Abstract
Purpose.
Oxidative stress induces retinal damage and contributes to vision loss in progressive retinopathies. Carcinine (β-alanyl-histamine) is a natural imidazole-containing peptide derivative with antioxidant activity. It is predicted to scavenge 4-hydroxynonenal (4-HNE), a toxic product of lipid oxidation. The aim of this study was to confirm the 4-HNE scavenging effect and evaluate the neuroprotective effect of carcinine in mouse retina subjected to oxidative stress.
Methods.
HPLC coupled with mass spectrometry was used to analyze carcinine and 4-HNE-carcinine adduct. Protection of retinal proteins from modification by 4-HNE was tested by incubating carcinine with retinal protein extract and 4-HNE. Modified retinal proteins were quantified by dot-blot analysis. Mice were treated with carcinine (intravitreal injection and gavage) and exposed to bright light to induce oxidative damage in the retina. Photoreceptor degeneration was measured by histology and electroretinography. Retinal levels of retinol dehydrogenase 12 (RDH12) were measured by immunoblot analysis, after exposure to bright light and in retinal explants after exposure to 4-HNE.
Results.
The ability of carcinine to form an adduct with 4-HNE, as well as to prevent and even reverse the adduction of retinal proteins by the toxic aldehyde was demonstrated in vitro. Carcinine, administered by intravitreal injection or gavage, strongly protected mouse retina against light-induced photoreceptor degeneration and had a protective effect on RHD12, a protein found specifically in photoreceptor cells.
Conclusions.
This study suggests that carcinine can be administered noninvasively to efficiently protect photoreceptor cells from oxidative damage. Carcinine could be administered daily to prevent vision loss in progressive retinopathies.
This study shows that the multifunctional antioxidant carcinine has a potent neuroprotective effect in mouse retina subjected to light-induced damage.
Introduction
Of all the tissues in the body, the retina is the most susceptible to oxidative damage because (1) retinal photoreceptor cells consume more oxygen than any other cell in the body, and this high oxygen metabolism leads to the production of damaging reactive oxygen species (ROS); (2) photoreceptor cells are exposed daily to bright light and are loaded with photosensitizers that contribute to the formation of ROS; and (3) photoreceptor cells have the largest percentage of long-chain polyunsaturated fatty acids (PUFAs) that are directly oxidized by ROS.1 Oxidation of PUFAs results in production of a complex mixture of toxic end products, including malondialdehyde and α,β-unsaturated aldehydes. Endogenous and dietary antioxidants scavenge ROS, maintaining the balance between production and inactivation of ROS. When this equilibrium is perturbed in diseases such as AMD, diabetic retinopathy (DR), or RP, oxidative damage overcomes cellular defenses and precipitates the progression of vision loss and photoreceptor cell death.2–4 Therefore, a good therapeutic strategy for these prevalent retinopathies is to scavenge the excess of ROS and/or enhance endogenous cellular defenses in order to slow down the irreversible loss of visual function.
The Age-Related Eye Disease Study (AREDS) showed that daily administration of exogenous antioxidants through diet supplementation reduces the risk of progression to advanced AMD and to severe loss of vision.5,6 A prescription of a specific AREDS formulation is now considered part of the standard care for AMD patients. However, despite the use of this formulation, many patients still develop advanced AMD.5,6 More efficient antioxidant formulations are needed to provide better neuroprotection in AMD and other degenerative retinopathies.
The imidazole-containing peptide derivatives carnosine (β-alanyl-histidine) and carcinine (β-alanyl-histamine) are found in several tissues such as muscle, liver, intestine, and brain in mammals, at high (millimolar) concentrations.7–9 These natural products have antioxidant properties, scavenging ROS in cells.10–19 They also have lipid-peroxidase activities, reducing lipid hydroperoxides produced by oxidation of membrane PUFAs.11,20 If not reduced, lipid hydroperoxides act as auto-amplificatory agents of the lipid peroxidation reaction, and their decomposition in the presence of ferrous ions leads to the formation of α,β-unsaturated aldehydes and other toxic end products.10,11,13 These reactive aldehydes are subject to Michael addition reactions with side chains of lysine, histidine, and cysteine residues, forming adduct with proteins that in most cases inactivates their functions and targets them to degradation.21 Of interest, carnosine has been shown to scavenge 4-hydroxynonenal (4-HNE), one of the most toxic and abundant end products of lipid peroxidation.12,22,23 Thus, multifunctional imidazole-containing peptide derivatives have high therapeutic potential because they can scavenge not only ROS but also the secondary toxic products that mediate and amplify the oxidative damage in cells.
Carcinine would be a better choice than carnosine for therapeutic applications because it is significantly more resistant to enzymatic hydrolysis than carnosine.24 Since carcinine contains an imidazole group, it is predicted to have a 4-HNE-scavenging activity similar to that of carnosine. In this study, we investigated the 4-HNE-scavenging activity of carcinine and its possible neuroprotective effect in mouse retina.
Methods
Materials
Goat polyclonal anti-HNE and mouse monoclonal anti-β-actin antibodies were obtained from Abcam (Cambridge, MA). Rabbit polyclonal anti-mouse RDH12 antibody was generated as previously described.25 The 4-HNE was obtained from Cayman Chemical (Ann Arbor, MI). Carcinine was a generous gift from Exsymol (Monaco, France). All other chemicals were from Sigma (St. Louis, MO) unless otherwise noted.
HPLC/Mass Spectrometry (MS) Analysis
To determine if carcinine can form an adduct with 4-HNE, 0.5 mM carcinine was incubated with 0 or 0.64 mM 4-HNE for 16 hours at room temperature. A 20-μL aliquot was dried under vacuum, resuspended in 15 μL reagent alcohol-water-triethylamine (TEA) (2:2:1), and dried again. Twenty microliters derivatization reagent alcohol-TEA-water-phenylisothiocyanate (PITC) (7:1:1:1) was added to the dried sample and sealed in vacuum vials for 20 minutes at room temperature. Reagent was removed with nitrogen for 10 minutes, and dried under vacuum. Samples were dissolved with 500 μL solvent A (0.09% formic acid, 0.01% trifluoroacetic acid (TFA), 2% acetonitrile, 97.9% water). Twenty microliters of 1:100 diluted sample was injected into HPLC/MS. To measure carcinine levels in the plasma and retina, four mouse retinas or 200 μL plasma were homogenized in 1 mL of cold 0.01 M HCl with a Polytron homogenizer (Kinematica AG, Lucerne, Switzerland). One microgram of the internal standard anserine was added to the samples. Samples were mixed with 1 mL acetonitrile and centrifuged at 1500g for 5 minutes to remove any solid debris. Supernatants were dried under vacuum. Samples were dissolved in 100 μL coupling solution (acetonitrile-pyridine-TEA-water; 10:5:2:3) and dried again. Samples were redissolved in 100 μL coupling solution, and 5 μL PITC was added for derivatization. After 5 minutes of incubation at room temperature, samples were evaporated and dissolved in 200 μL of 10% acetonitrile. Samples were filtered in microcentrifuge filter tubes (Corning Inc., Corning, NY) and diluted 2-fold with solvent A. Samples were analyzed on HPLC/MS (Paradigm MSRB capillary HPLC; Michrom Bioresources, Auburn, CA; HCT Ultra Ion trap MS; Bruker Daltonics, Billerica, MA). HPLC was run on column Magic MS C18, 5m, 200 A, 0.5 × 150 mm with solvent A and solvent B (0.09% formic acid, 0.0085% TFA, 95% acetonitrile, 4.9% water). A gradient of 2% to 18% of solvent B in 10 minutes, 18% to 60% in 6 minutes, and 60% for 2 minutes was used. The flow rate was 20 mL/min, and the detection wavelength was 215 nm. The ion trap MS was equipped with an electrospray ion source, where the spray voltage was set at 4 kV in the positive mode. The heater temperature was maintained at 300°C. The dry gas pressure was 10 L/min, and the nebulizer gas pressure was 30 psi. Results were analyzed with an Esquire data analysis system (Esquire Innovation, Inc., Temecula, CA).
Effect of Carcinine on Retinal Protein Modification by 4-HNE In Vitro
Mouse retinas were homogenized in Tissue Protein Extraction Reagent (T-PER) reagent from Pierce (Rockford, IL), and proteins were extracted as described25 according to the manufacturer's instructions. Protein concentration was measured using Coomassie reagent (Pierce). To measure the kinetics of 4-HNE modification, 2.5 μg retinal protein was diluted in 10 μL of PBS and incubated with 0.64-mM 4-HNE at room temperature for indicated times. To measure the inhibition of this reaction by carcinine, increasing amounts of carcinine (from 1 μg to 2 mg) were added to the 10 μL reaction before addition of 4-HNE. To measure the reversion of this reaction, retinal protein and 4-HNE were incubated overnight to allow complete modification of retinal proteins by 4-HNE. Then, 1-mg carcinine was added to the 10-μL reaction and incubated for indicated times. At the end of each reaction, 4-HNE-protein adducts were quantified by dot-blot analysis.
Dot-Blot Quantification of 4-HNE Protein Adduct
Dot-blot quantification was carried out as described.26 Each reaction containing 2.5 μg retinal protein was applied to a 96-well dot-blot apparatus (Bio-Rad, Hercules, CA) and then transferred to a nitrocellulose membrane (Bio-Rad). Sample loading was monitored by staining the membrane with Ponceau red. Membranes were blotted with anti-HNE antibody coupled with horseradish peroxidase (HRP; Abcam). Signals were quantified using SuperSignal Chemiluminescent Substrate (Pierce), and the digital Carestream Image Station 4000R (Kodak, Rochester, NY). Care was taken to ensure that the intensities of detected signals were within the linear range of the camera and that no pixels were saturated.
Treatment of Mice with Carcinine and Exposure to Bright Light
Seven-week-old BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were housed in a 12-hour dim light (10 lux)–dark cycle for 1 week before treatment. Carcinine was administered to 8-week-old mice by intravitreal injection or by gavage. Mice were injected intravitreally using a 36-gauge needle (World Precision Instruments, Sarasota, FL) through the temporal limbus of the eye. One eye received 1 μL PBS, and the other eye received 1 μL of 2 M carcinine diluted in PBS. Mice were returned to dim cyclic light for 48 hours for recovery before light damage was initiated. Light damage was then induced in mice by 5 hours exposure to 4000 lux of diffuse, cool, white fluorescent light, as previously described.25 Another group of 8-week-old mice was gavaged once a day for 5 days with 0, 0.2, 2, or 20 mg carcinine dissolved in 100 μL water. To measure carcinine levels in the plasma and retina, one group of mice was euthanized 4 hours after the last daily gavage on the fifth day of treatment. Immediately after CO2 inhalation, blood was collected from the left ventricle, centrifuged at low speed, and the plasma was frozen in liquid nitrogen for subsequent analysis. To avoid any blood contamination of retinal samples, mice were perfused with saline before dissection of the retinas. Retinas were subsequently dissected and frozen in liquid nitrogen. Another group of mice was exposed to 3000 lux of bright white fluorescent light for 4 hours, immediately after the daily gavage on the fifth day of treatment.25 Conditions of light exposure were different for mice that received intravitreal injection compared with those that received carcinine through gavage because we empirically determined that a stronger light exposure was needed after intravitreal injection to induce retinal damage. Mice were either euthanized immediately after light exposure for preparation of retinal protein or returned to dim cyclic light for 7 days to allow the retina to clear all dead cells and restore a well-organized morphology before ERG testing and histology. During this recovery period, carcinine gavage was continued. All mice were euthanized by CO2 inhalation, a method approved by the Panel of Euthanasia of the American Veterinary Medical Association. All procedures were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Measurement of Photoreceptor Survival
Whole eyes were enucleated after orientation of the superior half with a permanent dye. Eyes were embedded in paraffin, and sections were cut along the vertical meridian, through the optic nerve head (ONH). Paraffin sections stained with hematoxylin and eosin were prepared from each eye, and the number of rows of photoreceptor nuclei within the outer nuclear layer (ONL) were counted at a distance of 0.5 mm from the ONH to the inferior and superior retina as an assessment of photoreceptor survival.27
Electroretinography
To assess photoreceptor function, mice were dark adapted overnight, anesthetized by intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg), and after administration of one drop of local anesthetic to the cornea (0.5% proparacaine), the pupils were dilated with tropicamide (1%) and phenylephrine (2.5%). ERGs were performed as previously described.28 Briefly, mice were placed on a 37°C heating pad under a Ganzfield dome stimulator (LKC Technologies, Gaithersburg, MD). Solid gold electrodes were placed on the cornea to measure responses to light stimuli. A thin coating of hypromellose solution was used to keep the eyes moist. Flash intensities ranging from 0.001 to 1000 cd.s/m2 were administered and responses were recorded using the Espion E2 ERG System (Diagnosys, LLC, Lowell, MA). Amplitudes of the a-wave were determined as the minimum voltage recorded between 0 and 25 milliseconds after the flash. The b-waves were calculated as the difference between the a-wave and the maximum amplitude recorded around 110 milliseconds after the flash. Values from both eyes were averaged for each mouse. Cone b-waves were recorded after 5 minutes of light adaptation by averaging the responses to five 3700 cd.s/m2 flashes.
Retinal Explants
Retinas were dissected from 6- to 8-week-old pigmented wild-type mice. Retinas were individually incubated in 200 μL Dulbecco's modified Eagle's medium (DMEM) containing 0 or 5 mM carcinine and 0 or 150 μM 4-HNE, at 37°C under 5% CO2 for indicated times. Retinas (four per group) were removed from incubation, immediately washed in PBS, and frozen in liquid nitrogen for subsequent protein preparation.
Protein Preparation and Immunoblot
Frozen retinas were homogenized in T-PER buffer (Pierce), according to the manufacturer's instructions. Protein concentrations were measured, and immunoblot analysis using anti-RDH12 and anti-β-actin antibodies was performed using 30 μg protein. Signals were detected using SuperSignal Chemiluminescent Substrate and quantified using Kodak Image Station 4000R and Kodak Molecular Imaging Software as previously described.25 The levels of RDH12 were normalized to the levels of β-actin (used as loading control) and then expressed relative to those of RDH12 in control retinas, arbitrarily defined as 100%.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA). The quantitative data are expressed as averages and SEM for each group. One or 2-way ANOVAs were performed to assess differences between the means. From the ANOVAs, Bonferroni's Multiple Comparison post hoc test was performed with a 95% confidence interval to determine statistical significance. Three to five independent samples were used per analysis as indicated.
Results
Carcinine Forms an Adduct with 4-HNE In Vitro
To confirm that carcinine can form an adduct with 4-HNE, they were combined and incubated overnight in water. The product of the reaction was analyzed by HPLC/MS (Fig. 1). Under these HPLC conditions, the carcinine-only control had a retention time of 10 minutes, and a mass of 318.1 m/z (Fig. 1A). After incubation with 4-HNE, the retention time of the newly formed molecule was 17.5 minutes, and the mass was increased to the predicted 474.2 m/z, corresponding to the adduct formed between carcinine and 4-HNE (Fig. 1B). This result demonstrated that carcinine reacted with 4-HNE to form an adduct detectable by HPLC/MS.
Figure 1. .
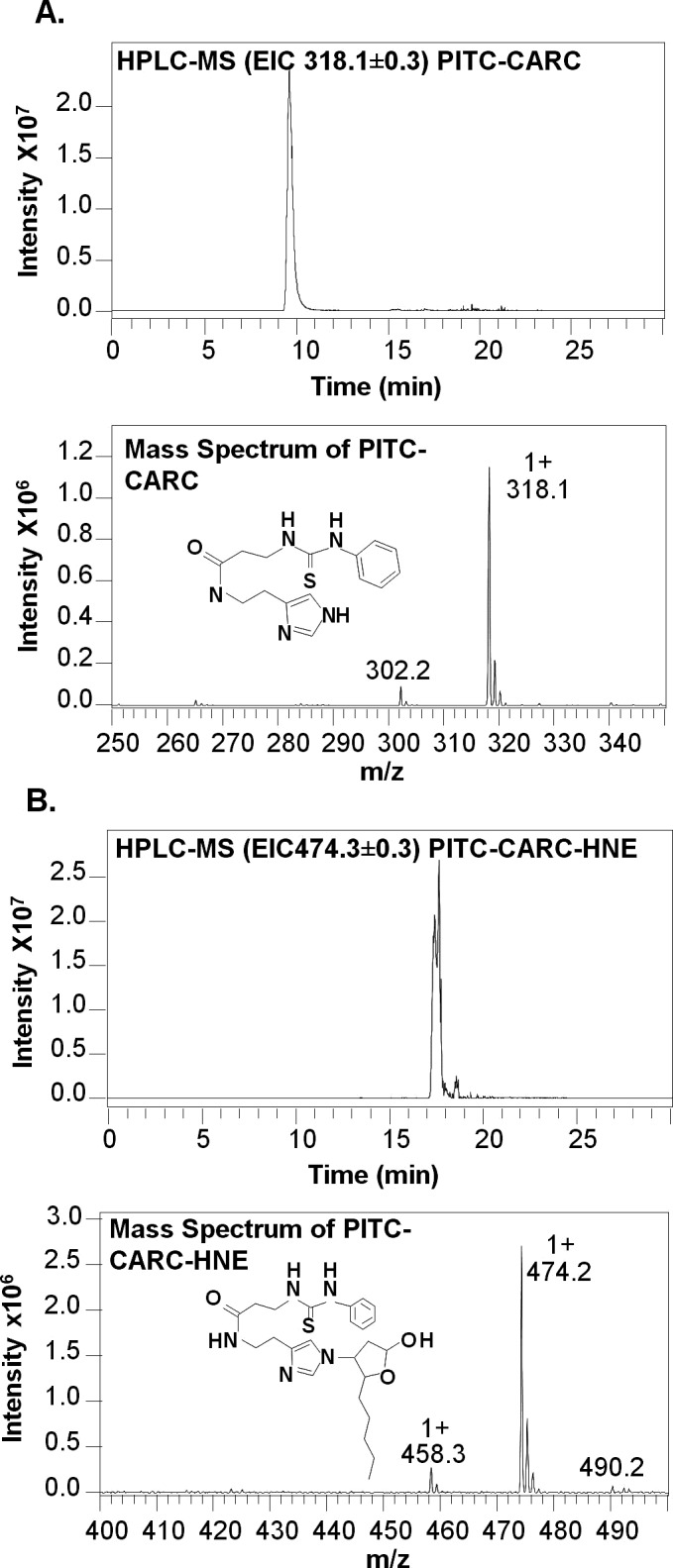
Carcinine forms an adduct with 4-HNE in vitro. Carcinine (0.5 mM) was incubated with 0 or 0.64 mM 4-HNE for 16 hours at room temperature, and samples were analyzed by HPLC/MS. (A) Representative chromatogram and corresponding mass spectrum obtained from carcinine incubated with 0 mM 4-HNE. (B) Representative chromatogram and corresponding mass spectrum obtained from carcinine incubated with 0.64 mM 4-HNE. Carcinine gives a peak at 318.1 m/z and 4-HNE-carcinine adduct at 474.2 m/z. This experiment was repeated three times with similar results.
Carcinine's Effects on 4-HNE–Modified Retinal Protein
Anti-HNE antibody specifically binds to 4-HNE-modified proteins but not to the 4-HNE-carcinine adduct (Fig. 2A). Therefore, this antibody was used for subsequent experiments for quantification of the 4-HNE-protein adduct. Note that carcinine is not detectable by Ponceau staining even though it is present on the membrane (Fig. 2A, lower panel). To determine the kinetics of 4-HNE-adduct formation, retinal proteins were incubated with a molar excess of 4-HNE in PBS at room temperature for indicated times. Adduct formation increased over time and reached its maximum level at 60 minutes of incubation and remained stable until 2 hours of incubation (Fig. 2B). In the next experiment, retinal proteins and increasing amounts of carcinine were incubated with 4-HNE for 90 minutes at room temperature. Carcinine inhibited the formation of adduct between 4-HNE and retinal proteins in a dose-dependent manner (Fig. 2C). Under these conditions, the inhibition of adduct formation reached 50% (IC50) with a carcinine concentration of 33.2 ± 0.6 μg/μL. Complete inhibition was achieved with a carcinine concentration of 0.1 mg/μL (1 mg per reaction).
Figure 2. .
Carcinine prevents 4-HNE modification of retinal proteins. (A) Carcinine (Car, 2.5 μg) or retinal proteins (Prot, 2.5 μg) were incubated with 0 (−) or 0.64 mM (+) 4-HNE for 16 hours at room temperature before dot-blot analysis. Protein loading is verified by staining the membrane with Ponceau red (lower blot). Signal is detected with anti-HNE antibody coupled with HRP (upper blot). Only retinal proteins modified by 4-HNE were detected by this method. (B) Representative dot-blot showing time-course of adduct formation between 4-HNE and retinal proteins. Ponceau staining (lower blot) and 4-HNE-protein adduct detected using anti-HNE antibody (upper blot) are shown. This experiment was repeated three times with similar results. (C) Representative dot-blot showing dose-response inhibition of adduct formation between 4-HNE and retinal proteins by carcinine. Results from three experiments, each done in triplicate, were quantified and plotted as percentage of adduct formed after normalizing to protein amount. Error bars correspond to SEM.
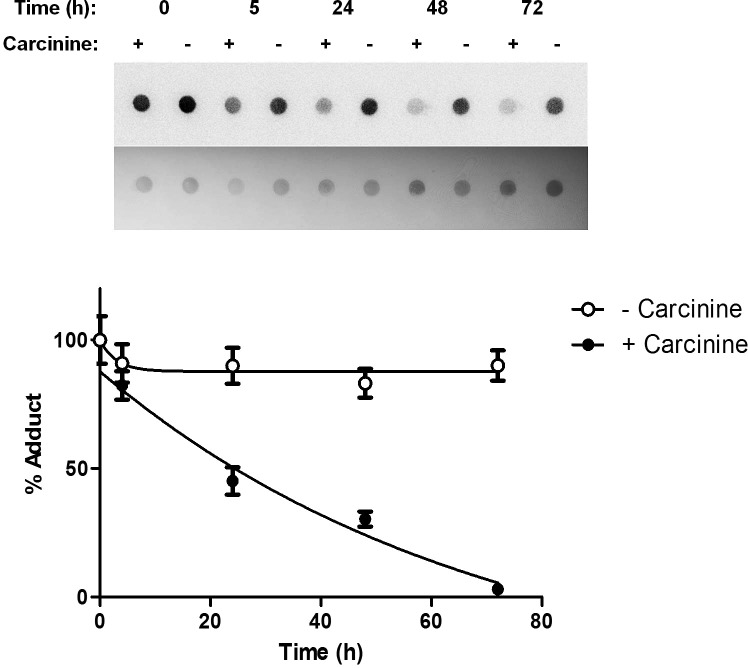
To determine if carcinine could reverse 4-HNE modification of retinal proteins after adduct formation had taken place, preformed adducts were incubated with 0 or 1 mg carcinine for increasing times (0 to 72 hours). Carcinine reversed 4-HNE modification of retinal proteins in a time-dependent manner (Fig. 3). This was a slow process that reached complete adduct reversion at 72 hours (Fig. 3). Taken together, these results demonstrated that carcinine could prevent and also reverse 4-HNE-adduction to retinal proteins.
Figure 3. .
Carcinine reverses 4-HNE modification of retinal proteins. Preformed 4-HNE-protein adducts were incubated for indicated times with 0 (−) or 1 mg (+) carcinine before dot-blot analysis. A representative dot-blot showing time-course of reversion is shown. Signals of the upper blot were quantified, normalized to protein amount (lower blot), and plotted as percentage of adducts remaining. Results from three experiments, each done in triplicate, were quantified and plotted. Error bars correspond to SEM.
Neuroprotective Effect of Intravitreal Injection of Carcinine on Light-Induced Retinal Degeneration In Vivo
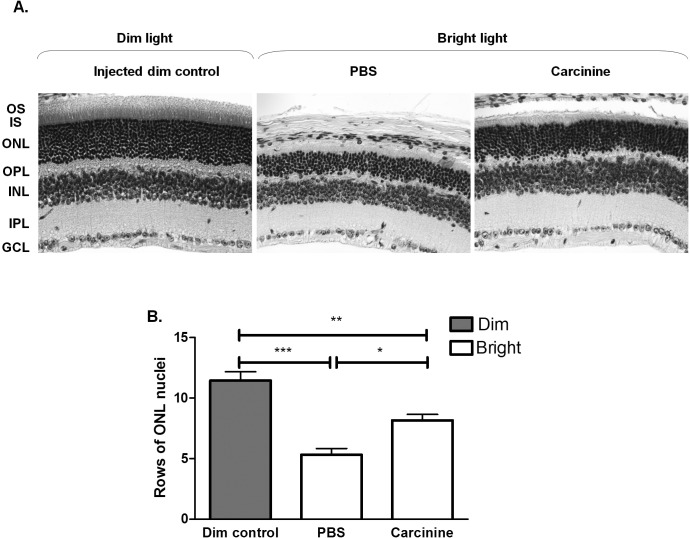
To determine if carcinine could provide neuroprotection to the photoreceptor cells in vivo, we used BALB/c mice exposed to bright light to stimulate oxidative damage in the retina. Mice received 1 μL intravitreal injection of 2 M carcinine in one eye and PBS only in the other eye. After 48 hours of recovery under dim cyclic light, mice were exposed to bright light (4000 lux for 5 hours). Representative retinal sections are shown in Figure 4A. A 53.5% loss of photoreceptor cell nuclei was induced by bright light in the central retina of the PBS-injected eyes (Fig. 4B). In the carcinine-injected eyes, only 28.7% loss of photoreceptor cell nuclei was induced by exposure to bright light, a result significantly different from the PBS-injected eyes. This result showed that carcinine protected photoreceptor cells against light-induced damage in vivo.
Figure 4. .
Intravitreal injection of carcinine protects photoreceptor cells from light-induced damage in mouse retina. (A) Representative pictures of retinal sections were taken at 0.5 mm from the optic nerve head (ONH) to the inferior retina at original magnification ×20. OS, photoreceptor outer segment; IS, photoreceptor inner segment; ONL, outer nuclear layer containing photoreceptor nuclei; OPL, outer plexiform layer, INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. (B) The numbers of nuclei in the ONL of PBS- and carcinine-injected eyes were counted on histologic sections from five different mice at 0.5 mm from the ONH to the inferior and superior retina. Averages and SEM were plotted for each group. One-way ANOVA was used to determine the differences between groups, and Bonferroni's post hoc test was performed to test for significance. *P < 0.05; **P < 0.01; ***P < 0.005.
Carcinine in Plasma and Retina after Oral Administration
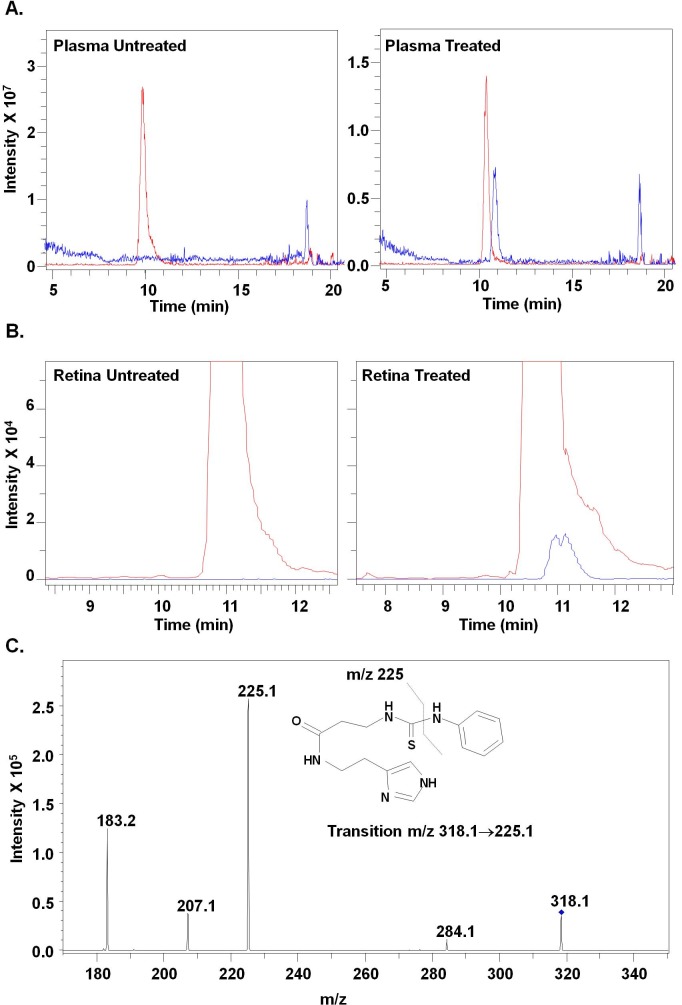
To determine if carcinine administered systemically could reach the retina and protect photoreceptors from oxidative damage, we treated BALB/c mice by gavage with increasing amounts of carcinine (0, 0.2, 2, and 20 mg per mouse per day for 5 days) and measured plasma and retinal levels of carcinine. Carcinine was found in the plasma of a mouse treated with 20 mg carcinine (1.1 ng carcinine per microliter of plasma; Fig. 5A), demonstrating that carcinine can be transported from the intestine to the blood in its intact form, without hydrolysis. Carcinine was not detected in the retina of untreated mice or in retinas from mice treated with 0.2 or 2 mg carcinine. Retinal carcinine content reached an average of 5 ± 1.1 ng per retina when mice were given 20 mg carcinine (Fig. 5B). Individual carcinine levels in the three analyzed samples were carcinine concentrations of 3.75, 5.72, and 5.52 ng per retina, respectively. The identity of the small peak shown in Figure 5B (right panel) was confirmed by the presence of the qualifier fragment ion at m/z 225.1, which is specifically derived from the fragmentation of carcinine (Fig. 5C). Presence of carcinine in the retina, only after treatment, demonstrated that it can cross the blood-retinal barrier and reach the retina after oral administration.
Figure 5. .
Carcinine in plasma and retina after oral administration. (A) HPLC/MS chromatogram and mass spectrum of plasma from mice gavaged with water alone (left panel) and carcinine (right panel). (B) HPLC/MS chromatogram and mass spectrum of retinas from mice gavaged with water alone (left panel) and carcinine (right panel). (C) Selected reaction monitoring (SRM) technique was applied on retinal extract from treated mice. Presence of the qualifier fragment ion at m/z 225.1, confirming the identity of carcinine, is shown. This analysis was done on three retinal samples from different mice.
Neuroprotective Effect of Oral Administration of Carcinine on Light-Induced Retinal Degeneration
To evaluate the neuroprotective effect of carcinine, BALB/c mice were treated by gavage with 20 mg carcinine per mouse per day for 5 days and then exposed to bright light (3000 lux) for 4 hours on the fifth day of treatment. The mice were then returned to dim cyclic light for 7 days of recovery, with continued carcinine treatment. Mice were euthanized after ERG recordings, and whole eyes were removed for histologic analysis.
To assess retinal function, we performed ERG in these mice. Rod a- and b-waves were plotted as a function of the log flash intensity (Fig. 6A). Water-gavaged mice lost almost all rod-mediated a- and b-wave responses after exposure to bright light (Fig. 6A). In this group, the maximum a-wave was 84 μV, and the maximum b-wave was 183 μV. Carcinine treatment significantly rescued these ERG responses. The maximum a-wave in the carcinine-gavaged group was 257 μV, and the maximum b-wave was 606 μV. These rescued ERG responses were not significantly different from those of dim-control mice for both a- and b-waves. Under these conditions of light exposure, the cone responses were not significantly decreased in water- or carcinine-gavaged mice (Fig. 6B).
Figure 6. .
Oral administration of carcinine prevents the loss of retinal function induced by exposure to bright light. (A) Dark-adapted (scotopic) rod a- and b-wave responses to increasing flash intensities were recorded on five mice per group. Averages and SEM are plotted. Results were compared by 2-way ANOVA. Bonferroni's post hoc test was performed to test for significance. **P < 0.01; ***P < 0.005. (B) Light-adapted (photopic) cone b-wave responses to 3700 cd.s/m2 were recorded on five mice per group. Averages and SEM are plotted. Results were compared by 1-way ANOVA and Bonferroni's post hoc test. There was no statistical difference between the groups.
Representative retinal sections are shown in Figure 6A. In water-gavaged mice, 78.1% of photoreceptor cell nuclei were lost after exposure to bright light (Fig. 7B). By contrast, only 17.3% of photoreceptor cell nuclei were lost after exposure to bright light in mice gavaged with carcinine. These results were consistent with the ERG results shown in Figure 6 and demonstrated that the rod photoreceptor cells rescued by carcinine treatment were functional. This demonstrated that carcinine provided significant neuroprotection to photoreceptor cells.
Figure 7. .
Oral administration of carcinine protects photoreceptor cells from light-induced damage in mouse retina. (A) Representative pictures of retinal sections were taken at 0.5 mm from the ONH to the inferior retina at original magnification ×20. (B) The numbers of nuclei in the ONL of water- and carcinine-gavaged mice were counted on histologic eye sections from five different mice at 0.5 mm from ONH in the inferior and superior retina. Averages and SEM were plotted for each group. One-way ANOVA was used to determine the differences between groups, and Bonferroni's post hoc test was performed to test for significance. ***P < 0.005.
To explore the mechanism of neuroprotection by carcinine, we quantified the expression level of the retinol dehydrogenase 12 (RDH12) protein, an enzyme involved in the detoxification of 4-HNE and all-trans retinal in photoreceptor cells.26,28,29 For this experiment, a different group of gavaged mice was euthanized immediately after 4 hours of exposure to bright light (3000 lux). Light exposure significantly decreased RDH12 protein level by 46.7% in water-gavaged mouse retina (Fig. 8B). This effect of bright light was completely blocked by gavage with 20 mg carcinine per mouse per day (Fig. 8B). We previously found a similar effect of carcinine on RDH12 after intravitreal injection.30 These results suggested that carcinine prevented the light-induced decrease of RDH12.
Figure 8. .
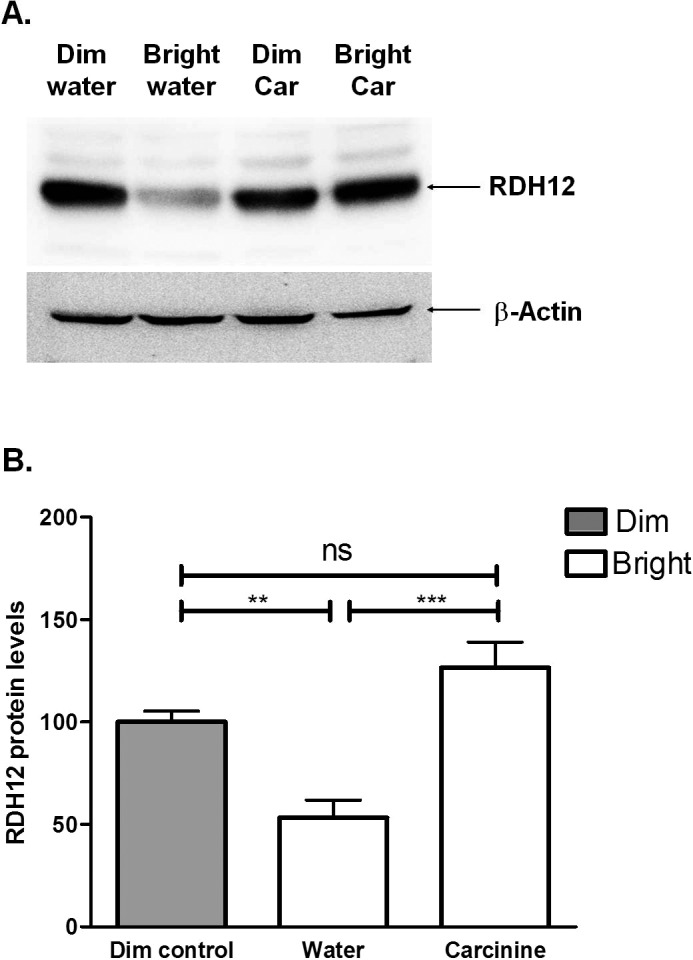
Oral administration of carcinine inhibits light-induced decrease of RDH12 protein in mouse retina. Mice were treated as described in Figure 6 and killed immediately after light exposure. (A) Representative immunoblot analysis of retinal protein with anti-RDH12 antibody (upper blot) and anti-actin antibody (lower blot). (B) RDH12 levels were normalized to β-actin and expressed relative to control retinas (dim controls), arbitrarily defined as 100. Averages and SEM from three mice per group are plotted. One-way ANOVA was used to determine the differences between groups, and Bonferroni's post hoc test was performed to test for significance. **P < 0.01; ***P < 0.005.
4-HNE Decreases RDH12 Protein Level in Retinal Explants, an Effect Reversed by Carcinine
To test if RDH12 could be modified by 4-HNE and targeted for degradation, mouse retinal explants were incubated with exogenous 4-HNE. We have previously determined that RDH12 is rapidly modified by 4-HNE after addition of exogenous 4-HNE to retinal explants.30 RDH12 protein levels were significantly decreased by 36% at 4 hours, and by 32.6% at 8 hours of 4-HNE (150 μM) exposure (Fig. 9). In the presence of carcinine, RDH12 protein levels were rescued to that of control retinas (incubated without 4-HNE) at 4 hours, and to a 71% higher level than in control retinas at 8 hours. These results support the hypothesis that 4-HNE modification of RDH12 can mediate the degradation of this protein in photoreceptor cells. Carcinine not only inhibited but also reversed the effect of 4-HNE.
Figure 9. .
The 4-HNE mediates a decrease of RDH12 protein level in retinal explants. Retinal explants were incubated for indicated times in 0 or 5 mM carcinine, with (150 μM) or without 4-HNE. Levels of RDH12 were normalized to β-actin and expressed relative to control retinas (incubated without 4-HNE), arbitrarily defined as 100. Averages and SEM from four retinas per group are plotted. One-way ANOVA was used to determine the differences between groups, and Bonferroni's post hoc test was performed to test for significance. **P < 0.01; ***P < 0.005.
Discussion
The adduction chemistry of carnosine to 4-HNE was previously determined and appears to involve both the amino group of the β-alanyl residue and the imidazole ring of the histidine residue, which act synergistically in trapping 4-HNE.12 Carcinine has a β-alanyl residue and a histamine that contains an imidazole ring. Therefore, the only difference between the two molecules is the carboxyl group of the histidine residue present in carnosine but not in carcinine. However, this carboxyl group is not involved in the 4-HNE adduction to carnosine,12 suggesting that carcinine should be able to form an adduct with 4-HNE. In the present study, this hypothesis is supported by the detection of this adduct by HPLC.
In cells, modification of proteins by 4-HNE typically inactivates their function, which is followed by their rapid degradation.21 Such degradation keeps the amount of the altered proteins at low levels during conditions of mild oxidative stress. However, during conditions of increased oxidative stress, 4-HNE attack extends to modification and inactivation of the proteasome and autophagy machineries, which leads to an accumulation of modified proteins.21,31 We found that carcinine prevented and even reversed adduct formation between retinal proteins and 4-HNE, in vitro. This is exciting because of the potential use of carcinine as a therapeutic to prevent and mainly to reverse oxidative damage. The amounts of 4-HNE-protein adduct were quantified by dot-blot analysis in whole retinas of water- and carcinine-gavaged mice kept under dim cyclic light or exposed to bright light. We found that carcinine gavage decreased the level of adduct by 17.6% ± 3.8% in dim-exposed retinas and by 10.2% ± 10.1% in bright light-exposed retinas (not shown). The effect of carcinine on decreasing the level of 4-HNE adduct was not significant, at least when measured in whole retinal extracts. A possible explanation is that a local scavenging of 4-HNE within photoreceptor cells could be masked by 4-HNE-protein adducts generated in other cells of the retina. Similar to 4-HNE, 4-hydroxyhexenal (4-HHE) is another important lipid peroxidation product generated in the retina that greatly contributes to the protein modification occurring in the retina. It would be interesting to evaluate the effect of carcinine on 4-HHE.
After oral administration, intact carcinine can enter the bloodstream and cross the blood-retinal barrier to reach the retina. This indicates that carcinine can be administered noninvasively, which is important when considering a daily, long-term treatment for progressive retinopathies. However, the amount of carcinine found within the retina is limited, suggesting a poor transportation through the blood-retinal barrier, a rapid degradation of carcinine within the retina, and/or a rapid reaction with other molecules. Although the amount of carcinine within the retina was low, it protected retinal structure and function from light-induced retinal degeneration. In this model, exposure to bright light induces oxidative modification of retinal proteins and apoptosis of photoreceptor cells.25,32 This effect can be blocked by antioxidants.33–35 We found that carcinine provided robust protection from light-induced retinal degeneration after gavage. This protection was also observed when we delivered carcinine directly into the eyeball by intravitreal injection.
The molecular mechanisms of neuroprotection mediated by carcinine were not clearly established. We hypothesized that by scavenging the light-induced ROS and 4-HNE produced within the photoreceptor cells, carcinine could protect intracellular proteins from modification and degradation. We previously showed that the level of photoreceptor-specific RDH12 decreases rapidly during exposure to bright light.25 This decrease occurs before the photoreceptor cells enter apoptosis and thus is not the result of a loss of photoreceptor cells.25 In addition, there is no decrease of Rdh12 mRNA, suggesting that the protein decline is due to its degradation rather than to a downregulation of gene expression.25 Therefore, oxidative modification of RDH12 such as 4-HNE-adduct formation could take place during exposure to bright light, targeting the modified protein for degradation.25 This hypothesis is supported by the finding that 4-HNE mediated a decrease of the RDH12 protein level in retinal explants, mimicking the effect of light exposure.
We found that carcinine gavage completely inhibited the decrease of RDH12 protein induced by exposure to bright light in mouse retina. It is possible that carcinine enters photoreceptor cells and exerts a stabilizing effect on RDH12 by scavenging 4-HNE, thus protecting RDH12 from modification and degradation. In support of this possibility, the decrease of RDH12 induced by 4-HNE was fully inhibited by carcinine in retinal explants. Alternatively, carcinine could bind to a specific receptor on the photoreceptor cells and exert its biological effect through intracellular neuroprotective signaling pathway(s). Carcinine is a natural compound that is well tolerated; even at the high doses administered in our study (up to 1g carcinine per kilogram of body weight), there were no adverse effects observed. However, we are currently investigating other routes of administration providing better bioavailability of carcinine in the retina. Because it is a multifunctional compound, attacking oxidative damage from different angles by combining antioxidant, lipid peroxidase, 4-HNE-scavenging activities, and reversion of 4-HNE-adduction, carcinine has the potential to be more powerful and more effective than classic antioxidants for progressive retinopathies by reversing the damage that has already occurred as well as preventing further damage. However, a side-by-side comparison with classic antioxidants will have to be performed in future studies. Another crucial step towards the utilization of carcinine in patients with progressive retinopathies will be to better understand, at the molecular level, the mechanism(s) of neuroprotection mediated by carcinine.
Acknowledgments
The authors thank Robert E. Anderson and John D. Ash for their helpful discussions. The authors also thank the personnel at the Animal, Imaging, and Molecular Modules of the Vision Research Core Facility at the University of Oklahoma Health Sciences Center.
Footnotes
Supported by the National Center for Research Resources Grant P20RR017703, the National Eye Institute of the National Institutes of Health Grants R21EY018907 and P30EY012190, the Oklahoma Center for the Advancement of Science and Technology, and a Research to Prevent Blindness, Inc. unrestricted grant to the University of Oklahoma Health Sciences Center, Department of Ophthalmology.
Disclosure: L.D. Marchette, None; H. Wang, None; F. Li, None; M.A. Babizhayev, P; A. Kasus-Jacobi, None
References
- 1. Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131 [DOI] [PubMed] [Google Scholar]
- 2. Zarbin MA, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina. 2010;30:1350–1367 [DOI] [PubMed] [Google Scholar]
- 3. Jarrett SG, Lewin AS, Boulton ME. The importance of mitochondria in age-related and inherited eye disorders. Ophthalm Res. 2010;44:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815 [DOI] [PubMed] [Google Scholar]
- 5. Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS Report No. 9 Arch Ophthalmol. 2001;119:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krishnadev N, Meleth AD, Chew EY. Nutritional supplements for age-related macular degeneration. Curr Opin Ophthalmol. 2010;21:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flancbaum L, Brotman DN, Fitzpatrick JC, Van Es T, Kasziba E, Fisher H. Existence of carcinine, a histamine-related compound, in mammalian tissues. Life Sci. 1990;47:1587–1593 [DOI] [PubMed] [Google Scholar]
- 8. Boldyrev AA, Koldobski A, Kurella E, Maltseva V, Stvolinski S. Natural histidine-containing dipeptide carnosine as a potent hydrophilic antioxidant with membrane stabilizing function: a biomedical aspect. Mol Chem Neuropathol. 1993;19:185–192 [DOI] [PubMed] [Google Scholar]
- 9. Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A. 1988;85:3175–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Babizhayev MA, Lozovskaya EL, Makareyeva EN, Lul'kin YA. Sapezhinskii II. Photoprotector and antioxidant properties of histamine-containing peptidomimetics in the photooxidation of glycyltryptophan. Biochemistry (Mosc). 1998;63:523–528 [PubMed] [Google Scholar]
- 11. Babizhayev MA, Seguin MC, Gueyne J, Evstigneeva RP, Ageyeva EA, Zheltukhina GA. L-carnosine (beta-alanyl-L-histidine) and carcinine (beta-alanylhistamine) act as natural antioxidants with hydroxyl-radical-scavenging and lipid-peroxidase activities. Biochem J. 1994;304(pt 2);509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aldini G, Carini M, Beretta G, Bradamante S, Facino RM. Carnosine is a quencher of 4-hydroxy-nonenal: through what mechanism of reaction? Biochem Biophys Res Commun. 2002;298:699–706 [DOI] [PubMed] [Google Scholar]
- 13. Babizhayev MA. Biological activities of the natural imidazole-containing peptidomimetics n-acetylcarnosine, carcinine and L-carnosine in ophthalmic and skin care products. Life Sci. 2006;78:2343–2357 [DOI] [PubMed] [Google Scholar]
- 14. Babizhayev MA. Antioxidant activity of L-carnosine, a natural histidine-containing dipeptide in crystalline lens. Biochim Biophys Acta. 1989;1004:363–371 [DOI] [PubMed] [Google Scholar]
- 15. Bellia F, Amorini AM, La Mendola D, et al. New glycosidic derivatives of histidine-containing dipeptides with antioxidant properties and resistant to carnosinase activity. Eur J Med Chem. 2008;43:373–380 [DOI] [PubMed] [Google Scholar]
- 16. Boldyrev AA. Does carnosine possess direct antioxidant activity? Int J Biochem. 1993;25:1101–1107 [DOI] [PubMed] [Google Scholar]
- 17. Decker EA, Livisay SA, Zhou S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry (Mosc). 2000;65:766–770 [PubMed] [Google Scholar]
- 18. Mozdzan M, Szemraj J, Rysz J, Nowak D. Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions. Basic Clin Pharmacol Toxicol. 2005;96:352–360 [DOI] [PubMed] [Google Scholar]
- 19. Reddy VP, Garrett MR, Perry G, Smith MA. Carnosine: a versatile antioxidant and antiglycating agent. Sci Aging Knowledge Environ. 2005;:pe12 [DOI] [PubMed]
- 20. Evstigneeva RP, Zheltukhina GA, Ageeva EA, Babizhaev MA. Lipoperoxidase activity of carnosine and carcinine [in Russian]. Dokl Akad Nauk. 1993;333:104–106 [PubMed] [Google Scholar]
- 21. Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orioli M, Aldini G, Benfatto MC, Facino RM, Carini MHNE. Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC-MS/MS profiling in Zucker obese rats. Anal Chem. 2007;79:9174–9184 [DOI] [PubMed] [Google Scholar]
- 23. Aldini G, Granata P, Carini M. Detoxification of cytotoxic alpha,beta-unsaturated aldehydes by carnosine: characterization of conjugated adducts by electrospray ionization tandem mass spectrometry and detection by liquid chromatography/mass spectrometry in rat skeletal muscle. J Mass Spectrom. 2002;37:1219–1228 [DOI] [PubMed] [Google Scholar]
- 24. Pegova A, Abe H, Boldyrev A. Hydrolysis of carnosine and related compounds by mammalian carnosinases. Comp Biochem Physiol B Biochem Mol Biol. 2000;127:443–446 [DOI] [PubMed] [Google Scholar]
- 25. Kanan Y, Wicker LD, Al-Ubaidi MR, Mandal NA, Kasus-Jacobi A. Retinol dehydrogenases RDH11 and RDH12 in the mouse retina: expression levels during development and regulation by oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchette LD, Thompson DA, Kravtsova M, Ngansop TN, Mandal NA, Kasus-Jacobi A. Retinol dehydrogenase 12 detoxifies 4-hydroxynonenal in photoreceptor cells. Free Radic Biol Med. 2010;48:16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nachman-Clewner M, Giblin FJ, Dorey CK, et al. Selective degeneration of central photoreceptors after hyperbaric oxygen in normal and metallothionein-knockout mice. Invest Ophthalmol Vis Sci. 2008;49:3207–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maeda A, Maeda T, Imanishi Y, et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurth I, Thompson DA, Rèuther K, et al. Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Mol Cell Biol. 2007;27:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kasus-Jacobi A, Marchette LD, Xu C, Li F, Wang H, Babizhayev MA. Mechanisms of RDH12-induced Leber congenital amaurosis and therapeutic approaches. Adv Ophthalmol. 2012;1:473–496 [Google Scholar]
- 31. Krohne TU, Stratmann NK, Kopitz J, Holz FG. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res. 2010;90:465–471 [DOI] [PubMed] [Google Scholar]
- 32. Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46:3859–3868 [DOI] [PubMed] [Google Scholar]
- 33. Organisciak DT, Darrow RM, Jiang YI, Marak GE, Blanks JC. Protection by dimethylthiourea against retinal light damage in rats. Invest Ophthalmol Vis Sci. 1992;33:1599–1609 [PubMed] [Google Scholar]
- 34. Tanito M, Masutani H, Nakamura H, Ohira A, Yodoi J. Cytoprotective effect of thioredoxin against retinal photic injury in mice. Invest Ophthalmol Vis Sci. 2002;43:1162–1167 [PubMed] [Google Scholar]
- 35. Tanito M, Masutani H, Nakamura H, Oka S, Ohira A, Yodoi J. Attenuation of retinal photooxidative damage in thioredoxin transgenic mice. Neurosci Lett. 2002;326:142–146 [DOI] [PubMed] [Google Scholar]