Abstract
Aqueous droplets in oil that are coated with lipid monolayers and joined through interface bilayers1,2 are useful for biophysical measurements on membrane proteins2–5. Further, functional networks of droplets that can act as light sensors, batteries and electrical components can be made by incorporating pumps, channels and pores into the bilayers2,6. These networks of droplets mimic simple tissues7, but so far have not been used in physiological environments because they have been constrained to a bulk oil phase. Here we form multisomes: networks of aqueous droplets with defined compositions within small drops of oil in water (Fig. 1a). The encapsulated droplets can communicate with each other and with the surrounding aqueous environment through membrane pores. The contents in the droplets can be released by changing the pH or temperature of the surrounding solution. Multisomes constitute a multi-compartment protocellular chassis7–9 with potential medical applications.
Multisomes of defined composition were constructed in three steps. First, a drop of oil containing dissolved lipid (diameter ~800 μm) was placed in a bulk buffer. Typically, the oil was a mixture of a silicone oil and hexadecane, and the lipid was 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC). Second, droplets of buffer (diameter ~300 μm) were pipetted into a different container filled with the same lipid in oil solution. Finally, after ~5 min (the incubation time, Supplementary Discussion 1), a predetermined number of aqueous droplets was transferred with a pipette into the oil drop. Within ~1 min of encapsulation, the inner droplets adhered to each other, forming internal interface bilayers, and to the surface of the oil drop, forming external interface bilayers, which separated them from the bulk aqueous phase (Fig. 1b–e). These structures were stable for at least 24 h. For simplicity, we use the term ‘multisome’ irrespective of the number of encapsulated droplets.
Fig. 1. Schematics and photographs of multisomes.
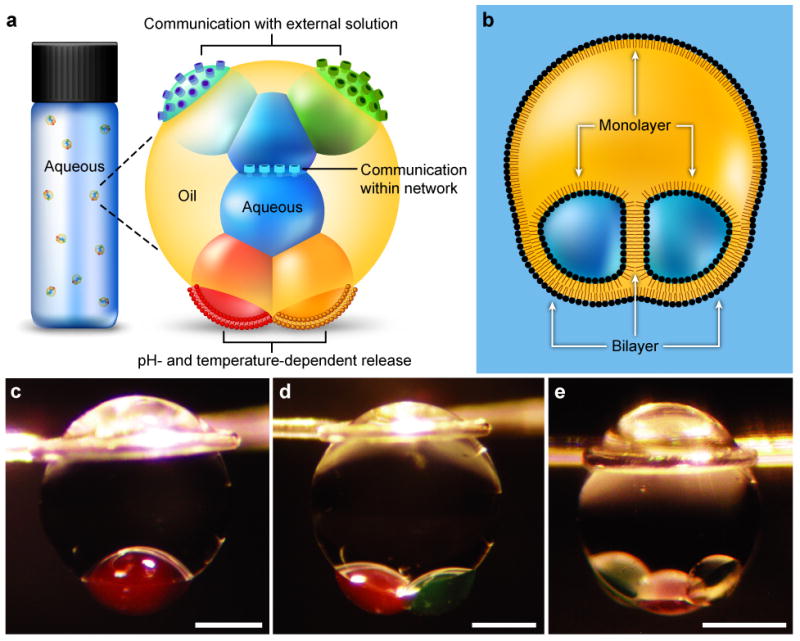
a, Illustration of a multisome. Aqueous droplets encapsulated in an oil drop are connected by lipid bilayers, which allow the droplets in the network to communicate through protein pores. Pores in bilayers at the surfaces of the aqueous droplets that protrude from the oil drop enable the network to communicate with the bulk solution. Multisomes can release the contents of encapsulated droplets by pH- or temperature-induced rupture of bilayers. b, Schematic of an encapsulated two-droplet network, illustrating the lipid monolayers and bilayers. c–e, Photographs of multisomes containing one (c), two (d) and three (e) inner droplets. Oil drops were suspended on wire loops to allow extended study. Aqueous droplets were dyed with 25 μM sulphorhodamine 101 (red) or fluorescein (green). Scale bars = 400 μm.
Multisomes are topologically related to other lipid-based structures, particularly vesosomes10, which consist of vesicles that contain multiple smaller vesicles, and multivesicular liposomes11, which are foam-like aggregates of aqueous compartments joined by lipid bilayers. However, vesosomes and multivesicular liposomes are made by bulk methods that yield poorly controlled structures. By contrast, here we assemble multisomes of defined structure. Further, the compartments of multisomes can employ protein pores to communicate with each other and the external environment and thereby form functional devices.
Following encapsulation but before bilayer formation, the oil drop and inner droplets minimize their surface energies by adopting spherical geometries. The geometries after bilayer formation represent a compromise between the favourable adhesion of apposing monolayers to form bilayers, and unfavourable distortions that expose a greater monolayer or bilayer surface area. By considering the range of possible geometries of a multisome, and calculating the energetic cost of each geometry relative to the state before bilayer formation, we may predict the most favourable geometry (see Supplementary Methods).
The structure was parameterized by three contact angles (Fig. 2a, Supplementary Methods). One angle is constrained by conservation of the oil drop and inner droplet volumes, while the other two angles remain free variables. We arbitrarily chose θ2 and θ3 as the free variables. The surface tensions of a monolayer and bilayer, γm and γb respectively, are measures of the energetic cost of creating a unit area of each kind of interface. The free energy of formation of a multisome of specified geometry is therefore given by the changes in monolayer and bilayer surface areas relative to the spherical states, weighted by the surface tension of each interface. In the Supplementary Methods we show that the free energy of formation of a multisome with contact angles (θ1, θ2, θ3) is:
where R1 and R2 are the radii of the oil drop and inner droplets, respectively, before bilayer formation, and the ri are the radii of curvature of the three spherical caps corresponding to the contact angles θi, and can be evaluated from the θi, R1 and R2. By using this formula, ΔF was evaluated for all combinations of θ2 and θ3 to produce a free energy landscape (Fig. 2b), using the following values: R1 = 400 μm, R2 = 200 μm, γm = 5 mN m−1 12,13 and γb/γm = 0.68. The minimum of the landscape represents the multisome geometry of lowest energy, which for the chosen parameters lies at (θ1, θ2, θ3) = (33°, 173°, 77°). The value of γb/γm was obtained from measurements of droplet interface bilayer contact angles (Supplementary Methods). Surface tensions in systems with high contact angles could be measured accurately using the method of Needham and Haydon14. The extension of this analysis to more complex multisomes is considered in Supplementary Discussion 2.
Fig. 2. Free energy landscape.
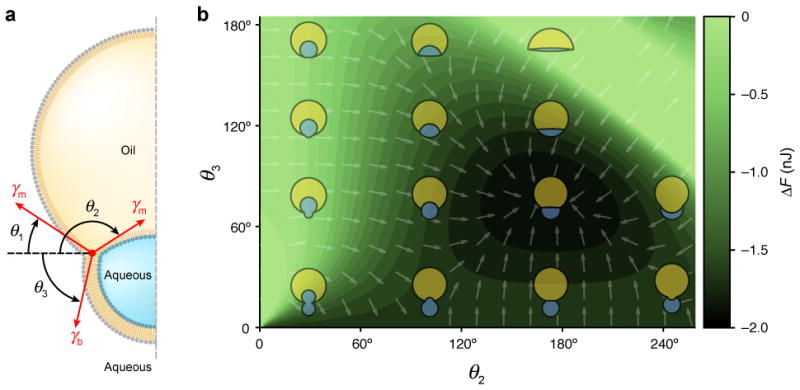
a, Schematic of a multisome with a single inner aqueous droplet, showing the definition of the contact angles θi relative to the horizontal. The arrows labelled γm and γb represent the monolayer and bilayer surface tensions, respectively. b, Free energy of bilayer formation for an encapsulated droplet, as a function of the contact angles θ2 and θ3. The landscape was computed assuming an oil drop radius of R1 = 400 μm, an aqueous droplet radius of R2 = 200 μm, a monolayer surface tension of γm = 5 mN m−1, and a ratio of bilayer to monolayer surface tensions of γb/γm = 0.68. Arrows indicate the direction of steepest descent. The geometry of the multisome is depicted at various points in the landscape, including the state of minimum free energy at (θ1, θ2, θ3) = (33°, 173°, 77°).
In addition to creating multisomes of defined structure, we aimed to produce functional droplet networks in an aqueous environment. By analogy with networks in bulk oil2, the incorporation of membrane pumps, channels and pores into multisome bilayers would allow control over the exchange of material, and electrical communication, between the various inner droplets and the external solution. To determine whether the presumed external bilayers of multisomes support the insertion of membrane proteins, we performed electrical measurements across the external bilayer of a multisome with a single inner droplet.
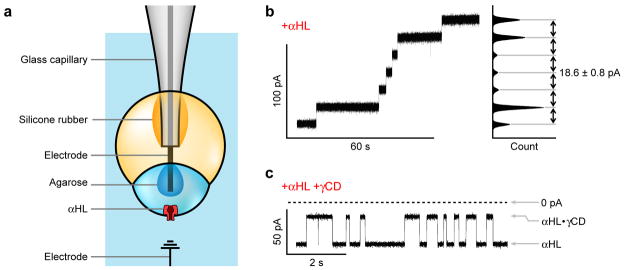
For this purpose, we made a glass-insulated Ag/AgCl electrode with an electrically exposed tip (see Methods). Immediately after an aqueous droplet had been transferred into the oil drop, the electrode tip was inserted into the inner droplet. Through micromanipulation of the electrode, the inner droplet was lowered until it adhered to the surface of the oil drop (Fig. 3a). When wild-type staphylococcal α-hemolysin (αHL) was included in the inner droplet, the ionic current began increasing in a stepwise manner within 1 min of adhesion, as recorded with the inserted electrode at +50 mV relative to the external aqueous solution (Fig. 3b). The amplitude of the steps was 18.6 ± 0.8 pA (mean ± s.d., n = 16), consistent with the expected current of ~18.6 pA for the αHL pore under the given conditions (500 mM KCl, pH 8.0)15. The current steps therefore corresponded to consecutive insertions of αHL pores into an external bilayer.
Fig. 3. Measurement of ionic currents through αHL pores.
a, Schematic of the measurement of ionic current flowing between an encapsulated droplet and the bulk aqueous solution, through an αHL pore inserted in the bilayer. b, Stepwise increase in current indicating consecutive insertions of αHL pores into the external bilayer, at +50 mV in 500 mM KCl at pH 8.0. The peaks in the current histogram were separated by 18.6 ± 0.8 pA (mean ± s.d., n = 16), as expected for insertions of individual wild-type αHL pores. c, Current blockades of a single wild-type αHL pore in the configuration shown in a after adding ~10 μM γ-cyclodextrin to the bulk solution, at −50 mV in 1 M KCl at pH 8.0. The current levels of the unoccupied pore and the pore with γ-cyclodextrin bound are indicated. The γ-cyclodextrin current blockades have an amplitude of 63.7 ± 2.0% (mean ± s.d., n = 673), and the dissociation rate of γ-cyclodextrin was 4.0 ± 0.6 s−1 (mean ± s.d.).
To confirm that the inserting pore was αHL, the experiment was repeated with a lower concentration of protein. After a few minutes, the current stepped from zero to a steady level, corresponding to the insertion of a single αHL pore into the external bilayer. Immediately afterwards, γ-cyclodextrin (~10 μM) was added to the external aqueous solution, which caused reversible blockades of the current through the pore (Fig. 3c) with a blocking amplitude of 63.7 ± 2.0% (mean ± s.d., n = 673) at −50 mV in 1 M KCl at pH 8.0. A least-squares linear fit to a logarithmic histogram of the dwell times gave a dissociation rate of 4.0 ± 0.6 s−1 (mean ± s.d.). A previous study, using an interface bilayer between two aqueous droplets in bulk oil, found a blocking amplitude of ~60% and a dissociation rate of 2.0 s−1 at pH 7.02. The higher dissociation rate seen here at pH 8.0 is consistent with the finding that the rate of dissociation of β-cyclodextrin from αHL increases with pH 16.
We also explored whether the inner droplets of a multisome can use pores to communicate passively with each other and with the external aqueous solution: that is, without driving an ion flux with an externally applied potential. First we tested whether an encapsulated droplet could communicate with the external solution. A multisome was made with a single inner droplet that contained αHL pores and fluo-4 (a Ca2+-sensitive dye) conjugated to 10,000 MW dextran. The addition of Ca2+ to the external solution caused the initially dark inner droplet to become fluorescent over ~1.5 h (Fig. 4a), after a delay (Supplementary Discussion 3). whereas a multisome without αHL showed no fluorescence increase (n = 6). We concluded that the fluorescence increase in the multisome containing αHL was caused by the diffusion of Ca2+ ions from the external aqueous solution into the inner droplet, through αHL pores in the external bilayer. The number of pores in the multisome bilayer was estimated using two aqueous droplets in bulk oil, joined by an interface bilayer. One of the droplets contained αHL at the same concentration as in the multisome experiment. From electrical measurements across this bilayer, the number of αHL pores inserted into the multisome bilayer is expected to be several thousand.
Fig. 4. Communication by diffusion through αHL pores.
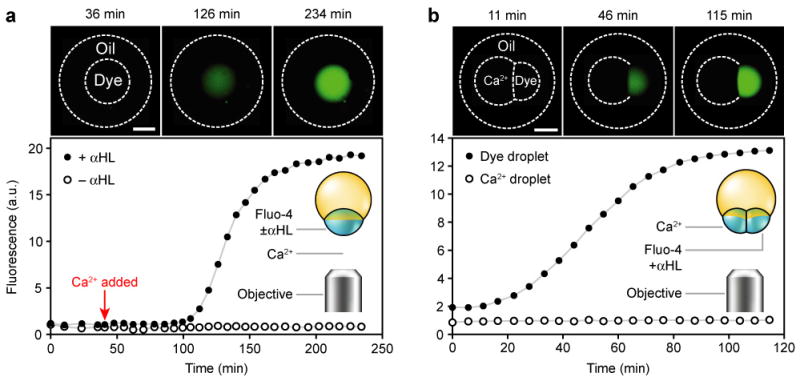
Fluorescence photographs and measurements of multisomes. Oil and inner droplets are outlined in the photographs where they are invisible. Inner droplets containing dextran-conjugated fluo-4 or Ca2+ are respectively labelled ‘Dye’ or ‘Ca2+’. a, Two multisomes with a single inner droplet each, in the same bulk solution; both multisomes contained the dye, and one also contained αHL. The photographs are of the droplet containing αHL, while the graph includes measurements from both droplets. Following the addition of Ca2+ to the external solution, the droplet containing αHL increased in fluorescence over ~1.5 h, while the droplet without protein did not. Scale bar = 300 μm. b, A multisome containing a two-droplet network, in which one droplet contained Ca2+ and the other contained the dye and αHL. The dye-containing droplet increased in fluorescence, while Ca2+-containing droplet did not. Scale bar = 300 μm.
We then tested whether two inner droplets in a multisome could communicate with each other in a similar way, by including dextran-conjugated fluo-4 and αHL in one droplet and Ca2+ in the other. The fluorescence of the droplet containing fluo-4 increased over ~1 h (Fig. 4b), demonstrating the diffusion of Ca2+ ions between the inner droplets through αHL pores in the internal bilayer. Communication by passive diffusion can therefore take place between a multisome and the external solution, and within the inner droplets of a multisome.
We also explored the possibility that the lipid bilayers themselves might be used to functionalize multisomes. We first rendered multisome bilayers pH-sensitive by employing a strategy commonly applied to liposomes. The approach uses a mixture of two lipids17: one, such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), that favours a non-bilayer state; and another, such as oleic acid (OA), that stabilizes the bilayer state at pH values above its pKa (~7.5 when incorporated in a bilayer18,19) but not at lower pH values, when a significant proportion of OA is protonated. Multisomes made with a mixture of DOPE and OA were stable for at least a day in bulk aqueous solution at pH 8.0 (n = 7). When the pH of the solution was decreased from 8.0 to ~5.5 by replacing half the solution with buffer at pH 3.0, the external bilayers suddenly ruptured, allowing the contents of the inner droplets to mix with the external solution (Supplementary Fig. S1). The droplets burst within <2 min of each other, and usually within <1 min (n = 8).
To determine whether the contents of the droplets mixed with each other before diffusing away into the external solution, multisomes with two inner droplets were made in which one inner droplet contained dextran-conjugated fluo-4, and the other contained Ca2+ (Fig. 5a). As shown by fluorescence microscopy, the contents of the two droplets indeed mixed when the pH of the external solution was lowered, before diffusing away (Fig. 5b).
Fig. 5. pH- and temperature-dependent release of encapsulated contents into the aqueous environment.
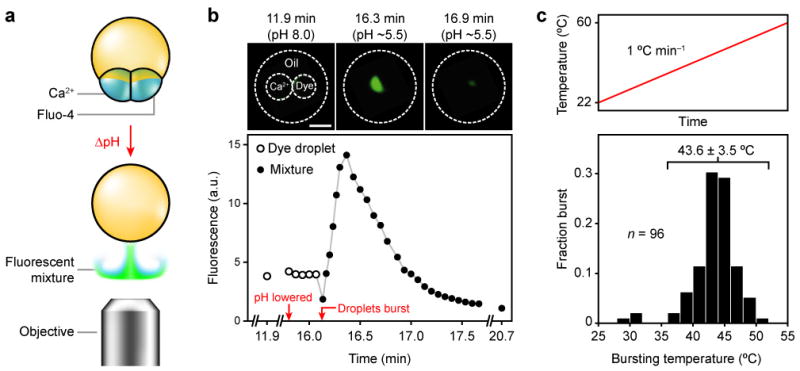
a, pH-dependent release. A multisome is made with a mixture of DOPE and OA, with one inner droplet containing Ca2+ and another dextran-conjugated fluo-4. Upon a decrease in pH, the multisome co-releases its contents into the bulk aqueous solution, where the two solutions mix to produce a signal monitored with a fluorescence microscope. b, Fluorescence photographs and measurements from the experiment depicted in a. Upon lowering the pH of the external aqueous buffer from 8.0 to ~5.5, the inner droplets burst simultaneously, producing a transient fluorescent cloud. Scale bar = 500 μm. c, Bursting temperatures of multisomes made with a mixture of DPPC and DSPC with a single inner droplet, subjected to a temperature ramp from room temperature at a rate of ~1 °C min−1 (top). Histogram of bursting temperatures (bottom). The bursting temperature was 43.6 ± 3.5 °C (mean ± s.d., n = 93), excluding the three multisomes that burst below 35 °C.
We also implemented temperature-triggered release from multisomes. Liposomes made from a lipid with a melting transition have a local maximum of permeability at around the melting temperature, attributable to the boundaries between the solid and fluid phases in the lipid bilayer (Supplementary Discussion 4)20. Multisomes made with a 3:1 (mol/mol) mixture of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and DPhPC with a single inner droplet were stable at room temperature for at least a day (n = 11). When multisomes were held at 37.2 ± 0.4 °C, 93% survived for at least 30 min (n = 46). Multisomes made with this lipid mixture were subjected to a temperature ramp of ~1 °C min−1, and the temperature at which each multisome burst was noted (Fig. 5c). Excluding the ~3% of multisomes that burst at ~30 °C, the bursting temperature was 43.6 ± 3.5 °C (mean ± s.d., n = 93). When multisomes with two inner droplets were heated in the same way, in 70% of cases the two inner droplets of each multisome burst within 0.1 °C of each other, at 42.9 ± 3.5 °C (mean ± s.d., n = 10). The two inner droplets never fused together to form a single, larger inner droplet in either the pH- or temperature-triggered experiments.
We envision several directions for the development of multisomes. For example, in basic science, the electrical recording platform developed here might be used to study pore-forming protein complexes that span two lipid bilayers, such as gap junctions21 and nuclear pores22, at the single-molecule level by positioning two multisomes with single inner droplets such that their bilayers are apposed. Further, unlike previous droplet networks in bulk oil2,6, the networks formed here function in an aqueous environment, which will enable prospective applications, including the engineering of synthetic tissue substitutes, and interfaces between tissues and electronics. Multisomes might also be developed as vehicles for combinatorial drug delivery, where the inner droplets would contain various drugs that could be released either gradually, by diffusion through pores in external bilayers, or by pH17- or temperature-triggered23 release. In this way multisomes might deliver multiple drugs with synergistic actions, or prodrugs with their activators24, in precise proportions.
The technique employed here was adequate for the initial production, manipulation and study of small numbers of structurally defined, functional multisomes. However, the practical application of multisomes is likely to require reproducible, high-throughput manufacture. For example, the production of multisomes on the sub-μm scale for drug delivery25 will likely require the development of new microfluidic techniques, and new design criteria (see Supplementary Discussion 5). Nevertheless, current microfluidic techniques should be capable of producing ~100-μm diameter structurally-defined multisomes with functional bilayer interfaces as presented here26–29 (see Supplementary Discussion 6). Multisomes of these dimensions would be useful for drug delivery in surgical30 or post-surgical treatments31 that require the gradual release of contents from local “depots”.
Methods
Lipids and oils
Lipids were purchased from Avanti Polar Lipids and dissolved in pentane (DPhPC, DOPE) or chloroform (DSPC) at 10 mg ml−1. Portions of these stock solutions were evaporated by using a nitrogen stream followed by at least 30 min under vacuum. The residues were re-solubilized in various oil mixtures. Except for the experiments on pH- and temperature-dependent release, the lipid used was DPhPC at 0.1–0.2 mg ml−1, and the oil was a 9:1 (v/v) mixture of silicone oil (Silicone Oil AR 20) and hexadecane (both from Sigma-Aldrich). The pH sensitivity experiments used a 2:1 (mol/mol) mixture of DOPE and OA (Sigma-Aldrich) at 10 mg ml−1 total concentration, in a 19:1 (v/v) mixture of silicone oil and hexadecane. The temperature sensitivity experiments used a 3:1 (mol/mol) mixture of DSPC and DPhPC at 1 mg ml−1 total concentration, in a 9:1 (v/v) mixture of silicone oil and hexadecane.
αHL pores
Wild-type αHL monomers were prepared by in vitro transcription/translation, and heptamerized by incubation with rabbit red blood cell membranes. The heptamers were then purified by SDS-PAGE32 to a final concentration of ~1 ng μl−1 33. For the electrical recording experiments, the protein concentration in the encapsulated droplets was 10 to 100 times lower.
The procedure used to prepare the αHL used in the fluorescence experiments has been described34. Briefly, a culture was grown from a single colony of the Wood 46 strain of Staphylococcus aureus. Spontaneously oligomerized heptamers of αHL were purified by cation exchange chromatography and gel electrophoresis, and stored at ~2 mg ml−1 in 20 mM sodium phosphate buffer with 150 mM NaCl and 0.3% (w/v) SDS at pH 8.0. This protein solution was added to the aqueous droplets at a 50-fold dilution.
Encapsulation
Multisomes were suspended on loops of silver wire or plastic (Supplementary Methods) in order to fix their positions while avoiding any interaction with the walls of the container that might disrupt the structure. A solution of lipids in oil was dispensed onto a loop submerged in buffer to make a suspended oil drop of volume ~0.5–2 μl. Micromachined acrylic wells were filled with the same oil solution, and aqueous droplets of volume ~0.5–70 nl were made in these wells using a 2 μl pipette with an electrophoresis gel-loading tip. The tip was filled with ~200 nl of the aqueous solution, and then immersed in the oil solution. Just enough of the solution was expelled to expose a small pendant droplet, and this droplet was separated from the pipette tip by pressing the tip against the bottom of the well.
After the incubation time, a pipette was used to transfer one or more of the aqueous droplets into the oil drop located in the bulk aqueous solution. In experiments using only DPhPC, the incubation time was ~10 min; using the DOPE/OA mixture, ~25 min; using the DSPC/DPhPC mixture, ~10 min. Multisomes with several inner droplets could be formed either by transferring an intact network of aqueous droplets joined by bilayers, or by transferring unconnected droplets, which then formed bilayers in situ.
Electrical measurements
Currents measured through multisome bilayers, and through droplet interface bilayers in bulk oil, showed occasional bursts of current leakage when pure silicone oil was used. These leaks were suppressed by mixing the oil with a small fraction of hexadecane.
Currents were measured by using Ag/AgCl electrodes with a patch-clamp amplifier (Axopatch 200B, Axon Instruments) and 16-bit digitizer (1322A, Molecular Devices). Data were acquired at 10 kHz with a 2 kHz low-pass Bessel filter, and for analysis were further filtered with a 400 Hz low-pass Bessel filter. Ag/AgCl electrodes were prepared by treating 25- or 100-μm diameter silver wire (Scientific Wire Company and Sigma Aldrich, respectively) with 25% sodium hypochlorite solution for at least 30 min. The preparation of glass-sheathed electrodes is described in the Supplementary Methods. Comparisons of conductance values to previous studies2,15 accounted for our use of the opposite convention for voltage polarity.
Fluorescence measurements
The buffer used was 25 mM Tris·HCl, 500 mM KCl, pH 8.0. The potassium salt of fluo-4 conjugated to a 10,000 MW dextran (Invitrogen) was dissolved in pure water, and added to droplets of buffer at a final dye concentration of 25 μM. Dye-containing droplets also contained 50 μM EDTA disodium salt. Ca2+-containing droplets consisted of buffer with 100 mM CaCl2.
Fluorescence microscopy was performed with a Nikon Eclipse TE2000-S inverted microscope with a Nikon CFI DL 10X objective, using a mercury arc lamp for illumination and the appropriate filter cube. Photographs were taken with a Hamamatsu C9100 EMCCD camera, with an exposure of 400 ms and gain of 179. Fluorescence intensities were measured using ImageJ software35. Fluorescence photographs were pseudo-coloured green.
pH sensitivity
The buffer used was 10 mM Tris·HCl, 10 mM succinic acid, 50 mM KCl, pH 8.0 or 3.0. Dye-containing droplets also contained 25 μM dextran-conjugated fluo-4 and 50 μM EDTA. Ca2+-containing droplets consisted of buffer with 10 mM CaCl2. Encapsulated droplets were allowed to equilibrate for ~15 min after formation, then half the volume of the external aqueous solution was replaced with an equal volume of the same pH 8.0 buffer. This was then repeated, but with an equal volume of pH 3.0 buffer.
Temperature sensitivity
A container made to hold 12 multisomes on plastic loops was placed on a heating block, and the temperature of the bulk aqueous solution was measured with a thermocouple. The bulk solution was continuously stirred to ensure temperature homogeneity. The heating block thermostat was controlled by using feedback from the temperature measurements to achieve the ramped and constant temperature regimes described in the text.
Supplementary Material
Acknowledgments
The authors thank Ellina Mikhailova for the α-hemolysin protein prepared by in vitro transcription/translation, and Quihong Li for the α-hemolysin from S. aureus. The authors also thank Mark Wallace for the loan of a microscope objective. This work was supported by grants from the National Institutes of Health and the European Commission’s Seventh Framework Programme Revolutionary Approaches and Devices for Nucleic Acid Analysis Consortium. G.V. was supported by an Engineering and Physical Sciences Research Council Life Sciences Interface Doctoral Training Centre studentship.
Footnotes
Supplementary information accompanies this paper at www.nature.com/naturenanotechnology. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/.
Author contributions
G.V., A.H. and H.B. planned the research. G.V. performed the experiments, data analysis and modelling. G.V. and H.B. wrote the paper.
Competing financial interests
The authors declare no competing financial interests.
Contributor Information
Gabriel Villar, Email: gabriel.villar@dtc.ox.ac.uk, Department of Chemistry, University of Oxford, Oxford OX1 3TA, UK.
Andrew J. Heron, Department of Chemistry, University of Oxford, Oxford OX1 3TA, UK
Hagan Bayley, Email: hagan.bayley@chem.ox.ac.uk, Department of Chemistry, University of Oxford, Oxford OX1 3TA, UK.
References
- 1.Funakoshi K, Suzuki H, Takeuchi S. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal Chem. 2006;78:8169–8174. doi: 10.1021/ac0613479. [DOI] [PubMed] [Google Scholar]
- 2.Holden MA, Needham D, Bayley H. Functional bionetworks from nanoliter water droplets. J Am Chem Soc. 2007;129:8650–8655. doi: 10.1021/ja072292a. [DOI] [PubMed] [Google Scholar]
- 3.Heron AJ, Thompson JR, Mason AE, Wallace MI. Direct detection of membrane channels from gels using water-in-oil droplet bilayers. J Am Chem Soc. 2007;129:16042–16047. doi: 10.1021/ja075715h. [DOI] [PubMed] [Google Scholar]
- 4.Syeda R, Holden MA, Hwang WL, Bayley H. Screening blockers against a potassium channel with a droplet interface bilayer array. J Am Chem Soc. 2008;130:15543–15548. doi: 10.1021/ja804968g. [DOI] [PubMed] [Google Scholar]
- 5.Heron AJ, Thompson JR, Cronin B, Bayley H, Wallace MI. Simultaneous measurement of ionic current and fluorescence from single protein pores. J Am Chem Soc. 2009;131:1652–1653. doi: 10.1021/ja808128s. [DOI] [PubMed] [Google Scholar]
- 6.Maglia G, et al. Droplet networks with incorporated protein diodes show collective properties. Nature Nanotech. 2009;4:437–440. doi: 10.1038/nnano.2009.121. [DOI] [PubMed] [Google Scholar]
- 7.Woolfson DN, Bromley EH. Synthetic biology. The Biochemist. 2011 Feb 19–25; [Google Scholar]
- 8.Channon K, Bromley EH, Woolfson DN. Synthetic biology through biomolecular design and engineering. Curr Opin Struc Biol. 2008;18:491–498. doi: 10.1016/j.sbi.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Solé RV, Munteanu A, Rodriguez-Caso C, Macía J. Synthetic protocell biology: from reproduction to computation. Philos T R Soc B. 2007;362:1727–1739. doi: 10.1098/rstb.2007.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker SA, Kennedy MT, Zasadzinski JA. Encapsulation of bilayer vesicles by self-assembly. Nature. 1997;387:61–64. doi: 10.1038/387061a0. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Turker MS, Chi EY, Sela S, Martin GM. Preparation of multivesicular liposomes. Biochim Biophys Acta. 1983;728:339–348. doi: 10.1016/0005-2736(83)90504-7. [DOI] [PubMed] [Google Scholar]
- 12.Yue BY, Jackson CM, Taylor JAG, Mingins J, Pethica BA. Phospholipid monolayers at non-polar oil/water interfaces. Part 1 – Phase transitions in distearoyl-lecithin films at the n-heptane aqueous sodium chloride interface. J Chem Soc Farad T. 1976;1(72):2685–2693. [Google Scholar]
- 13.Morisaku T, Yui H, Sawada T. Development of a new experimental system for monitoring biomembrane reactions: combination of laser spectroscopic techniques and biomembrane models formed at an oil/water interface. Anal Sci. 2004;20:1605–1608. doi: 10.2116/analsci.20.1605. [DOI] [PubMed] [Google Scholar]
- 14.Needham D, Haydon DA. Tensions and free energies of formation of “solventless” lipid bilayers – Measurement of high contact angles. Biophys J. 1983;41:251–257. doi: 10.1016/S0006-3495(83)84435-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoddart D, Heron AJ, Mikhailova E, Maglia G, Bayley H. Single-nucleotide discrimination in immobilized DNA oligonucleotides with a biological nanopore. Proc Natl Acad Sci USA. 2009;106:7702–7707. doi: 10.1073/pnas.0901054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu LQ, Bayley H. Interaction of the noncovalent molecular adapter, β-cyclodextrin, with the staphylococcal α-hemolysin pore. Biophys J. 2000;79:1967–1975. doi: 10.1016/S0006-3495(00)76445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond DC, Zignani M, Leroux JC. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39:409–460. doi: 10.1016/s0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton JA, Cistola DP. Transfer of oleic acid between albumin and phospholipid vesicles. Proc Natl Acad Sci USA. 1986;83:82–86. doi: 10.1073/pnas.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small DM, Cabral DJ, Cistola DP, Parks JS, Hamilton JA. The ionization behavior of fatty acids and bile acids in micelles and membranes. Hepatology. 1984;4:77S–79S. doi: 10.1002/hep.1840040814. [DOI] [PubMed] [Google Scholar]
- 20.Mills JK, Needham D. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. BBA-Biomembranes. 2005;1716:77–96. doi: 10.1016/j.bbamem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa S, Maeda S, Tsukihara T. Structural and functional studies of gap junction channels. Curr Opin Struc Biol. 2010;20:423–430. doi: 10.1016/j.sbi.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: bridging nuclear transport and gene regulation. Nature Rev Mol Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- 23.Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliver Rev. 2001;53:285–305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 24.Rautio J, et al. Prodrugs: design and clinical applications. Nature Rev Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 25.Devine DV, Wong K, Serrano K, Chonn A, Cullis PR. Liposome-complement interactions in rat serum: implications for liposome survival studies. Biochim Biophys Acta. 1994;1191:43–51. doi: 10.1016/0005-2736(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 26.Okushima S, Nisisako T, Torii T, Higuchi T. Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir. 2004;20:9905–9908. doi: 10.1021/la0480336. [DOI] [PubMed] [Google Scholar]
- 27.Chu LY, Utada AS, Shah RK, Kim JW, Weitz DA. Controllable monodisperse multiple emulsions. Angew Chem Int Edit. 2007;46:8970–8974. doi: 10.1002/anie.200701358. [DOI] [PubMed] [Google Scholar]
- 28.Seo M, Paquet C, Nie Z, Xu S, Kumacheva E. Microfluidic consecutive flow-focusing droplet generators. Soft Matter. 2007;3:986–992. doi: 10.1039/b700687j. [DOI] [PubMed] [Google Scholar]
- 29.Shum HC, Zhao Y, Kim SH, Weitz DA. Multicompartment polymersomes from double emulsions. Angew Chem Int Edit. 2011;50:1648–1651. doi: 10.1002/anie.201006023. [DOI] [PubMed] [Google Scholar]
- 30.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9:1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen C, et al. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J Pharm Sci. 2008;97:4622–4654. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- 32.Cheley S, et al. Spontaneous oligomerization of a staphylococcal α-hemolysin conformationally constrained by removal of residues that form the transmembrane β-barrel. Protein Eng. 1997;10:1433–1443. doi: 10.1093/protein/10.12.1433. [DOI] [PubMed] [Google Scholar]
- 33.Maglia G, Heron AJ, Stoddart D, Japrung D, Bayley H. Analysis of single nucleic acid molecules with protein nanopores. Method Enzymol. 2010;475:591–623. doi: 10.1016/S0076-6879(10)75022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maglia G, et al. DNA strands from denatured duplexes are translocated through engineered protein nanopores at alkaline pH. Nano Lett. 2009;9:3831–3836. doi: 10.1021/nl9020232. [DOI] [PubMed] [Google Scholar]
- 35.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.