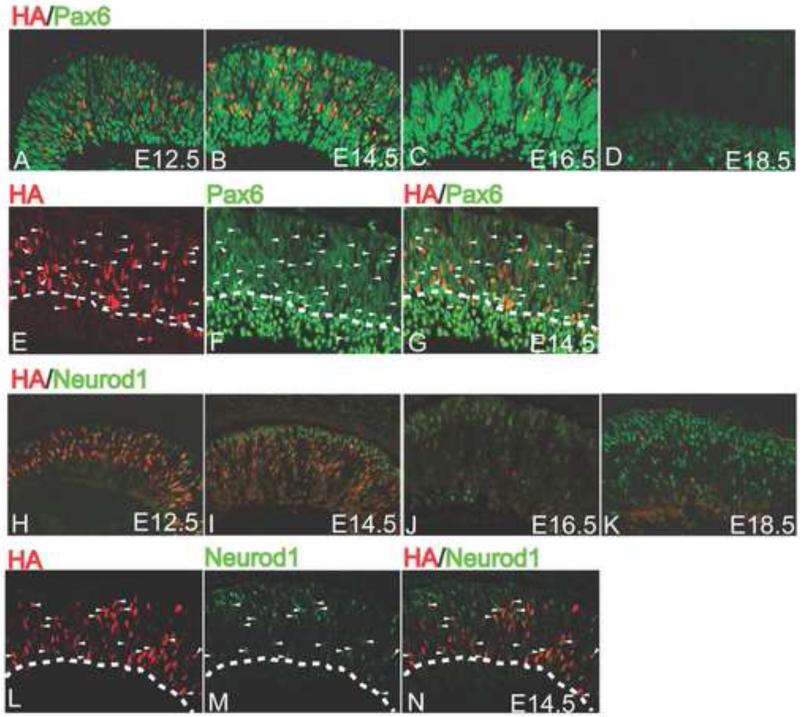
Pax6 and Atoh7 are essential for RGC development, yet the relationship between these two critical transcription factors during retinal development is only vaguely understood. A recent report describes a cis-regulatory element upstream of Atoh7 whose activity depends on Pax6, suggesting that Atoh7 is a direct Pax6 target gene (Riesenberg et al., 2009a). To further address this issue, we determined the extent to which Pax6 and Atoh7 were co-expressed during retinal development by co-labeling retinas at different developmental times with anti-Pax6 and anti-HA antibodies. At E12.5, almost 90% of the cells in the neuroblast layer of the retina that were expressing Atoh7-HA were also expressing Pax6 (Fig. 3; Fig. 5A). The fraction of Atoh7-HA-expressing cells that were also expressing Pax6 decreased to 78% at E13.5, and by E14.5, this fraction had decreased to 45% and continued to steadily decline to 42%, 30%, and 23% by E15.5, E16.5, and E17.5, respectively (Fig 3; Fig. 5A–5C). At E18.5 and E19.5, no co-labeling was observed (Fig. 3; Fig. 5D). These results reinforced the view that during the critical times when RPCs are acquiring competence to specify an RGC fate, the expression of both Pax6 and Atoh7 is required.
Figure 5.
Co-expression of Atoh7 with Pax6 (A–D) or Neurod1 (E–H). Retinas at the indicated developmental times were immunolabeled with anti-HA (red) and anti-Pax6 or anti-Neurod1 (green) antibodies.
3.4. Co-expression of Atoh7 with Neurod1
Neurod1 is a proneural bHLH factor that shares significant sequence similarity to Atoh7 (Vetter and Brown, 2001; Mao et al 2008b). In retinal development, Neurod1 has been shown to be necessary for amacrine cell formation and for the maintenance of rod photoreceptor cells (Inoue et al., 2002; Pennesi et al., 2003). Using a gene knock-in construct designed for gene replacement at the Atoh7 locus, we recently demonstrated that Neurod1 could partially replace the functions of Atoh7 when it is expressed at the same time and place as Atoh7 (Mao et al., 2008b). One interpretation of our results was that Neurod1 played some role in RGC development in addition to its other roles in amacrine cell development and rod photoreceptor maintenance. We hypothesized that the functions for Atoh7 and Neurod1 as well as for other proneural bHLH factors expressed during retinogenesis were far more synergestic and integrated than what had previously been suggested (Mao et. al., 2008b)
To gain further insight into the possible interactions between Atoh7 and Neurod1, we co-labeled developing retinas with anti-HA and anti-Neurod1 antibodies. At E12.5, we detected HA-positive cells and Neurod1-positive cells distributed throughout the neuroblast layer (Fig. 5E). By E14.5, Neurod1-positive cells were largely restricted to the outer nuclear layer and this pattern was maintained as development proceeded (Fig. 5G –5H). Throughout development, we found that 20-30% of HA-positive cells were also Neurod1-positive (Fig. 3; Fig. 5E–5H). The results indicated that a significant population of Atoh7-expressing RPCs was also expressing Neurod1, and provided further support to the notion that Neurod1 played a role in RGC development.
3.5. Generation and characterization of Atoh7-Neurod1 double knockout mice
Despite the importance of Atoh7 in specifying RGC fate, a small fraction of seemingly normal RGCs persist in Atoh7-null retinas (Lin et al., 2004; Moshiri et al., 2008). These experiments, together with our recent results showing that Neurod1 could replace Atoh7, suggested the possibility of an Atoh7-independent program that required Neurod1 to generate a small subpopulation of RGCs. To determine whether this was the case, we generated Atoh7-Neurod1 double knockout mice and compared their retinas with Atoh7-null retinas. Adult retinas from Atoh7-Neurod1 double knockout mice maintained their laminar integrity but overall were very thin when compared with Atoh7 -null or wild-type retinas (Fig. 6). Overall retinal thinness is often a sign of decreased RPC proliferation or increased apoptosis during retinal development.
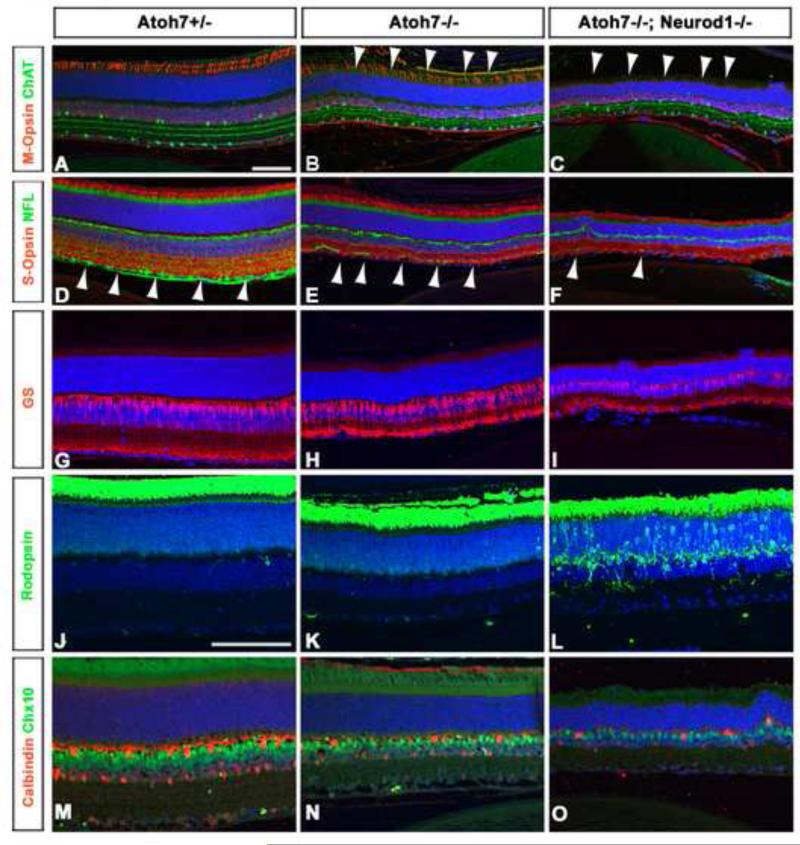
Figure 6.
Retinal cell types in Atoh7+/- (left panels), Atoh7-/- (middle panels) and Atoh7-Neurod1 double mutant (right panels) retinas at P35. Cell types were identified by immunohistochemical labelling with the indicated antibody: anti-Chat, amacrine cells (A–C, green), anti-M-opsin, M-cone photoreceptors (A– C, red), anti-NFL RGCs (D–F, green), anti-S-opsin, S-cone photoreceptors (D–F, red), anti-glutamine synthase (GS), Müller glial cells (G–I, red), anti-rhodopsin rod photoreceptors (J–L, green), Chx10, bipolar cells (M–O, green), and anti-calbindin, horizontal cells (M–O, red). Scale bar in panels A, J, 50 μm.
To determine whether any of the retinal cell types were affected in the double-mutant retinas, we immunohistochemically labeled adult retinas with a number of antibodies that labeled specific retinal cell types, namely, anti-Chat (amacrine cells, Fig. 6A–6C), anti-M-opsin (M-cone photoreceptors, Fig. 6A–6C), anti-NFL (RGCs, Fig. 6D–6F), anti-S-opsin (S-cone photoreceptors, Fig. 6D–6F), anti-glutamine synthase (GS) (Müller glial cells, Fig. 6G–6I), anti-rhodopsin, (rod photoreceptors, Fig. 6J–6L), Chx10 (bipolar cells, Fig. 6M–6O), and anti-calbindin (horizontal cells, Fig. 6M–6O).
We detected a significant decrease in the number of M-cone photoreceptors in double mutant retinas (none detectable) compared with Atoh7-null (65.33 ± 6.11) or wild-type retinas (91.67 ± 4.04) (Fig. 6A–6C). The loss of M-cone photoreceptors in Neurod1-knockout mice has recently been found to be associated with an overproliferation of S-cone photoreceptors at the expense of M-cone photoreceptors, yet we did not detect a difference in the number of M-cone cells between the Neurod1-/- and double mutants (Liu et al., 2008). Other additional defects were also seen in Atoh7-Neurod1 double mutant retinas; e.g. calbindin-positive amacrine cells were decreased in the inner nuclear layer of double mutant retinas (217± 25.53) compared to that in control retinas (272 ±7.55). These data suggested that Atoh7 does not compensate for Neurod1 in cone cell formation. In addition, the perinuclear condensation of rhodopsin that we observed in the rod photoreceptors of the double mutants (Fig. 6L) was a sign of rod photoreceptor degeneration, which has been previously reported in Neurod1-null retinas by Pennesi et al. (2003).
Importantly, we also observed a significant decrease in NFL expression in Atoh7-Neurod1 double mutant retinas compared with Atoh7-null retinas (Fig. 6E, 6F). Although NFL expression was strongly downregulated in Atoh7-null retinas compared with wild-type controls (Fig. 6D, 6E), the diminishment of NFL expression was even more pronounced in the double mutant retinas (Fig. 6F). These results indicated that the loss of RGCs was even greater in Atoh7-Neurod1 double mutant retinas than in Atoh7-null retinas. This was particularly interesting because RGCs develop normally in Neurod1-null mice (Pennesi et al., 2003).
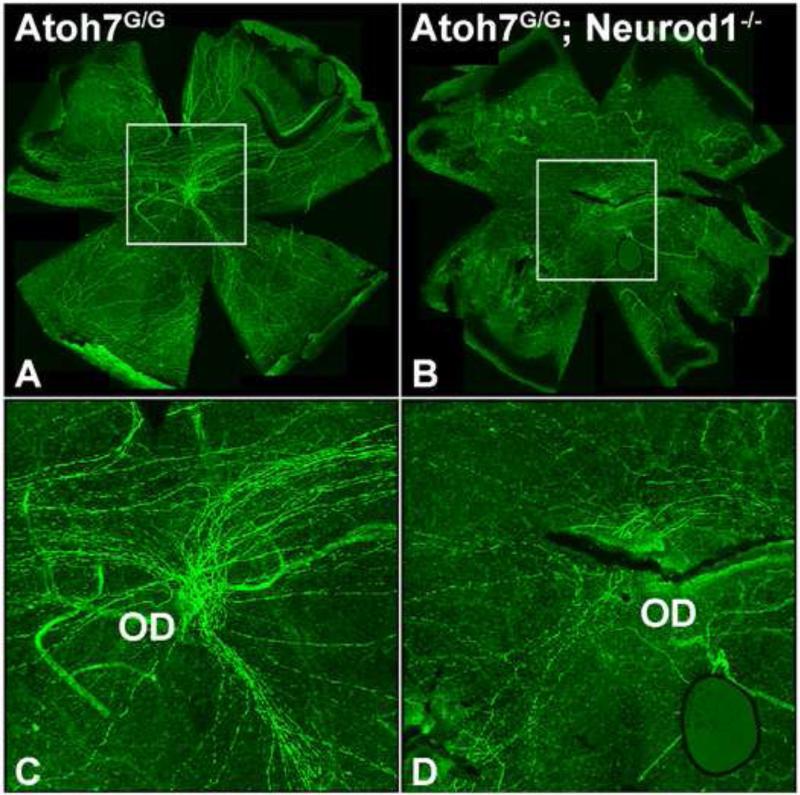
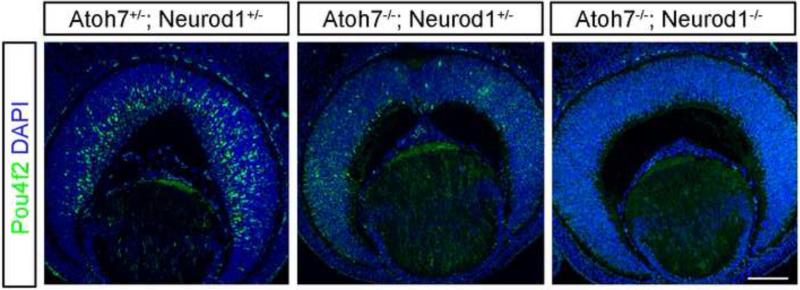
We determined the number of RGCs in double mutant retinas more directly using flat-mounted retina preparations that were immunolabeled with anti-NFL antibody to detect RGC axons (Fig. 7A–7D). We detected significantly fewer RGC axons in double mutant retinas than in Atoh7-null retinas (compare Fig. 7A, 7C with Fig. 7B, 7D). To determine whether the enhanced RGC phenotype is due to a specification or a maintenance defect, we immunostained E13.5 retinal sections with an anti-Pou4f2 antibody. We found that the remaining few Pou4f2-positive cells in the Atoh7 mutant were completely abolished in the Atoh7-Neuorod1 double mutant (Fig. 8B, C). Furthermore, we conducted apoptosis analysis on mutant retinas, and found a slight increase of dying cells in the Atoh7-Neurod1 double mutant compared to that of the control (Fig. 9). The results were consistent with our hypothesis that Neurod1 contributed to RGC development and that it was required for the formation of a subpopulation of RGCs that formed in the absence of Atoh7 .
Figure 7.
Atoh7-Neurod1 double-mutant retinas contain fewer RGC axon fibers than do Atoh7 mutant retinas. (A, B) Immunolabeling of flat-mounted retinas from P30 mice with anti-NFL antibody to reveal RGC axons. Genotypes are indicated on the upper left of each panel. (C, D) Enlarged images of boxed areas in panels A and B highlight the reduced number of axon fibers in Atoh7-Neurod1 double mutants. OD, optic disk.
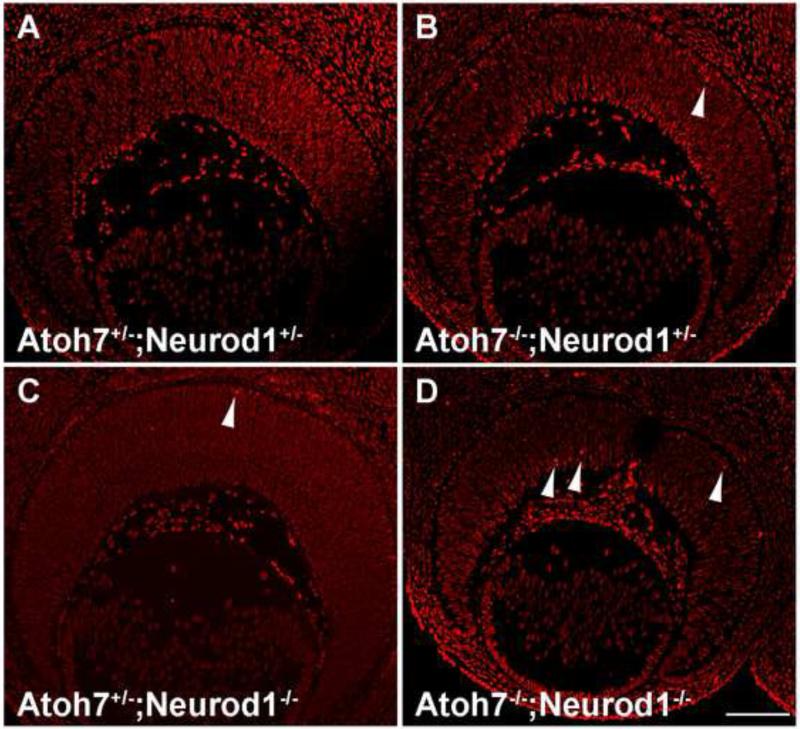
Figure 8.
Pou4f2 expression in E13.5 Atoh7 and Neurod1 mutant retinas. (A) Atoh7+/-; Neurod1+/-, (B) Atoh7-/-; Neurod1+/-, and (C) Atoh7-/-; Neurod1-/- retinas. Scale bar in C, 100 μm.
Figure 9.
Apoptosis analysis in E14.5 Atoh7 and Neurod1 mutant retinas. (A) Atoh7+/-; Neurod1+/-, (B) Atoh7-/-; Neurod1+/-, (C) Atoh7+/-; Neurod1-/-, and (D) Atoh7-/-; Neurod1-/- retinas. Note that a slight increase of cell death is always found in Atoh7-/-; Neurod1-/- retinas than in control retinas. Arrowheads indicate dying cells. Scale bar in D, 100 μm.
4. Discussion
The ability to generate mouse models using targeted gene knockout technology has resulted in major advances in our understanding of the mechanisms that regulate retinal development (e.g., Chalupa and Williams, 2008). In the present study, we made use of two genetically engineered mouse lines, one that expressed an Atoh7-HA allele at the Atoh7 locus and another that lacked both the Atoh7 and Neurod1 genes. The experiments described in this report relied on these mouse lines to provide new insights into RPCs and how they acquire the competency for specifying the retinal cell types. The results lead to the conclusion that RPCs are far more complicated in their expression of regulatory genes than previously thought. Furthermore, regulatory genes expressed in RPCs are likely to be synergistic and to operate in complex combinations to advance a competent RPC to a committed retinal cell type.
Immunolabeling with anti-HA antibody allowed us to determine the spatiotemporal expression pattern of Atoh7 and the fraction of RPCs that express Atoh7 during retinal development. We found that Atoh7 was expressed in a narrow window of time, with expression peaking at E14.5 and rapidly declining thereafter. At E12.5, the earliest time we examined, Atoh7-expressing RPCs already represented 20% of the cells in the neuroblast layer, and at its peak, Atoh7 was expressed in more than one-quarter of the cells. Thus, Atoh7 must be activated at very early times in retinal development and Atoh7-expressing RPCs must make a significant contribution to the initial but not final stages of retinogenesis. This is particularly interesting because most Atoh7-expressing RPCs are not destined to follow an RGC fate (Yang et al., 2003). Earlier reports have shown that Atoh7 has additional functions besides those for RGC fate specification, which are not easily revealed by knockout and other loss-of-function approaches (Brown et al., 2001, Wang et al., 2001, Le et al., 2006). We favor a model in which Atoh7-expressing RPCs play critical roles in broader aspects of retinal cell type specification, especially at early times in retinogenesis. Atoh7 is likely to work together with Pax6 and other transcription factors in defining one subpopulation of RPCs during these times.
An unexpected finding from our experiments was that in addition to postmitotic RPCs, a fraction of proliferating RPCs also express Atoh7. Although our results appear to contradict previous studies (Yang et al., 2003; Le et al., 2006), they are consistent with a model in which proliferating RPCs activate Atoh7 expression and that this activation is closely coupled to cell cycle exit. The time in the cell cycle when Atoh7 is expressed may be highly sensitive to the method of detection. Because a robust antibody for Atoh7 is not available and in situ hybridization is limited by the abundance of Atoh7 transcripts and technical difficulty in double labeling with cell cycle markers, less direct methods are required. Nevertheless, all experiments reported to date are consistent with a model in which the majority of Atoh7-expressing RPCs are just exiting or have exited the cell cycle.
Pax6 plays a broader role in retinal development than Atoh7 (Marquardt and Gruss, 2002) and is positioned genetically upstream of Atoh7 in a model for a RGC gene regulatory network that we have proposed (Mu and Klein, 2008; Mu et al., 2008). The Pax6-HA co-labeling experiments further support the view Pax6 and Atoh7 must be co-expressed in the same RPC in order for it to acquire competency for an RGC fate. It is also likely that co-expression of Pax6 and Atoh7 is important for the formation of other retinal cell types, although this remains to be shown.
Kageyama and co-workers have proposed that retinal cell-fate specification is regulated by combinations of bHLH and homeobox genes. In their model, homeobox genes confer positional identity whereas bHLH genes promote neuronal cell fate specification (Hatakeyama et al., 2001; Hatakeyama and Kageyama, 2004; Ohsawa and Kageyama, 2008). Some bHLH factors are known to function as transcriptional activators (e.g., Atoh7, Neurod1) and others as transcriptional repressors (e.g., Hes1, Hes5). In addition, bHLH factors regulate each other's expression (Mu et al., 2005; Le et al., 2006; Ohsawa and Kageyama, 2008). In RPCs of the developing retina, these interactions occur in dynamic spatiotemporal patterns. It is therefore likely that an exceeding complicated transcriptional network regulates retinal development.
Earlier work suggested that Neurod1-expressing and Atoh7-expressing RPCs are largely distinct populations with differing spatiotemporal expression patterns (Inoue et al., 2002; Mu et al., 2005). Our results indicate that there is a substantial number of RPCs that co-express Neuod1 and Atoh7. Thus, three distinct subpopulations of RPCs co-exist during the early stages of retinal development, Atoh7-expressing RPCs, Neurod1-expressing RPCs, and Atoh7-Neurod1-expressing RPCs.
We showed previously that a Neurod1 knock-in construct inserted at the Atoh7 locus resulted in partial, but not full, activation of the RGC gene regulatory network and restoration of RGCs and the optic nerve (Mao et al., 2008b). The close relatedness of Neurod1 and Atoh7 sequences in their bHLH domains indicates that these transcription factors may have extensive overlap in the gene regulatory networks that they activate. However, Neurod1 is thought to play different roles in retinal cell-fate specification than those played by Atoh7. Neurod1 and a related bHLH gene, Math3, are required together for amacrine cell-fate specification (Inoue et al., 2002). The fact that many RPCs co-express Neurod1 and Atoh7, and that fewer RGCs are present in Atoh7-Neurod1 double knockout retinas compared with Atoh7 single knockout retinas, strongly argues that Neurod1 plays a role in RGC development. Neurod1 may synergize with Atoh7 to promote RGC specification, and in addition, Neurod1 may be required for the development of a subpopulation of RGCs that form independently of Atoh7.
Recently, we demonstrated a role for the neuronal transcriptional repressor REST/NRSF in retinal development (Mao et al., 2010). REST/NRSF is an engrailed domain-containing transcriptional repressor that represses the expression of neuronal differentiation genes in non-neuronal cells, pluripotent stem cells, and neuronal progenitors (Kagalwala et al., 2008). Six3-Cre-mediated deletion of a floxed REST/NRSF allele leads to the efficient deletion of REST/NRSF in the developing retina beginning at E10.5 (Mao et al., 2010). We found that in the absence of REST/NRSF, many of the genes that are normally expressed in differentiated RGCs are prematurely activated in proliferating RPCs (Mao et al., 2010). Most importantly, the premature expression of RGC genes was independent of Atoh7, as shown by the expression of some of the genes in RPCs of Atoh7-REST/NRSF double knockout retinas. These results led to the hypothesis that a distinct pathway for formation of a subpopulation of RGCs exists in the developing retina. This pathway is suppressed by REST/NRSF and does not depend on the presence of Atoh7. From the results presented here, we suggest that Neurod1 is required for the Atoh7-indenpendent pathway to operate.
In summary, our results provide further demonstration of the complexity of the gene regulatory networks that are required for retinal development. Emerging new technologies using next generation genomic and cDNA sequencing offer a potentially valuable way to identify the downstream components of retinal gene regulatory networks. A more detailed picture of these networks and the crucial nodes within them should eventually lead to a more comprehensive understanding of the molecular programs that regulate retinal cell-fate specification and to how these programs are altered by the continuously changing extrinsic environment of the developing retina.
Research Highlights.
Spatiotemporal expression pattern of the bHLH gene Atoh7 in retinal development
Atoh7 is expressed in both postmitotic and proliferating retinal progenitor cells
Pax6 and Atoh7 are co-expressed in RPCs during early stages of retinal development
Distinct subpopulations of RPCs express Atoh7, Neurod1, or both Atoh7-Neurod1
Atoh7-Neurod1 double mutant retinas reveal a role for Neurod1 in RGC specification
Acknowledgements
We thank Ming-Jer Tsai (Baylor College of Medicine) for providing the Neurod1 mice. We acknowledge The University of Texas MD Anderson Cancer Center DNA Analysis Facility for DNA sequencing, the Genetically Engineered Mouse Facility and the Research Animal Support Facility for providing their services. The Core Facilities are supported in part by a National Cancer Institute Cancer Center Core Grant (CA016672). The work was supported by grants to W.H.K. from the National Eye Institute (EY011930 and EY010608-139005) and from the Robert A. Welch Foundation (G-0010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu. Rev. Cell Dev. Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, Taylor BA, Hankin MH, McInnes RR. Ocular retardation mouse caused by Chx10 homeobox-null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Williams RW. Eye, Retina, and Visual System of the Mouse, Chapter IV, Development of the Mouse Eye. The MIT Press; Cambridge, MA: 2008. [Google Scholar]
- Dyer MA. Regulation of proliferation, cell fate specification and differentiation by the homeodomain proteins, Prox1, Six3, and Chx10 in the developing retina. Cell Cycle. 2003;2:350–357. [PubMed] [Google Scholar]
- Fu X, Kiyama T, Li R, Russell M, Klein WH, Mu X. Epitope-tagging Math5 and Pou4f2: new tools to study retinal ganglion cell development in the mouse. Dev. Dyn. 2009;238:2309–2317. doi: 10.1002/dvdy.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Semin. Cell, Dev. Biol. 2004;15:83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Tomita K, Inoue T, Kageyama R. Roles of homeobox and bHLH genes in specification of a retinal cell type. Development. 2001;128:1313–1322. doi: 10.1242/dev.128.8.1313. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Kagalwala MN, Singh SK, Majumder S. Stemness is only a state of the cell. Cold Spring Harb. Symp. Quant. Biol. 2008;73:227–234. doi: 10.1101/sqb.2008.73.042. [DOI] [PubMed] [Google Scholar]
- Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Wroblewski E, Patel S, Riesenberg AN, Brown NL. Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev. Biol. 2006;295:764–778. doi: 10.1016/j.ydbio.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Liang L, Sandell JH. Focus on molecules: homeobox protein Chx10. Exp. Eye Res. 2008;86:541–542. doi: 10.1016/j.exer.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:474–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Liu H, Etter P, Hayes S, Jones I, Nelson B, Hartman B, Forrest D, Reh T. NeuroD1 regulates expression of thyroid hormone receptor 2 and cone opsin in the developing mouse retina. J. Neurosci. 2008;28:749–756. doi: 10.1523/JNEUROSCI.4832-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Mao CA, Kiyama T, Pan P, Furuta Y, Hadjantonakis AK, Klein WH. Eomesodermin, a target gene of Pou4f2, is required for retinal ganglion cell and optic nerve development in the mouse. Development. 2008a;135:271–280. doi: 10.1242/dev.009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CA, Tsai WW, Barton MC, Klein WH. Neuronal transcriptional repressor REST suppresses an Atoh7-independent program for initiating retinal ganglion cell development. De. Biol. 2010 doi: 10.1016/j.ydbio.2010.10.008. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CA, Wang SW, Pan P, Klein WH. Rewiring the retinal ganglion cell gene regulatory network: Neurod1 promotes retinal ganglion cell fate in the absence of Math5. Development. 2008b;135:3379–3388. doi: 10.1242/dev.024612. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Paden R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Gruss P. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci. 2002;25:32–38. doi: 10.1016/s0166-2236(00)02028-2. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Gonzalez E, Tagawa K, Wang M, Frishman LJ, Wang SW. Near complete loss of retinal ganglion cells in the math5/brn3b double knockout elicits severe reductions of other cell types during retinal development. Dev. Biol. 2008;316:214–227. doi: 10.1016/j.ydbio.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Fu X, Beremand PD, Thomas TL, Klein WH. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6942–6947. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Fu X, Sun H, Beremand PD, Thomas TL, Klein WH. A gene network downstream of transcription factor Math5 regulates retinal progenitor cell competence and ganglion cell fate. Dev. Biol. 2005;280:467–481. doi: 10.1016/j.ydbio.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Mu X, Klein WH. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin. Cell Dev. Biol. 2004;15:115–123. doi: 10.1016/j.semcdb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Mu X, Klein WH. Gene regulatory networks and retinal ganglion cell Development. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. The MIT Press; Cambridge, MA: 2008. pp. 321–332. [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;4:1192, 90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Oran-Karni V, Fahy C, Elgart M, Remizova L, Yaron D, Xie Q, Cveki A, Ashery-Paden R. Dual requirement for Pax6 in retinal progenitor cell. Development. 2008;135:4037–4047. doi: 10.1242/dev.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–1990. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Cho JH, Wu SH, Zhang J, Wu SM, Tsai MJ. BETA2/NeuroD1 null mice: a new model for transcription factor-dependent photoreceptor degeneration. J. Neurosci. 2003;23:453–461. doi: 10.1523/JNEUROSCI.23-02-00453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg AN, Lee TT, Willardsen MI, Blackburn DC, Vetter ML, Brown NL. Pax6 regulation of Math5 during mouse retinal neurogenesis. Genesis. 2009a;47:175–187. doi: 10.1002/dvg.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg AN, Liu Z, Kopan R, Brown NL. Rbpj cell autonomous regulation of retinal ganglion cell and cone photoreceptor fates in the mouse retina. J. Neurosci. 2009b;29:12865–12877. doi: 10.1523/JNEUROSCI.3382-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter ML, Brown NL. The role of basic helix-loop-helix genes in vertebrate retinogenesis. Semin. Cell Dev. Biol. 2001;12:491–498. doi: 10.1006/scdb.2001.0273. [DOI] [PubMed] [Google Scholar]
- Willardsen MI, Suli A, Pan Y, Marsh-Armstrong N, Chien CB, El-Hodiri H, Brown NL, Moore KB, Vetter ML. Temporal regulation of Ath5 gene expression during eye development. Dev. Biol. 2009;326:471–481. doi: 10.1016/j.ydbio.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev. Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]