Abstract
Exposure to a moderate or high total-body dose of radiation induces not only acute bone marrow suppression but also residual (or long-term) bone marrow injury. The induction of residual bone marrow injury is primarily attributed to the induction of hematopoietic cell senescence by ionizing radiation. However, the mechanisms underlying radiation-induced hematopoietic cell senescence are not known and thus were investigated in the present study. Using a well-established long-term bone marrow cell culture system, we found that radiation induced hematopoietic cell senescence at least in part via activation of p38 mitogen-activated protein kinase (p38). This suggestion is supported by the finding that exposure to radiation selectively activated p38 in bone marrow hematopoietic cells. The activation was associated with a significant reduction in hematopoietic cell clonogenic function, an increased expression of p16INK4a (p16), and an elevated senescence-associated β-galactosidase (SA-β-gal) activity. All these changes were attenuated by p38 inhibition with a specific p38 inhibitor, SB203580 (SB). Selective activation of p38 was also observed in bone marrow hematopoietic stem cells (HSCs) after mice were exposed to a sublethal total-body dose (6.5 Gy) of radiation. Treatment of the irradiated mice with SB after total-body irradiation (TBI) increased the frequencies of HSCs and hematopoietic progenitor cells (HPCs) in their bone marrow and the clonogenic functions of the irradiated HSCs and HPCs. These findings suggest that activation of p38 plays a role in mediating radiation-induced hematopoietic cell senescence and residual bone marrow suppression.
INTRODUCTION
Bone marrow suppression is one of the major concerns for cancer patients undergoing chemotherapy and/or radiotherapy. We and others have demonstrated that ionizing radiation causes residual (or long-term) bone marrow injury in part by inducing hematopoietic cell senescence, particularly in the hematopoietic stem cell (HSC) population (1–3). However, the mechanisms underlying radiation-induced HSC senescence are not well understood. Our recent studies showed that the induction of HSC senescence by radiation is attributed to an increased production of reactive oxygen species (ROS) (4). Interestingly, it has been shown that activation of the p38 mitogen-activated protein kinase (p38) pathway can mediate oxidative stress-induced HSC senescence and premature exhaustion under various pathological conditions (5, 6). Therefore, it is of great interest to determine the role of p38 in radiation-induced hematopoietic cell senescence and residual bone marrow suppression.
p38 is activated in a sequential order [mitogen-activated or extracellular signal-regulated kinase kinase (MEKK)-MAPK kinase 3/6 (MKK3/6)-p38] after exposure to stress including radiation (7). Activation of p38 regulates a variety of cellular processes such as inflammation, cell cycle arrest and apoptosis in a cell type-specific manner. There is an increasing body of evidence demonstrating that p38 plays a critical role in the induction of senescence in response to a variety of stimuli via upregulating p16Ink4a (p16) (8, 9). For example, it was shown that a high level of Ras or Raf activation in human normal fibroblasts induced senescence by stimulating a sustained activation of p38, which in turn upregulated the expression of p16 (9). Activation of the p38 pathway also contributes to the induction of p16 and cellular senescence after DNA damage resulting from exposure to genotoxic and oxidative stress and telomere shortening due to extensive replication (5, 10, 11). Furthermore, activation of p38 by ectopic transfection of MKK3 and/or MKK6 increases p16 expression and induces senescence. In contrast, inhibition of p38 activity or downregulation of p38 expression attenuates the induction of p16 and cellular senescence by oncogenic stress, DNA damage and telomere shortening (5, 10–12).
In addition, activation of p38 has been implicated in bone marrow suppression in various pathological conditions, including aplastic anemia and myelodysplastic syndromes (13, 14). Furthermore, recently it was shown that mutation of the ATM gene and knockout of the FoxO3 gene induced premature senescence/exhaustion of HSCs (15–17). The induction of HSC senescence/exhaustion was associated with an elevated production of ROS, a selective activation of p38, and an upregulation of p16 in HSCs. Pharmacological inhibition of p38 activity rescued the defects of HSCs from ATM mutants and FoxO3 knockout mice (15–17). These findings indicate that p38 plays an important role in regulation of HSC self-renewal under stress conditions. Particularly, its activation by oxidative stress can mediate the induction of HSC senescence via regulation of p16 (5). These interesting observations prompted us to examine whether radiation causes hematopoietic suppression in part by inducing hematopoietic cell senescence through activation of the p38 pathway and if pharmacological inhibition of p38 can attenuate radiation-induced residual bone marrow injury.
The long-term bone marrow cell culture assay originally established by Dexter et al. has been used widely to examine the functions of HSCs and hematopoietic progenitor cells (HPCs) in vitro (18–20). Using this assay, we found that p38 was selectively activated in irradiated hematopoietic cells and that this activation was sustained up to 5 weeks after irradiation. Inhibition of p38 activity with a specific inhibitor, SB203580 (SB), attenuated radiation-induced suppression of bone marrow hematopoietic cell function in an association with a significant reduction in p16 expression and senescence-associated β-galactosidase (SA-β-gal) activity. Moreover, our in vivo data show that inhibition of p38 attenuates radiation-induced residual bone marrow suppression. These results suggest that p38 activation plays a role in mediating radiation-induced hematopoietic cell senescence and bone marrow suppression and that pharmacological inhibition of the p38 pathway with a specific inhibitor can be further exploited for amelioration of radiation-induced residual bone marrow injury.
METHODS AND MATERIALS
Reagents
Phycoerythrin (PE)-conjugated anti-Sca-1 (Clone E13-161.7, rat IgG2a), APC-conjugated anti-c-kit (Clone 2B8, rat IgG2b), biotin-conjugated anti-CD3e (Clone 145-2C11, Hamster IgG1), anti-CD45R/ B220 (Clone RA3-6B2, rat IgG2a), anti-Gr-1 (Clone RB6-8C5, rat IgG2b), anti-Mac-1 (Clone M1/70, rat IgG2b), anti-Ter-119 (Clone Ter-119, rat IgG2b), purified rat anti-CD 16/CD32 (Clone 2.4G2, Fcγ receptor blocker, rat IgG2b), and fluorescein isothiocyanate (FITC)-conjugated streptavidin were purchased from BD Pharmingen (San Diego, CA). Antibodies against phosphorylated-p38 (p-p38), phosphorylated-Erk (p-Erk), and phosphorylated-JNK (p-JNK) were purchased from Cell Signaling Technology (Beverly, MA). Alexa Fluor-555-conjugated goat anti-rabbit IgG antibody was obtained from Invitrogen (Carlsbad, CA). SB203580 (4-[4-fluorophenyl]-2-[4-methylsulfinylphenyl]-5-[4-pyridyl] 1H-imidazole, or SB), a p38 specific inhibitor, was purchased from Calbiochem (San Diego, CA). Methocult® GF M3434 medium was purchased from Stem Cell Technologies (Vancouver, Canada).
Mice
Male C57BL/6 mice were purchased from The Charles River Laboratories through the National Cancer Institute. Mice were housed four to a cage at the Medical University of South Carolina (MUSC) AAALAC-certified animal facilities. Animals received food and water ad libitum. All mice were used at approximately 8–10 weeks of age. The Institutional Animal Care and Use Committee of MUSC approved all experimental procedures used in this study.
Isolation of Bone Marrow Mononuclear Cells
The femora and tibiae were harvested from the mice immediately after they were euthanized with CO2. Bone marrow cells were flushed from the bones into Hank’s buffered saline solution (HBSS) containing 2% FCS using a 21-gauge needle and syringe. Cells from at least three mice were pooled and centrifuged through Histopaque 1083 (Sigma, St. Louis, MO) to isolate bone marrow mononuclear cells. The isolated cells were resuspended in complete IMDM supplemented with 10% FCS, 2 mM L-glutamine, 10 μM Hepes buffer, 100 U/ml penicillin, and 100 μg/ml streptomycin at 4 × 106 cells/ml.
Irradiation of Bone Marrow Mononuclear Cells
Bone marrow mononuclear cells (5 × 106/ml) suspended in complete IMDM were exposed to 4 Gy of radiation in a J. L. Shepherd Model 143 137Cs γ irradiator (J. L. Shepherd, Glendale, CA) at a dose rate of 2.4 Gy/min. Cells were incubated in 60-mm petri dishes at 33°C in 95% air/5% CO2 and 100% humidity for various times as indicated in individual experiments.
Total-Body Irradiation (TBI) and SB Treatment
Mice were exposed to a sublethal dose (6.5 Gy) of radiation in a J. L. Shepherd Model 143 137Cs γ irradiator at a dose rate of 2.4 Gy/min. Twenty-four hours after TBI, SB was administered by s.c injection at dose of 15 mg/kg (diluted in DMSO) body weight every other day for 30 days. An equal volume (0.1 ml/mouse) of DMSO was injected as vehicle control. Thirty days after TBI, mice were euthanized by CO2 suffocation followed by cervical dislocation, and the femora and tibiae were immediately harvested from the mice for isolation of bone marrow mononuclear cells as described above.
Long-term Bone Marrow Cell Culture
Long-term bone marrow cell culture was performed according to the method of Dexter et al. with modifications as described previously (18–20). Briefly, bone marrow cells at 5 × 106/ml were exposed to 4 Gy radiation or sham-irradiated as a control. The irradiated or control cells were placed in a 60-mm dish with CAFC medium as described previously (3). These dishes were incubated at 33°C in a humidified incubator with 95% air/5% CO2. The cells were fed weekly by removal of half the medium and replacement with the same volume of fresh medium. After 2 or 5 weeks of culture, hematopoietic cells were collected as described previously (3). The recovered hematopoietic cells were analyzed by immunofluorescence staining, phenotyping with flow cytometry, colony-forming cell (CFC) assay, reverse transcription polymerase chain reaction (RT-PCR), and SA-β-gal staining as described below.
Immunofluorescence Microscopy
The activation of p38, extracellular signal-regulated kinases (Erk) and c-Jun N-terminal kinase (JNK) was analyzed by phospho-p38, phospho-Erk and phospho-JNK immunofluorescence staining. Briefly, after 5 weeks of long-term bone marrow cell culture, hematopoietic cells were collected and cytospun onto slides at 1,000 rpm × 5 min. Cells were fixed with 4% PFA and permeabilized with 0.2% Triton X-100. After blocking with 5% normal goat serum, cells were incubated with anti-p-p38 (1:100), anti-p-Erk (1:100) or anti-p-JNK (1:100) overnight at 4°C. After extensive washing, the first antibodies were visualized with Alexa Fluor-555-conjugated goat anti-rabbit IgG antibody (1:200). The nuclei of the cells were then stained with Hoechst 33342. Slides were mounted with VECTASHIELD, and images of the cells were viewed and acquired by a Zeiss Axio Observer (Carl Zeiss Microimaging Inc., GmbH, Jena, Germany). The images were captured using AxioVision (4.7.1.0) software (Carl Zeiss Imaging Solutions GmbH, Jena, Germany). The collected images were then displayed in Adobe Photoshop V6.0.
Phenotypic Analysis of Hematopoietic Cells by Flow Cytometry
Briefly, 2 × 105 hematopoietic cells harvested from 5 weeks of long-term bone marrow cell culture or mouse bone marrow were incubated with biotin-conjugated antibodies against CD3ε, CD45R/ B220, Gr-1, Mac-1 and Ter-119 and then with streptavidin-FITC to stain the lineage-positive cells. The cells were washed with PBS and incubated with anti-CD16/CD32 antibody to block Fc receptors. Finally, the cells were stained with anti-Sca-1-PE and anti-c-kit-APC antibodies and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The data were analyzed using CellQuest software (Becton Dickinson). Cells stained negative for lineage markers and c-kit but positive for Sca1 were considered as HPCs (lineage−Sca1+c-kit− cells, or LSK− cells) and those negative for lineage markers but positive for Sca1 and c-kit as HSCs (lineage−Sca1+c-kit+ cells, or LSK+ cells).
Hematopoietic Cell Clonogenic Assays
Colony-forming cell (CFC) and cobblestone area-forming cell (CAFC) assays were used to determine the clonogenic functions of HPCs and HSCs, respectively, as described previously (1,3, 4).
Apoptosis Assay
Bone marrow mononuclear cells were stained with FITC-Annexin V from an apoptosis assay kit (BD Biosciences) according to the manufacturer’s protocol. Apoptotic cells were determined by flow cytometry analysis on a FACSCalibur (Becton Dickinson), and the data were analyzed using CellQuest software (Becton Dickinson) as described previously (21).
Semiquantitative RT-PCR
Total RNA was isolated from hematopoietic cells harvested from 5-week long-term bone marrow cell cultures using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. RNA yield and quality were determined with a NanoDrop (Thermo Scientific, Wilmington, DE). First-strand cDNA was synthesized from 1 μg of total RNA using a SuperScript III first-strand synthesis system (Invitrogen) according to the manufacturer’s manual. Two microliters of cDNA was used for the PCR amplification using 2 U of Pfx DNA polymerase (Invitrogen). The housekeeping gene GAPDH cDNA was amplified simultaneously as an internal quantitative control, and all of the samples were normalized to the expression of GAPDH as described previously (3).
SA-β-Gal Staining
SA-β-gal activity was determined with a SA-β-gal staining kit from Cell Signaling Technology (Beverly, MA) according to the manufacturer’s instructions as described previously (3). Briefly, nonadherent and adherent hematopoietic cells harvested from 5-week long-term bone marrow cell cultures were cytospun onto slides. They were fixed in 2% (v/v) formaldehyde and 0.2% glutaraldehyde and then incubated in SA-β-gal staining solution [1 mg/ml 5-bromo-4-chloro-3-indolyl β-D-galactosidase, 40 mM citric acid (pH 6.0), 40 mM sodium phosphate (pH 6.0), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM sodium chloride, and 2 mM magnesium chloride] at 37°C for 10 h. Senescent cells were identified as blue-stained cells by standard light microscopy, and a total of 1000 cells were counted in five random fields on a slide to determine the percentage of SA-β-gal-positive cells.
Analysis of p38 Activation in Bone Marrow HSCs and HPCs by Phosphospecific Flow Cytometry
Approximately 1 × 106 cells freshly isolated bone marrow mononuclear cells were first stained with PE-conjugated anti-lineage antibodies (B220, Thy 1, Ter-119, Gr-1 and CD11b) after blocking with anti- CD16/CD32 antibody. The cells were then fixed with CytoFix/Cytoperm (BD Pharmingen, San Diego, CA), permeabilized with Cytoperm™ Plus (BD Pharmingen), and stained with APC-c-Kit, PE-Cy7-Sca-1 and mouse anti-p-p38 antibody (1:100) according to the manufacturers’ instructions. Phosphorylated p38 (p-p38) staining was detected by Alexa Fluor-488 conjugated rabbit anti-mouse IgG (1:200). Cells were analyzed on a FACSCalibur and the data were analyzed using CellQuest software.
Statistical Analysis
The differences between treated and control groups were examined by unpaired Student’s t tests. Differences were considered significant at P < 0.05. All of these analyses were done using GraphPad Prism from GraphPad Software (San Diego, CA).
RESULTS
Radiation Selectively Activates p38 in Bone Marrow Hematopoietic Cells In Vitro
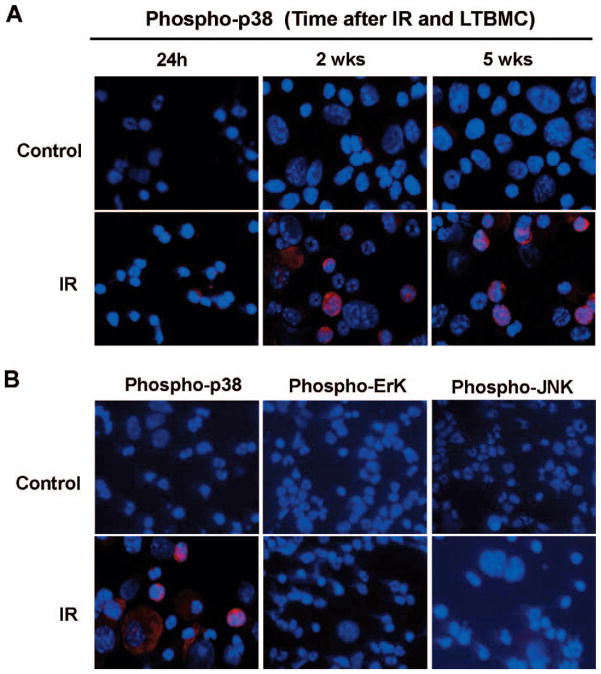
We exposed bone marrow mononuclear cells to 4 Gy radiation and examined the kinetics of p38 activation in irradiated bone marrow cells to determine whether activation of p38 is involved in radiation-induced hematopoietic cell injury using a long-term bone marrow cell culture system. As shown in Fig. 1A, we found that an increase in p38 phosphorylation was detected within 24 h after irradiation in bone marrow hematopoietic cells and that its activation was sustained up to 5 weeks after the exposure. Next we investigated whether radiation causes selective activation of p38 in bone marrow hematopoietic cells by examining Erk and JNK phosphorylation in hematopoietic cells harvested from 5-week long-term bone marrow cell cultures by immunofluorescence staining. The data showed that phospho-p38, but not phospho-Erk and phosphor-JNK, was detected in irradiated bone marrow hematopoietic cells, indicating that radiation can selectively activate p38 in hematopoietic cells (Fig. 1B).
FIG. 1.
Radiation selectively activates p38 in bone marrow hematopoietic cells. Panel A: Bone marrow mononuclear cells either were not irradiated (Control) or were exposed to 4 Gy of radiation. At different times after long-term bone marrow cell culture, hematopoietic cells were harvested and analyzed for p38 activation using a phospho-p38 specific antibody and visualized with Alexa Fluor-555-conjugated goat anti-rabbit IgG antibody (red). The nuclei of the cells were stained with Hoechst 33342 (blue). Panel B: After 5 weeks of long-term bone marrow cell culture, irradiated (IR) or unirradiated (Control) hematopoietic cells were harvested and analyzed for p38, Erk and JNK activation using phospho-p38, -Erk and -JNK specific antibodies and visualized with Alexa fluor-555-conjugated goat anti-rabbit IgG antibody (red). The nuclei of the cells were stained with Hoechst 33342 (blue). Representative photomicrographs of phospho-p38, -Erk and -JNK immunofluorescence staining are shown.
Inhibition of p38 Attenuates Radiation-Induced Suppression of Bone Marrow Hematopoietic Cell Function
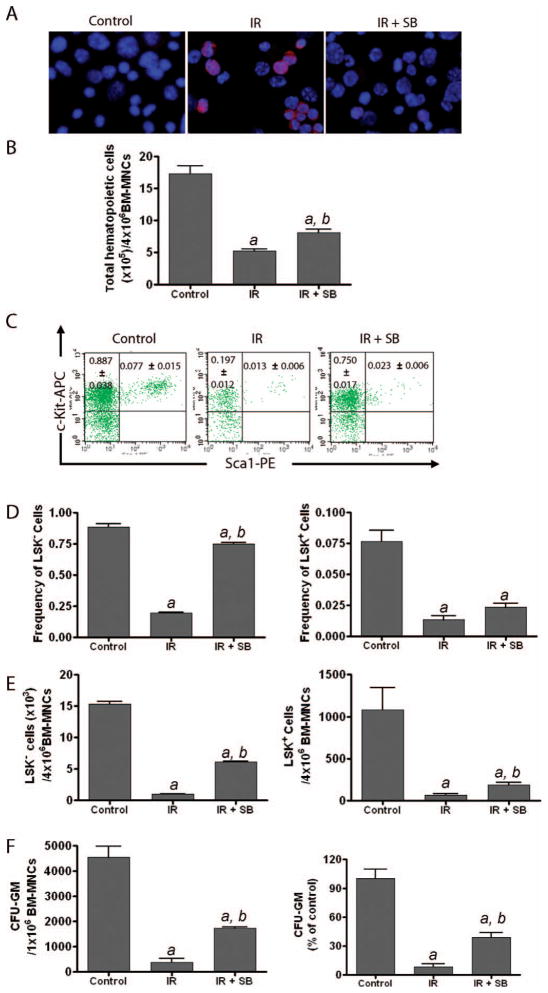
Since radiation exposure has been shown to induce sustained activation of p38 in hematopoietic cells (Fig. 1), we decided to further determine if inhibition of p38 can attenuate radiation-induced bone marrow suppression. To this end, we treated bone marrow cells with SB, a specific p38 inhibitor, after irradiation for the duration of the 5-week long-term bone marrow cell culture to determine whether SB treatment inhibits radiation-induced p38 activation and consequently attenuates radiation-induced suppression of hematopoietic cell function. As shown in Fig. 2A, SB treatment significantly inhibited radiation-induced activation of p38 in bone marrow hematopoietic cells harvested from long-term bone marrow cell cultures, suggesting that SB treatment can inhibit radiation-induced p38 activation in bone marrow cells.
FIG. 2.
Inhibition of p38 attenuates radiation-induced suppression of bone marrow hematopoietic cell function. Bone marrow mononuclear cells (BM-MNCs) either were not irradiated (Control) or were exposed to 4 Gy radiation. After irradiation, the cells were cultured in the presence of SB (5 μM, IR+SB) or DMSO (IR) for up to 5 weeks in long-term bone marrow cell culture. Panel A: Two weeks after long-term bone marrow cell culture, hematopoietic cells were collected and p38 activation was determined by immunofluorescence microscopy using a phospho-p38 specific antibody and visualized with Alexa Fluor-555-conjugated goat anti-rabbit IgG antibody (red). The nuclei of the cells were stained with Hoechst 33342 (blue). Representative photomicrographs of phospho-p38 immunofluorescence staining are shown. Panel B: The numbers of total hematopoietic cells harvested after 5 weeks of long-term bone marrow cell culture are presented as means ± SE of three independent experiments. Panel C: Representative flow cytometry analyses of HSCs (LSK+ cells) and HPCs (LSK− cells) in hematopoietic cells harvested from 5-week long-term bone marrow cell cultures. Panel D: Frequencies of HSCs (LSK+ cells) and HPCs (LSK− cells) in hematopoietic cells harvested from 5-week long-term bone marrow cell cultures are presented as means ± SE of three independent experiments according to the flow cytometry analysis presented in panel C. Panel E: Numbers of HSCs (LSK+ cells) and HPCs (LSK− cells) in hematopoietic cells harvested from 5-week long-term bone marrow cell cultures were calculated according to the total numbers of hematopoietic cells harvested from each long-term bone marrow cell culture and the frequencies of HSCs (LSK+ cells) and HPCs (LSK− cells) in the whole cell population. The data are presented as means ± SE of three independent experiments. Panel F: The clonogenic function of hematopoietic cells harvested from 5-week long-term bone marrow cell cultures was determined by CFC assays. The numbers of CFU-GM expressed as per 106 input bone marrow mononuclear cells in long-term bone marrow cell cultures are presented as means ± SE of three independent experiments on the left. On the right, the data from irradiated (IR) and IR+SB cultures are also expressed as percentages of control (unirradiated cells). a, P < 0.001 compared to control; b, P < 0.001 compared to irradiated.
Next we investigated whether inhibition of p38 with SB can suppress radiation-induced reduction of HSCs and HPCs in irradiated bone marrow cells after 5 weeks of long-term bone marrow cell culture. The data showed that exposure of bone marrow cells to radiation led to a significant decrease of HSCs (lineage−Sca1+c-kit+ cells, or LSK+ cells) and HPCs (LSK− cells) (Fig. 2B–E). However, the numbers of HSCs and HPCs were markedly increased in long-term bone marrow cell cultures treated with SB compared to those treated with DMSO (Fig. 2B–E).
In addition, we performed the CFC assay to examine whether irradiated hematopoietic cells can maintain their clonogenic function after incubation with SB in long-term bone marrow cell cultures. As expected, radiation exposure significantly reduced the production of colony forming units-granulocyte-macrophage (CFU-GM) by bone marrow hematopoietic cells harvested from a 5-week long-term bone marrow cell culture. The addition of SB to long-term bone marrow cell cultures markedly increased the production of CFU-GM. In fact, the number of CFU-GM generated by the irradiated bone marrow hematopoietic cells with SB treatment after 5 weeks of long-term bone marrow cell culture was more than threefold greater than that produced by the irradiated cells treated with DMSO (Fig. 2F). These results suggest that inhibition of p38 attenuates radiation-induced suppression of bone marrow hematopoietic cell function.
Inhibition of p38 MAPK Reduces Radiation-Induced Senescence but not Apoptosis in Hematopoietic Cells
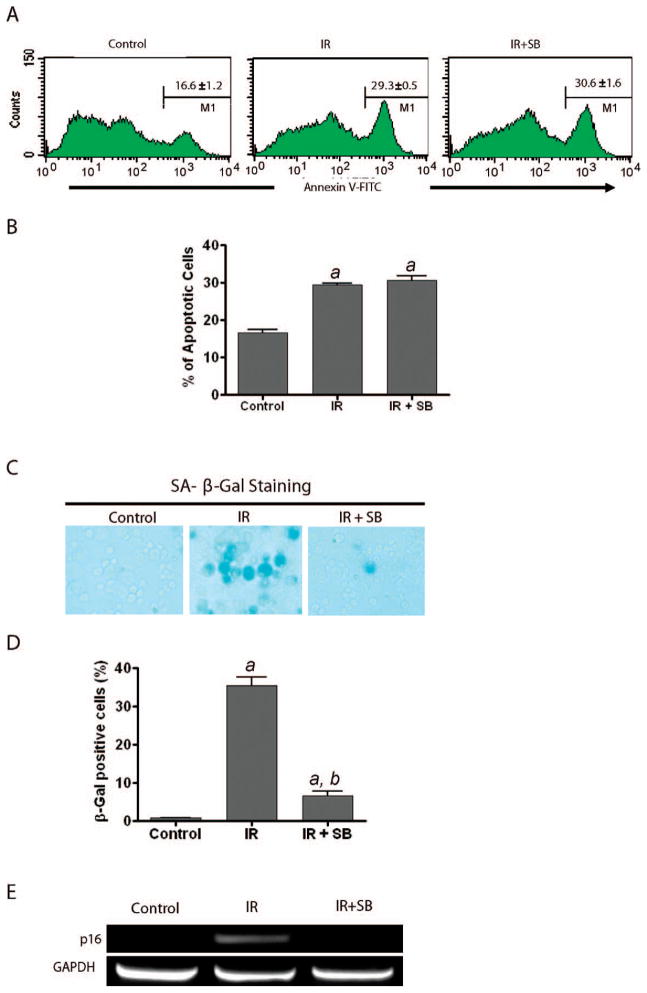
To gain insights into the mechanisms of action of SB, we examined the effects of SB on radiation-induced apoptosis and senescence in hematopoietic cells. We incubated bone marrow mononuclear cells with SB at 30 min prior to radiation exposure and analyzed apoptotic cells by Annexin V-FITC staining and flow cytometry. The results showed that the percentage of Annexin V positively stained cells (apoptotic cells) was not altered in irradiated bone marrow cells treated with either SB or vehicle (Fig. 3A and B), suggesting that inhibition of p38 has no effect on radiation-induced apoptosis in bone marrow hematopoietic cells.
FIG. 3.
Inhibition of p38 suppresses the induction of senescence but not apoptosis in hematopoietic cells by radiation. Panels A and B: Percentage of apoptotic cells was determined by Annexin V staining and flow cytometry at 24 h after bone marrow mononuclear cells were exposed to radiation in the presence of 5 μM SB (IR+SB) or without SB (IR). Unirradiated bone marrow mononuclear cells were included as a control. Representative flow cytometry analyses of apoptotic (Annexin V-positive) hematopoietic cells are shown in panel A, and the percentages of apoptotic hematopoietic cells are shown in panel B as means ± SE of three independent experiments according to the flow cytometry analysis presented in panel A. Panels C and D: Bone marrow mononuclear cells either were not irradiated (Control) or were exposed to 4 Gy radiation. After irradiation, the cells were cultured in the presence of SB (5 μM, IR+SB) or DMSO (IR) for 5 weeks in long-term bone marrow cell cultures. Induction of senescence in the hematopoietic cells harvested from 5-week long-term bone marrow cell cultures was determined by SA-β-gal staining. Representative photomicrographs of SA-β-gal staining are shown in panel C. The cells with blue staining represent senescent hematopoietic cells. Percentages of SA-β-gal-positive senescent cells are presented in panel D as means ± SE (n = 3). Panel E: The cells were treated as described above. The expression of p16 and GAPDH mRNAs in hematopoietic cells harvested from 5-week long-term bone marrow cell cultures was analyzed by RT-PCR. Representative results of DNA electrophoresis of the amplified PCR products for all the mRNA are shown. a, P < 0.001 compared to control; b, P < 0.001 compared to irradiated.
Next we examined whether p38 inhibition attenuates radiation-induced suppression of bone marrow hematopoietic cells by inhibiting induction of senescence. Since increased SA-β-gal staining is a hallmark of cellular senescence (22), we used SA-β-gal staining to identify senescent cells in hematopoietic cells harvested from a 5-week long-term bone marrow cell culture. As shown in Fig. 3C and D, the percentage of SA-β-gal-positive cells was markedly increased in bone marrow hematopoietic cells after exposure to radiation and was significantly reduced by treatment with SB. These results suggest that p38 inhibition can inhibit radiation-induced hematopoietic cell senescence.
It has been demonstrated that p16 is not only a critical biomarker of senescence but also a very important effector that mediates the induction of senescence. Therefore, we compared the expression of p16 in bone marrow hematopoietic cells after irradiation with or without SB treatment during long-term bone marrow cell culture using RT-PCR. The results showed that exposure to radiation significantly increased the expression of p16 mRNA in bone marrow hematopoietic cells harvested from 5-week long-term bone marrow cell cultures (Fig. 3E). These results suggest that p38 inhibition inhibits radiation-induced senescence in bone marrow hematopoietic cells by suppressing p16 expression.
Inhibition of p38 Mitigates Radiation-Induced Residual Bone Marrow Injury
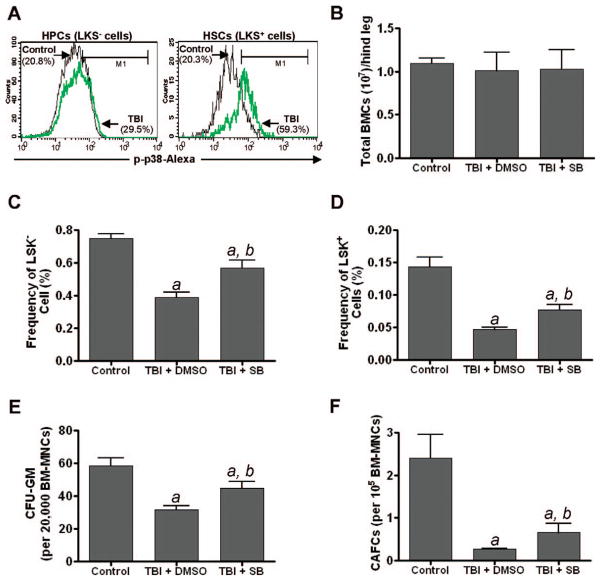
Our previous studies showed that total-body exposure of mice to radiation induced residual bone marrow suppression in part by induction of HSC senescence (1, 4). Activation of p38 has been implicated in mediating the induction of HSC senescence under various pathological conditions (5, 14). The data presented above also demonstrate that p38 activation plays an important role in mediating radiation-induced senescence in hematopoietic cells in vitro. Therefore, we examined whether total-body exposure of mice to radiation induces p38 activation in HSCs and whether treatment of mice with the p38 inhibitor SB can mitigate radiation-induced residual bone marrow injury. As shown in Fig. 4A, total-body exposure of mice to radiation resulted in a slight increase in p38 activation in HPCs (LSK− cells) but a dramatic increase in p38 activation in HSCs (LSK+ cells), which is in agreement with numerous previous reports showing that activation of p38 occurs primarily in HSCs but not in HPCs (5, 6). To determine whether activation of p38 mediates radiation-induced residual bone marrow injury, mice were treated with SB (15 mg/kg, s.c.) or vehicle (DMSO) at 24 h after TBI and then every other day for 30 days. After the last treatment, bone marrow mononuclear cells were harvested from the irradiated mice and a group of unirradiated mice as a control. It was found that at 30 days after TBI the number of total bone marrow mononuclear cells from these irradiated mice treated with vehicle or SB was back to a level similar to that seen in unirradiated normal mice (Fig. 4B). However, the frequencies of HSCs and HPCs and their clonogenic functions measured by the frequencies of CFACs and CFU-GM, respectively, remained low in vehicle-treated and irradiated mice compared to those in the control group of unirradiated mice (Fig. 4C–F), demonstrating that total-body exposure of mice to radiation induced residual bone marrow injury as shown in our previous reports (1–4). The reduction in the frequencies of HSCs and HPCs and their clonogenic functions was significantly attenuated by SB treatment (Fig. 4C–F). These findings suggest that activation of p38 plays an important role in mediating radiation-induced residual bone marrow suppression, probably by induction of HSC senescence. Specific p38 inhibitors have the potential to be used as novel therapeutic agents to mitigate radiation-induced residual bone marrow toxicity.
FIG. 4.
Inhibition of p38 MAPK mitigates radiation-induced residual bone marrow injury. Panel A: C57BL/6 mice were total-body exposed to 6.5 Gy radiation or sham-irradiated (Control). One month after TBI, p38 activation in HSCs and HPCs was determined by flow cytometry after membrane permeabilization and immunostaining with Alexa Fluor-488-conjugated anti-phospho-p38 antibody. Alexa Fluor-488-conjugated isotype control antibody was included as control for setting up the positive gate. Representative flow cytometry analyses of phospho-p38 positively stained cells are shown. Panels B–F: C57BL/6 mice were total-body exposed to 6.5 Gy radiation or were sham-irradiated (Control). Twenty-four hours after TBI, the irradiated mice were treated with SB (15 mg/kg, s.c.) or vehicle (DMSO) every other day for 30 days. After the last treatment, bone marrow mononuclear cells were harvested from the irradiated mice and control unirradiated mice for analyses. The numbers of total bone marrow -mononuclear cells are presented in panel B. The frequencies of HPCs (LSK− cells) and HSCs (LSK+ cells) in bone marrow mononuclear cells are presented in panels C and D and those of CFUs-GM and day-35 CAFCs are presented in panels E and F, respectively. All the data are presented as means ± SE of three independent experiments. a, P < 0.001 compared to control; b, P < 0.001 compared to irradiated.
DISCUSSION
Currently, there are more than 11 million cancer survivors in the U.S., and most of them are expected to survive 5 years or more after being diagnosed with cancer. Unfortunately, long-term cancer survivors are at increased risk of developing treatment-related late effects, including residual bone marrow injury induced by radiation and/or chemotherapy (23–26). Unlike acute myelosuppression, residual bone marrow damage is latent and sometimes can even be worsened by treatment with various hematopoietic growth factors (HGFs) that are widely used to ameliorate chemotherapy- and radiation-induced acute bone marrow suppression (27–29). Although residual bone marrow damage is latent, it is long-lasting, shows little tendency for recovery, and can lead to the development of hypoplastic anemia or a myelodysplastic syndrome at later times or after additional hematopoietic stress such as subsequent cycles of cancer treatment or bone marrow transplantation (24–26). Therefore, effective treatments that can mitigate chemotherapy- and radiation-induced long-term bone marrow suppression are needed.
Our previous studies demonstrated that radiation causes long-term bone marrow injury in part by inducing hematopoietic cell senescence, particularly in HSCs (1, 4). Activation of the p38 pathway has been shown to suppress hematopoietic cell function in part by induction of cellular senescence under various pathological conditions (5, 6). Therefore, using the well-established long-term bone marrow cell culture as an in vitro model of hematopoiesis, we first examined whether activation of p38 mediates radiation-induced hematopoietic cell senescence. Our results showed that exposure of bone marrow hematopoietic cells to radiation selectively activated p38 and that this activation was sustained up to 5 weeks after irradiation. Inhibition of p38 activity with SB, a specific p38 inhibitor, attenuated radiation-induced suppression of bone marrow hematopoietic cell function. This effect is likely attributed to the inhibition of radiation-induced hematopoietic cell senescence because SB treatment reduced the expression of p16 and SA-β-gal activity in bone marrow hematopoietic cells after exposure to radiation but had no significant effect on radiation-induced hematopoietic cell apoptosis. This finding is in agreement with previous observations demonstrating that p38 functions as a key molecule mediating diverse stimulus-induced cellular senescence via upregulation of p16 (5, 8, 10–12). However, the mechanisms by which radiation activates p38 in hematopoietic cells remain to be elucidated. It has been well established that oxidative stress can activate p38 in HSCs to induce HSC senescence and premature exhaustion (5, 6). Our recent studies also showed that radiation-induced HSC senescence and long-term bone marrow suppression are partially attributable to the induction of sustained oxidative stress in HSCs (4, 30). It will be interesting to determine if radiation activates p38 in hematopoietic cells via induction of oxidative stress.
We also examined whether inhibition of the p38 pathway can mitigate radiation-induced residual bone marrow injury because it has been well established that TBI induces residual bone marrow injury primarily by induction of HSC senescence (1, 4, 30). Our results showed that total-body exposure to radiation selectively activated p38 in HSCs and that treatment of the mice with SB after TBI attenuated radiation-induced residual bone marrow injury. These findings confirmed that p38 plays a role in mediating radiation-induced residual bone marrow suppression, probably by induction of HSC senescence. However, treatment with SB alone did not completely restore the bone marrow hematopoietic function of the irradiated mice, indicating that activation of p38 is not the sole mechanism by which radiation causes bone marrow suppression. Other factors, such as damage to the HSC niche, may also contribute to radiation-induced residual bone marrow injury and remain to be investigated.
Although it has been well documented that p38 activation plays a critical role in mediating the induction of senescence in a variety of cells (5, 8, 9, 11, 12), our present study demonstrates for the first time that activation of p38 is involved in radiation-induced hematopoietic cell senescence and residual bone marrow injury. Long-term bone marrow suppression also occurs in cancer patients after treatment with certain chemotherapeutic agents such as carboplatinum, busulfan and bis-chloronitrosourea (23–26). It would be of great clinical interest to determine whether various chemotherapeutic agents also induce long-term bone marrow suppression via activation of p38 and whether specific p38 inhibitors can also be used to mitigate chemotherapy-induced residual bone marrow injury.
Acknowledgments
The authors thank Mrs. Aimin Yang for her excellent technical assistance and Mr. Richard Peppler and Dr. Haiqun Zeng for the flow cytometry analysis and cell sorting. This study was supported in part by grants from the National Institutes of Health and the Winthrop W. Rockefeller Endowment for Leukemia Research.
References
- 1.Wang Y, Schulte BA, Larue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–66. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le ON, Rodier F, Fontaine F, Coppe JP, Campisi J, Degregori J, et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010;9:398–409. doi: 10.1111/j.1474-9726.2010.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–9. [PubMed] [Google Scholar]
- 4.Wang Y, Liu L, Pazhanisamy SP, Meng A, Zhou D. Total body irradiation selectively induces persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–56. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, et al. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23:1358–65. doi: 10.1096/fj.08-110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong J, Hong L, Liao R, Deng Q, Han J, Sun P. p38alpha and p38gamma mediate oncogenic ras-induced senescence through differential mechanisms. J Biol Chem. 2009;284:11237–46. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Probin V, Wang Y, Bai A, Zhou D. Busulfan selectively induces cellular senescence but not apoptosis in WI38 fibroblasts via a p53-independent but extracellular signal-regulated kinase-p38 mitogen-activated protein kinase-dependent mechanism. J Phar-macol Exp Ther. 2006;319:551–60. doi: 10.1124/jpet.106.107771. [DOI] [PubMed] [Google Scholar]
- 11.Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–44. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- 12.Deng Q, Liao R, Wu BL, Sun P. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J Biol Chem. 2004;279:1050–9. doi: 10.1074/jbc.M308644200. [DOI] [PubMed] [Google Scholar]
- 13.Navas TA, Mohindru M, Estes M, Ma JY, Sokol L, Pahanish P, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108:4170–7. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsoulidis E, Li Y, Yoon P, Sassano A, Altman J, Kannan-Thulasiraman P, et al. Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes. Cancer Res. 2005;65:9029–37. doi: 10.1158/0008-5472.CAN-04-4555. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 16.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283:25692–705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- 18.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–44. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 19.Frimberger AE, Stering AI, Quesenberry PJ. Characterization of engraftable hematopoietic stem cells in murine long-term bone marrow cultures. Exp Hematol. 2001;29:643–52. doi: 10.1016/s0301-472x(01)00629-4. [DOI] [PubMed] [Google Scholar]
- 20.Epperly MW, Rugo R, Cao S, Wang H, Franicola D, Goff JP, et al. Investigation of the effects of aging on homologous recombination in long-term bone marrow cultures. In Vivo. 2009;23:669–77. [PMC free article] [PubMed] [Google Scholar]
- 21.Meng A, Wang Y, Brown SA, Van ZG, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol. 2003;31:1348–56. doi: 10.1016/j.exphem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 23.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–39. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 24.Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985;5:101–10. [PubMed] [Google Scholar]
- 25.Gale RP. Myelosuppressive effects of antineoplastic chemotherapy. In: Testa NG, Gale RP, editors. Hematopoiesis: Long-term effects of chemotherapy and radiation. New York: Marcel Dekker; 1988. pp. 63–73. [Google Scholar]
- 26.Lohrmann HPE, Schreml W. Long-term hematopoietic damage after cytotoxic drug therapy for solid tumors. In: Testa N, Gale RP, editors. Hematopoiesis: Long-term effects of chemotherapy and radiation. New York: Marcel Dekker; 1988. pp. 325–37. [Google Scholar]
- 27.van Os R, Robinson S, Sheridan T, Mislow JM, Dawes D, Mauch PM. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood. 1998;92:1950–6. [PubMed] [Google Scholar]
- 28.Gardner RV, Begue R, McKinnon E. The effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on primitive hematopoietic stem cell (PHSC) function and numbers, after chemotherapy. Exp Hematol. 2001;29:1053–9. doi: 10.1016/s0301-472x(01)00685-3. [DOI] [PubMed] [Google Scholar]
- 29.van Os R, Robinson S, Sheridan T, Mauch PM. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000;18:120–7. doi: 10.1634/stemcells.18-2-120. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Wang Y, Pazhanisamy SK, Shao L, Meng A, Batinic-Haberle I, et al. Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic Biol Med. 2011;51:30–7. doi: 10.1016/j.freeradbiomed.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]