Abstract
Ex vivo expansion of hematopoietic stem cells (HSCs) depends on HSC self-renewing proliferation and functional maintenance, which can be negatively affected by HSC differentiation, apoptosis, and senescence. Therefore, inhibition of HSC senescence may promote HSC expansion. To test this hypothesis, we examined the effect of inhibition of p38 mitogen-activated protein kinase (p38) on the expansion of human umbilical cord blood (hUCB) CD133+ cells because activation of p38 has been implicated in the induction of HSC senescence under various physiological and pathological conditions. Our results showed that ex vivo expansion of hUCB CD133+ cells activated p38, which was abrogated by the p38 specific inhibitor SB203580 (SB). Inhibition of p38 activity with SB promoted the expansion of CD133+ cells and CD133+CD38− cells. In addition, hUCB CD133+ cells expanded in the presence of SB for 7 days showed about threefold increase in the clonogenic function of HSCs and engraftment in non-obese diabetic/severe combined immunodeficient mice after transplantation compared to the input cells. In contrast, the cells expanded without SB exhibited a significant reduction in these HSC functions. The enhancement of ex vivo expansion of hUCB HSCs is primarily attributable to SB-mediated inhibition of HSC senescence. In addition, inhibition of HSC apoptosis and upregulation of CXCR4 may also contribute to the enhancement. However, p38 inhibition had no significant effect on HSC differentiation and proliferation. These findings suggest that inhibition of p38 activation may represent a novel strategy to promote ex vivo expansion of hUCB HSCs.
Keywords: Human umbilical cord blood, Hematopoietic stem cells, Ex vivo expansion, p38, Senescence
Introduction
Human umbilical cord blood (hUCB) is recognized as a rich source of hematopoietic stem cells (HSCs). Due to its ready availability through banking and relatively lower risk of graft-versus-host disease after transplantation in a HLA-mismatched recipient, hUCB has been increasingly used in clinics for the treatment of various hematopoietic disorders and malignancies [1]. However, the low number of HSCs in each unit of hUCB has limited its use primarily for pediatric patients and also leads to an increase in transplantation failure and risks of transplantation complications due to a delay in donor cell engraftment [2–4]. Therefore, extensive efforts have been made to expand HSCs ex vivo to overcome the shortcomings of hUCB. Most of the current HSC expansion methods require ectopic expression of a HSC regulatory transcription factor, culture of the cells with a variety of hematopoietic growth factors, or co-culture of the cells with stromal elements [1, 5]. These methods have many drawbacks which limit their clinical practice because of concerns of HSC transformation by gene transfection, high costs of the hematopoietic growth factors, and difficulty to standardize stromal elements to meet the FDA regulation. Therefore, increasing efforts have been devoted to identify small molecules that can promote HSC self-renewal and ex vivo expansion to overcome the shortcomings of these existing methods.
p38 mitogen-activated protein kinase (p38) is a member of the mitogen-activated protein kinase super family. It was originally discovered as a stress-activated kinase but has now been shown to be involved in the regulation of a wide range of physiologic and pathologic processes, such as cell proliferation, differentiation, apoptosis, and senescence [6–9]. It was reported that p38 is required for the fetal development of hematopoiesis primarily via regulation of erythropoietin production but is dispensable for hematopoiesis in adults [6, 10]. However, activation of p38 has been implicated in bone marrow (BM) suppression in various pathological conditions, such as myelodysplastic syndromes and Fanconi anemia [11, 12]. Furthermore, recently, it was shown that ATM mutation and FoxO3 deletion in mice induced premature exhaustion/ senescence of HSCs [13, 14]. The induction of HSC exhaustion/senescence was associated with an elevated production of reactive oxygen species (ROS), a selective activation of p38, and an upregulation of p16Ink4a (p16) in HSCs. Pharmacological inhibition of p38 activity rescued the defects of HSCs from ATM mutated and FoxO3 knockout mice. In addition, our recent studies showed that exposure of mice to a sublethal dose of total body irradiation (TBI) also selectively activated p38 in bone marrow HSCs and p38 inhibition with a specific inhibitor reduced IR-induced hematopoietic cell senescence and TBI-induced residual BM suppression [9]. These findings indicate that p38 is dispensible for HSC self-renewal and normal hematopoiesis in a homeostatic condition, but it plays an important role in the regulation of HSC self-renewal under stress conditions. Particularly, its activation by oxidative stress can mediate the induction of HSC senescence via regulation of p16 [13].
It has been well established that culture of mammalian cells in vitro in a normoxic condition (20% of O2) induces oxidative stress that can potentially activate p38 in HSCs [15, 16]. In addition, many hematopoietic suppressive cytokines can be produced by the progeny of HSCs during ex vivo expansion and some of them, such as tumor growth factor-β, can stimulate p38 activation [11, 14, 17, 18]. Therefore, we assume that p38 activation may occur in HSCs during ex vivo expansion, which could impair their ability to self-renew and maintain their stemness by induction of HSC senescence. This assumption is supported by our recent study in which we found that p38 inhibition promoted the ex vivo expansion of the mouse bone marrow HSCs [19]. However, it has been shown that mouse and human HSCs sometimes respond differently to a HSC expansion stimulating agent [20]. Therefore, in the present study, we examined whether p38 inhibition can also promote ex vivo expansion of hUCB HSCs. Our results showed that hUCB CD133+ cells enriched with HSCs exhibited a time-dependent activation of p38 during ex vivo expansion, which was abrogated by SB203580 (SB), a specific p38 inhibitor. More importantly, inhibition of p38 with SB promoted hUCB HSC expansion and engraftment after transplantation into non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. The effect of SB on hUCB HSCs is likely attributed to the inhibition of induction of HSC senescence and apoptosis and improvement of HSC homing via increasing the expression of C-X-C chemokine receptor type 4 (CXCR4) on HSCs. Since numerous small molecules of p38 inhibitors are widely available and some of them have passed phase I and II clinical trials [21], our finding has the potential of clinical translation to improve ex vivo expansion of hUCB HSCs for transplantation.
Materials and methods
CD133+ cell preparation and ex vivo expansion
hUCB was obtained from full-term elective caesarean section volunteers at the Wuhan Union Hospital (Wuhan, Hubei, China) with the consent of the patients and the approval of the local ethics committee equivalent to the Institutional Review Board in the USA. The mononuclear cell population was separated by Ficoll gradient centrifugation, and CD133+ cells were enriched using the CD133+ Microbead Kit (Miltenyi Biotech, Inc. Bisley, Germany) as described previously [21]. The purity of hUCB CD133+ cells was >90%. They were seeded in wells of 12-well plates at 3×104 cells/ml and cultured at 37°C, 5% CO2, and 100% humidity in StemSpan® H3000 medium supplemented with 100 ng/ml recombinant human stem cell factor (Peprotech Co., UK), 100 ng/ml recombinant human thrombopoietin (Peprotech Co.), and 100 ng/ml recombinant human Flt3 ligand (Peprotech Co.) (STF–HSC expansion medium) in the presence of vehicle (0.1% DMSO) or 10 μM SB (Cat # 559389, Calbiochem, San Diego, CA, USA) for 7 days. Half of the culture medium was replaced by the freshly prepared STF–HSC expansion medium along with or without SB every 3 days of culture.
Analysis of p38 activation in CD133+ cells by immunofluorescence microscopy
Freshly isolated CD133+ cells and CD133+ cells harvested from 4 to 7 days of culture were fixed in 4% paraformalde-hyde and then permeabilized with 0.1% Triton X-100 (30 min). After being blocked with 0.3% bovine serum albumin, the cells were incubated with anti-phosphorylated p38 (p-p38) antibody (1:100, Cat # 9211, Cell Signaling Technology Ltd., Beverly, MA, USA) overnight at 4°C. After extensive washing, the cells were incubated with Alexa 555-conjugated goat anti-rabbit antibody (1:200) (Cat # A21430, Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. Hoechst33342 (Cat # B2261, Sigma, St. Louis, MO, USA) was used for nuclei counter staining. Slides were mounted with Vecta shield and cells were viewed and images of the cells were acquired using a Nikon fluorescence microscope equipped with an F-601-AF camera (Nikon, Japan) and processed using the Adobe Photoshop V7.01 software.
Flow cytometric analysis
Freshly isolated and expanded CD133+ cells were stained with the following antibodies against human cells: CD133-APC (Cat # 17-1338-41, Clone TMP4), CD38-PerCP (Cat # 303519, Clone HIT2), CD45-PECy5 (Cat # 304009, Clone HI30), CD33-PE (Cat # 303403, Clone WM53), CD19-FITC (Cat # 302205, Clone HIB19), and CXCR4-PECy5 (Cat # 306508, Clone 12 G5). All of the antibodies were purchased from BioLegend (San Diego, CA, USA) except CD133-APC which was obtained from e-Bioscience (San Diego, CA, USA). Appropriate isotype control antibodies were used as controls for the staining. After staining, cells were analyzed on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA) and the data were analyzed using the Cell Quest software (Becton Dickinson).
Colony-forming cell (CFC) assay
Freshly isolated and expanded CD133+ cells were suspended in Methocult GF H4435 methylcellulose medium (StemCell Technologies, Vancouver, Canada) and triplicate cultures were set up for each specimen according to the manufacturer’s instruction. After 12 to 14 days of culture, the colonies of colony-forming unit-granulocyte macrophage (CFU-GM), burst-forming unit-erythroid, and CFU-granulocyte-erythrocyte-monocyte-megakaryocyte (CFU-GEMM) were counted with an inverted microscope at×400 magnification.
Cobblestone area forming cell (CAFC) assay
Feeder cell stromal layers were prepared by seeding 103/well FBMD-1 stromal cells in each well of flat-bottom 96-well plates (Falcon, Lincoln Park, NJ, USA). One week later, freshly isolated hUCB CD133+ cells or their progenies harvested from 7-day expansion cultures were resuspended in CAFC medium (Iscove’s MDM supplemented with 20% horse serum, 10−5 M hydrocortisone, 10−5 M 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin) and were overlaid on these stromal layers in six dilutions and threefold apart. Twenty wells were plated for each dilution to allow limiting dilution analysis of the precursor cells forming hematopoietic clones under the stromal layer. Cultures were fed weekly by changing one half of the media. The frequencies of CAFC were determined on day 28. Wells were scored positive if at least one phase dark hematopoietic clone (containing five or more cells) was seen. The frequency of day 28 CAFCs representing hematopoietic progenitor and stem cells was calculated using the L-Calc program (StemCell Technologies) as described previously [21].
Transplantation and analysis of human cell engraftment
NOD/SCID mice were obtained from the Institute of Laboratory Animal Sciences (Beijing, China) and bred in the Laboratory Animal Services Center at Huazhong University of Science and Technology. Female NOD/SCID mice at 8 to 10 weeks of age were sublethally irradiated (2.75 Gy) in a 60Co irradiator. Twelve hours after irradiation, 6×104 freshly isolated CD133+ cells or the total cells harvested from cultures initiated with 6×104 input CD133+ cells after they were expanded for 7 days ex vivo with or without p38 inhibition as described above were intravenously injected into an irradiated mouse via the tail vein. Ten weeks after the transplantation, the mice were sacrificed and bone marrow cells from both femurs and tibiae were collected for the analysis of human cell engraftment by flow cytometry.
Analysis of cell division by carboxyfluorescein diacetate succinimidyl ester (CFSE) staining
Freshly isolated CD133+ cells (5×105/ml) were labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester (Invitrogen) for 10 min at 37°C as described previously [22, 23]. The CFSE-labeled cells were cultured in STF–HSC expansion medium with vehicle (0.1% DMSO) or 10 μM SB. After 7 days of culture, the cells were harvested and stained with anti-CD133-APC and analyzed by flow cytometry to measure the fluorescence intensity of CFSE in CD133+ cells.
Apoptotic assay
Apoptosis was assayed by staining the cells harvested from a day-7 culture of CD133+ cells with APC-conjugated anti-CD133 antibody and followed by Annexin V-FITC and propidium iodide staining using the Annexin V-FITC Apoptosis Detection Kit (Cat # APOAF, Sigma) according to the manufacturer’s instructions. Apoptotic cells were determined by flow cytometric analysis on a FACS Calibur (Becton Dickinson) and the data were analyzed using the CellQuest software (Becton Dickinson).
Senescence-associated β galactosidase (SA-β-gal) staining
SA-β-gal activity in freshly isolated CD133+ cells and cells harvested from a day-7 culture of CD133+ cells was determined using a SA-β-gal staining kit (Cat # 9860) from Cell Signaling according to the manufacturer’s instructions. Senescent cells were identified as blue-stained cells under a standard light microscope [24]. A minimum of 1,000 cells were counted in 10 random fields to determine the percentage of SA-β-gal-positive cells.
Real-time RT-PCR
First-strand cDNA was reverse-transcribed with total RNA extracted from freshly isolated CD133+ cells and the cells harvested from a day-7 culture of CD133+ cells using a RNeasy Mini Kit (Qiagen Sciences, Germantown, MD, USA) according to the manufacturer’s instructions. The pre-designed PCR primers for human p16INK4a (p16), p21WAF1 (p21), and β-actin were obtained from Invitrogen. The relative mRNA expression was calculated using the comparative CT (2−ΔΔCt) method as previously described [24]. The sequences of the primers used are: p21—forward 5′-TTAGCAGCG GAACAAGGAGT-3′, reverse 5′-AACGGGAACCAG GACACAT-3′; p16—forward 5′-CCCAACGCACCG AATAGTT-3′, reverse 5′-GCTCCTCAGCCAGGTCCAC-3′; and β-actin—forward 5′-GTCCACCGCAAATGCTTCTA-3′, reverse 5′-TGCTGTCACCTTCACCGTTC-3′.
Cellular migration assay
The assays were performed in duplicate using 5-μm pore polycarbonate transwell culture inserts (Cat # 3421, Costar, Cambridge, MA, USA) as described before [21]. An aliquot of 600 μl serum-free medium containing 100 ng/ml recombinant human stromal cell-derived factor-1 (Peprotech Co.) was added to the lower chambers of the transwells. Freshly isolated or 7-day expanded CD133+ cells (1×105) were loaded into the upper chamber of the transwells. After a 4-h incubation at 37°C in a humidified 5% CO2 environment, the upper chamber was removed and the cells in the bottom chamber were harvested and analyzed by flow cytometry after staining with anti-CD133-APC antibody. The migration rate of CD133+ cells was determined as a percentage of the number of CD133+ cells in the bottom chamber vs. the input as described before [21]. Spontaneous migration without stromal cell-derived factor 1 (SDF-1) addition was also determined as controls.
Statistical analysis
Data are expressed as mean±standard error (SE). Statistical differences were evaluated using analysis of variance, paired t test or Wilcoxon signed rank test, depending on the design and distribution of data, with significance at p ≤ 0.05. The SPSS 17.0 software package was used for the statistical analyses.
Results
Ex vivo expansion of hUCB CD133+ cells activates p38
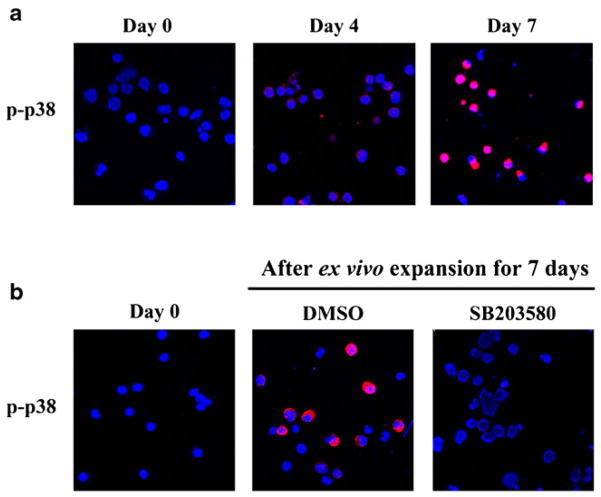
Activation of p38 was detected by immunofluorescence staining using antibodies specifically against p-p38. As shown in Fig. 1a, all the freshly isolated hUCB CD133+ cells were negative for p-p38 staining. After 4 days of culture, almost all the expanded cells exhibited weak staining of p-p38. The intensity of p-p38 staining was dramatically increased after the cells were cultured in vitro for 7 days, indicating that ex vivo expansion of hUCB CD133+ cells causes a time-dependent activation of p38. The activation was abrogated when the cells were cultured with SB (Fig. 1b).
Fig. 1.
Ex vivo expansion of hUCB CD133+ cells activates p38. (a), the activation was abrogated when the cells were cultured with SB (b). hUCB CD133+ cells were cultured in STF–HSC expansion medium with DMSO (0.1%) or SB (10 μM). Activation of p38 was determined by immunofluorescence staining with anti-phosphorylated p38 (p-p38) primary antibody and Alexa fluor-555-conjugated secondary antibody (red). The nuclei of the cells were stained with Hoechst33342 (blue). Representative photomicrographs of the immunofluorescence staining are shown
Inhibition of p38 promotes ex vivo expansion of hUCB CD133+ and CD133+CD38− cells
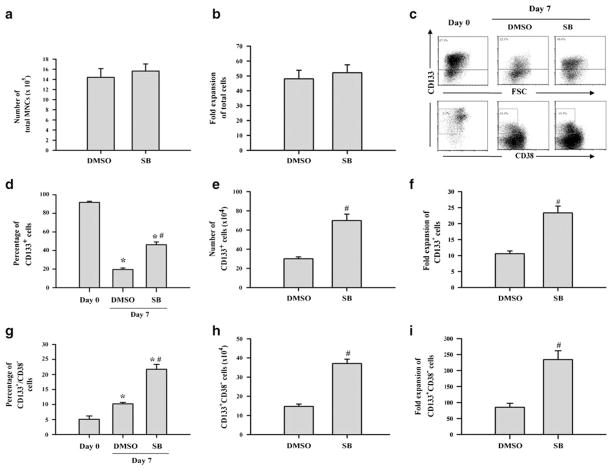
To determine if activation of p38 inhibits human HSC ex vivo expansion, hUCB CD133+ cells were cultured in STF–HSC expansion medium in the presence of vehicle (0.1% DMSO) or SB. After 7 days of culture, the expanded cells were harvested for numeration and analysis of CD133 and CD38 expression by flow cytometry. As shown in Fig. 2a, b, the total number of mononuclear cells (MNCs) was similarly expanded for about 50-fold after 7 days of culture regardless of whether hUCB CD133+ cells were cultured with or without SB (p>0.05). The percentages of CD133+ cells were reduced from about 90% in the freshly isolated CD133+ cells to about 20% and 45% in the expanded cells without and with SB in the culture (Fig. 2c, d), respectively. In contrast, the percentages of CD133+CD38− cells were increased from about 5% in the freshly isolated CD133+ cells to about 10% and 20% in the expanded cells without and with SB in the culture (Fig. 2c, g), respectively. These findings indicate that hUCB CD133+ cells cultured with SB generated significantly more CD133+ and CD133+CD38− cells compared to the cells cultured without SB (p<0.01). In fact, the number of CD133+ cells increased 10- and 23-fold and that of CD133+CD38− cells increased 85- and 234-fold after 7 days of culture in the absence or presence of SB (Fig. 2e, f, h, and i), respectively, compared to those in the input cells (day 0), indicating that p38 inhibition increased the production of CD133+ and CD133+CD38− cells.
Fig. 2.
Inhibition of p38 promotes ex vivo expansion of hUCB CD133+ and CD133+CD38− cells. hUCB CD133+ cells were cultured in STF–HSC expansion medium with DMSO (0.1%) or SB (10 μM) for 7 days. The cells harvested from the cultures were numerated and phenotypically analyzed by flow cytometry. Production (a) and fold expansion (b) of MNCs. Representative flow cytometric analyses (c) and percentage of CD133+ cells (d) and CD133+CD38− cells (g) in freshly isolated hUCB CD133+ cells (day 0) and day-7 expanded cells with DMSO or SB. Production and fold expansion of CD133+ cells (e, f) and CD133+CD38− cells (h, i) after 7 days of expansion. The data are presented as mean±SE (n = 5 independent cultures). All the numbers were calculated based on the cultures with 3×104 input hUCB CD133+ cells. *p<0.01 vs. day 0 cells and #p<0.01 vs. DMSO
Inhibition of p38 promotes the ex vivo expansion of HSCs but not that of hematopoietic progenitor cells (HPCs)
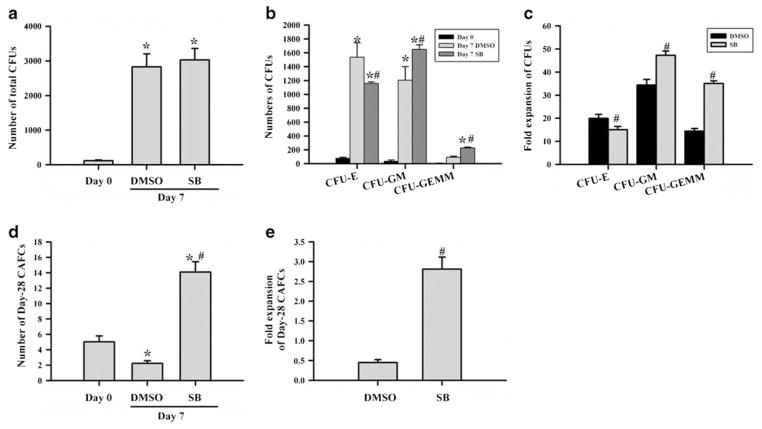
Human CD133+ and CD133+CD38− cells contain both HSCs and HPCs [25, 26]. To determine if inhibition of p38 actually promotes the expansion of hUCB HPCs and HSCs, we analyzed the numbers of CFUs and day-28 CAFCs in the expanded cells as surrogates for HPCs and HSCs, respectively. As shown in Fig. 3a, the number of total CFUs increased more than 24-fold when CD133+ cells were expanded ex vivo in the presence of vehicle (0.1% DMSO) for 7 days compared to that in the input cells. The number of total CFUs was slightly greater after the cells were cultured with SB for the same duration than that without SB, but the difference was not statistically significant (p>0.05). However, the cells expanded in the presence of SB generated significantly more CFU-GEMMs than the cells without SB (p<0.01) (Fig. 3b, c), indicating that inhibition of p38 promotes the expansion of more primitive hematopoietic cells.
Fig. 3.
Inhibition of p38 promotes ex vivo expansion of HSCs but not that of HPCs. hUCB CD133+ cells were cultured in STF–HSC expansion medium with DMSO (0.1%) or SB (10 μM) for 7 days. The number of CFUs and day-28 CAFCs was quantified in freshly isolated hUCB CD133+ cells (day 0) and day-7 expanded cells with DMSO or SB by CFC and day-28 CAFCs assays to measure the expansion of HPCs and HSCs, respectively. Number of total CFUs (a), different types of CFUs (b), and day-28 CAFCs (d) and fold of expansion of various types of CFUs (c) and day-28 CAFCs (e) are presented as mean±SE (n = 5 independent cultures). The numbers of CFUs and CAFCs were calculated based on the culture with 200 and 1,000 input hUCB CD133+ cells, respectively. *p<0.01 vs. day 0 cells and #p<0.01 vs. DMSO
As expected, the cells expanded in the presence of STF without SB resulted in about 50% reduction of day-28 CAFCs compared to the input cells (Fig. 3d, e), indicating a significant reduction in HSCs as shown in numerous previous studies [19, 27]. In contrast, with SB, HSCs had an almost threefold increase in day-28 CAFCs compared to the input cells (Fig. 3d, e). More importantly, this represents a more than sixfold increase in day-28 CAFCs compared to the cells cultured without SB, indicating that p38 inhibition promotes ex vivo HSC expansion.
Inhibition of p38 increases the engraftment of ex vivo expanded hUCB CD133+ cells in NOD/SCID mice
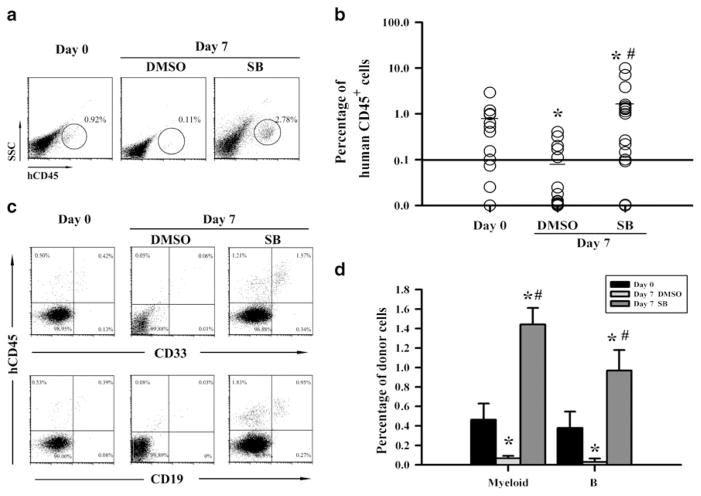
At present, the analysis of human hematopoietic cell engraftment in NOD/SCID mice after transplantation is considered as a more reliable assay for human HSCs. Therefore, to validate whether inhibition of p38 promotes hUCB HSCs ex vivo expansion, we transplanted 6×104 freshly isolated hUCB CD133+ cells or the cells harvested from the cultures with the same number of input hUCB CD133+ cells in the presence or absence of SB into nonlethally irradiated NOD/ SCID mice. Ten weeks after the transplantation, human hematopoietic cell engraftment in the mouse BM was determined by flow cytometry. As shown in Fig. 4a, b, the average human cell engraftment in BM of the mice transplanted with freshly isolated hUCB CD133+ cells was about 0.89%. The engraftment reduced to less than 0.1% after the cells were cultured in vitro without SB. In contrast, ex vivo expansion of hUCB CD133+ cells with SB resulted in more than threefold increase in human cell engraftment compared to the freshly isolated hUCB CD133+ cells. Moreover, the engraftment was multilineages, including both myeloid cells and B lymphocytes, in mice transplanted with fresh hUCB CD133+ cells or the cells expanded with SB (Fig. 4c, d). These findings confirm that inhibition of p38 can promote hUCB HSC ex vivo expansion.
Fig. 4.
Inhibition of p38 increases the engraftment of ex vivo expanded hUCB CD133+ cells in NOD/SCID mice. Nonlethally irradiated NOD/ SCID mice were transplanted with 6×104 freshly isolated hUCB CD133+ cells (day 0) or the cells expanded from the same number of hUCB CD133+ cells for 7 days with DMSO (0.1%) or SB (10 μM). Human hematopoietic cell engraftment in the mouse BM was determined 10 weeks after transplantation by flow cytometry. Representative flow cytometric analyses of human CD45+ hematopoietic cells (a) and CD45+/ CD33+ myeloid cells and CD45+/CD19+ B cells (c) and percentage of CD45+ cells (b) and CD45+/CD33+ myeloid cells and CD45+/CD19+ B cells (d) in mouse BM cells are shown. In b, each data point represents an individual mouse, the short line for each data set is the mean engraftment and the solid line shows the positive engraftment threshold (set at 0.1%). The data presented in d are mean (± SE) engraftment of human myeloid and B cells (n = 12, 16, and 17 for day 0, DMSO-expanded, and SB-expanded cells, respectively). *p<0.01 vs. day 0 cells and #p<0.01 vs. DMSO
The mechanisms of action of SB in promoting ex vivo expansion of hUCB HSCs
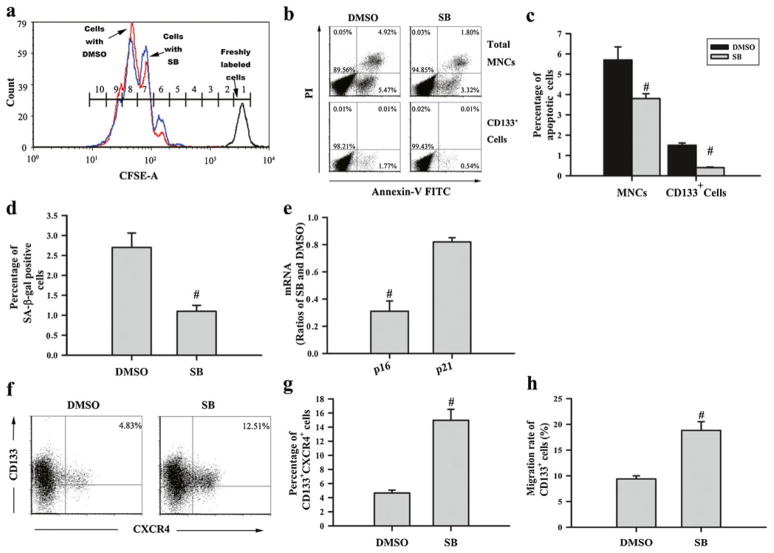
HSC expansion depends on HSC self-renewal proliferation and functional maintenance, which can be negatively affected by HSC differentiation, apoptosis, and senescence [20, 28–30]. To investigate the mechanisms through which p38 inhibition promotes hUCB HSC expansion, we examined the effects of SB on hUCB CD133+ cell proliferation, apoptosis, and senescence. This is because the data presented in Fig. 3a–c have already demonstrated that p38 inhibition does not inhibit HSC differentiation and activation of p38 has been implicated in the regulation of cell proliferation, apoptosis, and senescence, particularly in HSCs [31, 32]. To determine the effect of p38 inhibition on HSC proliferation, we labeled hUCB CD133+ cells with the cell division monitoring dye CFSE and then cultured the cells with or without SB. The fluorescence intensity of CFSE staining of the cells after 7 days of culture was determined by flow cytometry in comparison with that of uncultured cells to quantify the number of cell divisions. As shown in Fig. 5a, the cells from both groups had undergone the same number of cell divisions, suggesting that inhibition of p38 does not promote hUCB CD133+ cell proliferation. This finding is in agreement with the observation that the same number of MNCs was produced by hUCB CD133+ cells when they were cultured with or without SB (Fig. 2a, b). In contrast, significantly lesser hUCB CD133+ cells were stained positive for Annexin V and SA-β-gal when they were cultured with SB compared to the cells cultured without SB (Fig. 5b–d), suggesting that inhibition of p38 inhibits hUCB HSC apoptosis and senescence during ex vivo expansion. The effect of SB on HSC senescence is further supported by the finding that culture of hUCB CD133+ cells with SB reduced their expression of p16 and p21 mRNA (Fig. 5e), since induction of p16 and p21 in HSCs can mediate the induction of HSC senescence [33, 34].
Fig. 5.
Effects of p38 inhibition on hUCB CD133+ cell proliferation, apoptosis, senescence, expression of CXCR4, and migration. hUCB CD133+ cells were cultured in STF–HSC expansion medium with DMSO (0.1%) or SB (10 μM) for 7 days. They were harvested from the culture and analyzed by various assays as described below: a representative analysis of cell divisions by CFSE staining and flow cytometry. The blue line, red line and black line represent the cells cultured with SB, DMSO, or without culture after CFSE labeling, respectively. Numbers in the histogram indicate the number of divisions (divisions 0–9 from right to left) underwent by the cells after 7 days of culture. b Representative analyses of apoptosis in expanded MNCs and CD133+ cells by Annexin V-FITC staining and flow cytometry. c Percentage of apoptotic cells in expanded MNCs and CD133+ cells. d Percentage of SA-β-gal-positive senescent cells in hUCB CD133+ cells after ex vivo expansion. e The ratio of p16 and p21 mRNA expression in expanded hUCB CD133+ cells with and without SB. f Representative analyses of CXCR4 expression in expanded CD133+ cells by flow cytometry. g Percentage of CXCR4-positive cells in hUCB CD133+ cell after ex vivo expansion. h Percentage of expanded hUCB CD133+ cells migrated through the transwell in response to SDF-1 attraction. The data presented in the bar graphs are mean±SE from five independent cultures. #p<0.05 vs. DMSO
In addition, we examined the effect of p38 inhibition on CXCR4 expression in hUCB CD133+ cells after ex vivo expansion to determine if upregulation of CXCR4 expression may also contribute to the enhanced engraftment of the cells expanded with SB. As shown in Fig. 5f, g, about 15% of hUCB CD133+ cells cultured with SB were stained positive for CXCR4 while less than 5% of the cells cultured without SB were positive for CXCR4, confirming that inhibition of p38 increased CXCR4 expression in hUCB CD133+ cells. Moreover, significantly more hUCB CD133+ cells migrated toward SDF-1 through the transwell after they were expanded with SB than the cells without SB (Fig. 5h) (p<0.01). These results demonstrate that inhibition of p38 can increase CXCR4 expression in hUCB CD133+ cells during ex vivo expansion, which may also contribute to the enhanced engraftment of the cells expanded with SB after transplantation by increasing HSC migration and homing.
Discussion
HSCs expand when they can undergo self-renewing proliferation and retain their functions. This occurs if HSC proliferation can be maintained by the stimulation with various hematopoietic growth factors while HSC differentiation, cell death (apoptosis), and senescence are suppressed by a variety of intrinsic and extrinsic factors [35]. Numerous strategies have been explored to promote ex vivo HSC expansion by inhibition of HSC differentiation in order to generate a large number of HSCs to overcome the shortage of HSCs for transplantation and to facilitate hematopoietic engraftment after transplantation. So far, only a few of these strategies have been proven to be successful and cost effective [36]. We reasoned that inhibition of HSC senescence may be exploitable as an alternative strategy to suppression of HSC differentiation for promotion of HSC expansion. This may be achievable by targeted inhibition of p38 activity using a small molecular inhibitor because activation of p38 has been implicated in mediating the induction of HSC senescence under various pathological conditions [9, 11, 12, 37]. This hypothesis is supported by our recent finding in which we showed that mouse BM HSCs exhibited a specific activation of p38 after short-term ex vivo expansion and suppression of p38 activity with a specific p38 inhibitor, e.g., SB, promoted their expansion ex vivo in association with a significant inhibition of HSC senescence [19]. This finding prompted us to extend our studies to examine if inhibition of p38 activity can also promote ex vivo expansion of hUCB HSCs.
As shown in this report, hUCB CD133+ cells cultured in STF–HSC expansion medium exhibited a time-dependent activation of p38. The activation is likely attributable to oxidative stress resulting from culture of hUCB HSCs in vitro in a normoxic condition (20% of O2) [15, 16]. This suggestion is supported by the findings from a separate study which was published recently by our group [38]. In that study, we found that hUCB CD133+ cells exhibited a significant increase in ROS production and p38 activation after ex vivo expansion. Interestingly, inhibition of p38 with SB not only inhibited the activation of p38 but also reduced the production of ROS in the cells after ex vivo expansion. A similar finding was also presented recently at the 53rd Annual Meeting of American Society of Hematology [39]. These findings suggest that ex vivo expansion of hUCB CD133+ cells elevates ROS production that activates p38, which in turn induces further increase in ROS production. Therefore, inhibition of p38 may promote hUCB HSCs ex vivo expansion in part by disrupting the vicious cycle of oxidative stress. Indeed, in the present study, we found that p38 activation in hUCB CD133+ cells after ex vivo expansion was abrogated by the addition of SB. Moreover, after hUCB CD133+ cells were cultured in STF–HSC expansion medium for 7 days, the numbers of CD133+ cells and CD133+CD38− cells expanded 10- and 85-fold, respectively, compared with input cells. However, significantly greater expansion of CD133+ cells (23-fold) and CD133+CD38− cells (234-fold) was observed when SB was included in the culture, suggesting that inhibition of p38 can promote ex vivo expansion of HPCs and HSCs. Interestingly, the cells cultured without SB for 7 days exhibited a more than 20-fold increase in CFUs but a significant reduction in day-28 CAFCs and hematopoietic engraftment after transplantation. This finding suggests that STF can stimulate HPC expansion but cannot maintain HSC self-renewal as shown in numerous previous studies [1]. In contrast, hUCB CD133+ cells cultured with SB for 7 days in STF–HSC expansion medium exhibited threefold and sixfold expansion of day-28 CAFCs and produced about threefold and 30-fold greater human hematopoietic cell engraftment after transplantation into sublethally NOD/SCID mice compared with input cells and the cells cultured without SB, respectively. These findings confirm that p38 inhibition is capable of promoting ex vivo expansion of hUCB HSCs. Similar findings were also observed in mouse bone marrow HSCs [19], suggesting that p38 activation is a conserved mechanism that limits ex vivo expansion of HSCs across different species. The role of p38 in the regulation of HSC ex vivo expansion was also confirmed by a recent study presented at the 53 rd Annual Meeting of American Society of Hematology [39], in which Baudet and Marsson reported that a forward RNAi screen identified p38 as a druggable target for hUCB HSC ex vivo expansion. In addition, they showed that incubation of hUCB CD34+ cells with several different p38 inhibitors including SB under a similar culture condition as ours (e.g., serum-free medium supplemented STF) resulted in a significantly greater expansion of HSCs than the cells cultured without p38 inhibition.
The promotion of hUCB HSC expansion by p38 inhibition is unlikely to be attributable to the increase in hUCB CD133+ cell proliferation, as CD133+ cells underwent similar numbers of cell division and produced similar amounts of total progeny when they were cultured with or without SB. p38 inhibition did not reduce HSC differentiation because hUCB CD133+ cells produced similar numbers of CFUs after 7 days of expansion in the presence or absence of SB. Because it has been shown that p38 functions as a key molecule mediating diverse stimuli-induced cellular senescence via upregulation of p16 in a variety of cells, including HSCs [13, 14], we hypothesize that p38 inhibition promotes hUCB HSC ex vivo expansion probably by inhibiting HSC senescence. This hypothesis is supported by the finding that hUCB CD133+ cells cultured with SB showed significant reduction in SA-β-gal staining and expressed less p16 mRNA than those without SB. SA-β-gal is a widely used biomarker of senescent cells [40] and increased expression of p16 has been implicated in the induction of cellular senescence downstream of p38 activation [11, 12, 37]. In addition, significantly fewer apoptotic cells were detected in CD133+ cells after hUCB CD133+ cells were cultured with SB than the cells without SB, indicating that inhibition of HSC apoptosis may also contribute to the increase in hUCB HSC expansion induced by p38 inhibition. In addition, p38 inhibition increased the expression of CXCR4 in the expanded hUCB CD133+ cells. CXCR4 plays an important role in HSC homing after transplantation via interaction with SDF-1 [41–43]. Upregulation of CXCR4 expression by p38 inhibition may also contribute to the enhanced engraftment of the cells cultured with SB after transplantation. This suggestion is partially supported by the finding that significantly more hUCB CD133+ cells migrated through the transwell in response to SDF-1 attraction after they were expanded in the presence of SB compared with the cells without SB.
All these findings demonstrate that p38 inhibition can exert multiple effects on hUCB HSCs to promote their ex vivo expansion and engraftment after transplantation. Because numerous small molecules of p38 inhibitors are widely available and inexpensive and some of them have already passed phase I and II clinical trials for the treatment of various human diseases [21], p38 inhibition with a specific inhibitor has the potential to become a viable, cost-effective, and safe approach to promote hUCB HSC ex vivo expansion for clinical transplantation. Furthermore, p38 inhibitor may be combined with other small molecules that inhibit HSC differentiation such as SR-1 discovered recently by Boitano et al. [20]. The combination may be more effective in promoting HSC self-renewal and expansion than individual agents because they can inhibit both HSC senescence and differentiation pathways [4, 17]. Such a combined approach may lead to a greater expansion of hUCB HSCs to make HSC transplantation available to more patients and reduce the complications associated with HSC transplantation by decreasing engraftment times to allow more rapid immune reconstitution after transplantation, which is being investigated by our group.
Acknowledgments
The authors thank Mr. Zhihui Liang and Mrs. Huifen Zhu for the flow cytometric analysis. This study was supported in part by a grant from the Chinese National Natural Science Foundation (no. 30871097) to Dr. Lingbo Liu and a grant from the National Institute of Allergy and Infectious Diseases of the United States (R01-AI080421) to Dr. Daohong Zhou.
Footnotes
Conflicts of interest The authors declare no conflicts of interest.
Contributor Information
Jing Zou, Email: liulingbo64@yahoo.com, Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Ping Zou, Email: xys387@163.com, Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Jie Wang, Email: bantoumingblur1985@163.com, Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Lei Li, Email: bud_lee89@yahoo.com.cn, Institute of Pediatrics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Yong Wang, Email: wangy@musc.edu, Department of Pathology, Medical University of South Carolina, Charleston, SC 29425, USA.
Daohong Zhou, Email: dzhou@uams.edu, Division of Radiation Health, Department of Pharmaceutical Sciences and Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Lingbo Liu, Email: liulingbo64@yahoo.com, Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
References
- 1.Kelly SS, Sola CB, de Lima M, Shpall E. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44:673–681. doi: 10.1038/bmt.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, Takahashi TA, Ortega J, Filipovich A, Locatelli F, Asano S, Fagioli F, Vowels M, Sirvent A, Laporte JP, Tiedemann K, Amadori S, Abecassis M, Bordigoni P, Diez B, Shaw PJ, Vora A, Caniglia M, Garnier F, Ionescu I, Garcia J, Koegler G, Rebulla P, Chevret S. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, Bearman SI, Nieto Y, Freed B, Madinger N, Hogan CJ, Slat-Vasquez V, Russell P, Blunk B, Schissel D, Hild E, Malcolm J, Ward W, McNiece IK. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 4.Peffault DLR, Purtill D, Ruggeri A, Sanz G, Michel G, Gandemer V, Maury S, Kurtzberg J, Bonfim C, Aljurf M, Gluckman E, Socie G, Passweg J, Rocha V. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by Eurocord and the Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2011;17:78–85. doi: 10.1016/j.bbmt.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Devine SM, Lazarus HM, Emerson SG. Clinical application of hematopoietic progenitor cell expansion: current status and future prospects. Bone Marrow Transplant. 2003;31:241–252. doi: 10.1038/sj.bmt.1703813. [DOI] [PubMed] [Google Scholar]
- 6.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 7.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 8.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Liu L, Zhou D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res. 2011;176:743–752. doi: 10.1667/rr2727.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somervaille TC, Linch DC, Khwaja A. Different levels of p38 MAP kinase activity mediate distinct biological effects in primary human erythroid progenitors. Br J Haematol. 2003;120:876–886. doi: 10.1046/j.1365-2141.2003.04204.x. [DOI] [PubMed] [Google Scholar]
- 11.Katsoulidis E, Li Y, Yoon P, Sassano A, Altman J, Kannan-Thulasiraman P, Balasubramanian L, Parmar S, Varga J, Tallman MS, Verma A, Platanias LC. Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes. Cancer Res. 2005;65:9029–9037. doi: 10.1158/0008-5472.CAN-04-4555. [DOI] [PubMed] [Google Scholar]
- 12.Saadatzadeh MR, Bijangi-Vishehsaraei K, Kapur R, Haneline LS. Distinct roles of stress-activated protein kinases in Fanconi anemia-type C-deficient hematopoiesis. Blood. 2009;113:2655–2660. doi: 10.1182/blood-2008-09-181420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell Signal. 2006;18:174–182. doi: 10.1016/j.cellsig.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Shao L, Li H, Pazhanisamy SK, Meng A, Wang Y, Zhou D. Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol. 2011;94:24–32. doi: 10.1007/s12185-011-0872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanovic Z, Dello SP, Trimoreau F, Faucher JL, Praloran V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;40:1482–1488. doi: 10.1046/j.1537-2995.2000.40121482.x. [DOI] [PubMed] [Google Scholar]
- 17.Verma A, Deb DK, Sassano A, Uddin S, Varga J, Wickrema A, Platanias LC. Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-beta on normal hematopoiesis. J Biol Chem. 2002;277:7726–7735. doi: 10.1074/jbc.M106640200. [DOI] [PubMed] [Google Scholar]
- 18.Nagata Y, Moriguchi T, Nishida E, Todokoro K. Activation of p38 MAP kinase pathway by erythropoietin and interleukin-3. Blood. 1997;90:929–934. [PubMed] [Google Scholar]
- 19.Wang Y, Kellner J, Liu L, Zhou D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011;20:1143–1152. doi: 10.1089/scd.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alakel N, Jing D, Muller K, Bornhauser M, Ehninger G, Ordemann R. Direct contact with mesenchymal stromal cells affects migratory behavior and gene expression profile of CD133+ hematopoietic stem cells during ex vivo expansion. Exp Hematol. 2009;37:504–513. doi: 10.1016/j.exphem.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Araki H, Yoshinaga K, Boccuni P, Zhao Y, Hoffman R, Mahmud N. Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007;109:3570–3578. doi: 10.1182/blood-2006-07-035287. [DOI] [PubMed] [Google Scholar]
- 24.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Et A. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao CL, Feng YH, Lin XZ, Chu IM, Hsieh TB, Hwang SM. Characterization of serum-free ex vivo-expanded hematopoietic stem cells derived from human umbilical cord blood CD133(+) cells. Stem Cells Dev. 2006;15:70–78. doi: 10.1089/scd.2006.15.70. [DOI] [PubMed] [Google Scholar]
- 27.Araki H, Baluchamy S, Yoshinaga K, Petro B, Petiwala S, Parajuli R, Milhem M, Lavelle D, DeSimone J, Mahmud N. Cord blood stem cell expansion is permissive to epigenetic regulation and environmental cues. Exp Hematol. 2009;37:1084–1095. doi: 10.1016/j.exphem.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauvageau G, Iscove NN, Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–7232. doi: 10.1038/sj.onc.1207942. [DOI] [PubMed] [Google Scholar]
- 29.Hofmeister CC, Zhang J, Knight KL, Le P, Stiff PJ. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39:11–23. doi: 10.1038/sj.bmt.1705538. [DOI] [PubMed] [Google Scholar]
- 30.Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18:1760–1768. doi: 10.1038/sj.leu.2403515. [DOI] [PubMed] [Google Scholar]
- 31.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 32.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 33.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akala OO, Clarke MF. Hematopoietic stem cell self-renewal. Curr Opin Genet Dev. 2006;16:496–501. doi: 10.1016/j.gde.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083–6090. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cirillo PF, Pargellis C, Regan J. The non-diaryl heterocycle classes of p38 MAP kinase inhibitors. Curr Top Med Chem. 2002;2:1021–1035. doi: 10.2174/1568026023393390. [DOI] [PubMed] [Google Scholar]
- 38.Zou J, Zou P, Lou Y, Xiao Y, Wang J, Liu L. The cross-talk between ROS and p38MAPKα in the ex vivo expanded human umbilical cord blood CD133(+) cells. J Huazhong Univ Sci Technol Med Sci. 2011;31:591–595. doi: 10.1007/s11596-011-0566-1. [DOI] [PubMed] [Google Scholar]
- 39.Baudet A, Larsson J. A forward RNAi screen identifies p38 MAP kinase as a druggable target for expansion of human hematopoietic stem cells. Blood. 2011;118(21):1016. abstract #2352. [Google Scholar]
- 40.Sauvageau G, Humphries RK. Medicine. The blood stem cell Holy Grail? Science. 2010;329:1291–1292. doi: 10.1126/science.1195173. [DOI] [PubMed] [Google Scholar]
- 41.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 42.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 43.Mobest D, Goan SR, Junghahn I, Winkler J, Fichtner I, Hermann M, Becker M, de Lima-Hahn E, Henschler R. Differential kinetics of primitive hematopoietic cells assayed in vitro and in vivo during serum-free suspension culture of CD34+ blood progenitor cells. Stem Cells. 1999;17:152–161. doi: 10.1002/stem.170152. [DOI] [PubMed] [Google Scholar]