Abstract
This study documents the identity of an intriguing transduction mechanism of the [Ca2+]i signals by the photoreceptor ROS-GC1. Despite their distal residences and operational modes in phototransduction, the two GCAPs transmit and activate ROS-GC1 through a common Ca2+ transmitter switch (Ca2+TS). A combination of immunoprecipitation, fluorescent spectroscopy, mutational analyses and reconstitution studies has been used to demonstrate that the structure of this switch is 657WTAPELL663. The two Ca2+ signaling GCAP pathways converge in Ca2+TS, get transduced, activate ROS-GC1, generate the LIGHT signal second messenger cyclic GMP and yet functionally perform divergent operations of the phototransduction machinery. The findings define a new Ca2+-modulated photoreceptor ROS-GC transduction model; it is depicted and discussed for its application to processing the different shades of LIGHT.
Keywords: Phototransduction, ROS-GC1, Ca2+, GCAP1, GCAP2, Membrane guanylate cyclase
Introduction
Vertebrate rods and cones are highly differentiated neurons that generate electric signals in response to captured photons. The biochemical process by which light signal is converted into electrical signal is called phototransduction. The second messenger of the PHOTON signal is cyclic GMP.
Source of this cyclic GMP is the photoreceptor ROS-GC membrane guanylate cyclase. Its landmark discovery demonstrated that ROS-GC differed in its key feature from, at the time the only known, surface receptor membrane guanylate cyclase family of not being a peptide hormone receptor [reviewed in: 1] but being modulated by the physiological concentrations of free Ca2+ present in rods and cones; it was inhibited by nM range of [Ca2+]i, a key feature of this machinery. It acquired this inhibitory feature through its unique composition: Ca2+ sensor (GCAP) and the transducer ROS-GC. GCAP captured the [Ca2+]i signal, transmitted it to and inhibited ROS-GC [reviewed in: 2].
With these findings, the membrane guanylate cyclase family branched into two subfamilies: peptide hormone receptor, regulated by the extracellular peptide hormone signal; and phototransduction-linked ROS-GC, modulated by the intracellular [Ca2+]i signal. These findings also demonstrated that the prototype transduction template of the receptor ANF-RGC membrane guanylate cyclase at both the structural and functional levels was not applicable to ROS-GC [reviewed in: 1].
Further broadening of the field came with the identification of Ca2+-modulated ONE-GC present in the cilia of the olfactory neurons [3]. With the discovery that in addition to being Ca2+-modulated ONE-GC is also modulated by odorant uroguanylin [4,5], the third subfamily of membrane guanylate cyclase was disclosed; it inherited the features of both Receptor and ROS-GC subfamilies. However, importantly, the Ca2+-modulated inhibitory feature of ROS-GC was missing and it inherited only the Ca2+-modulated stimulatory feature.
With these advancements, the membrane guanylate cyclase transduction field has now been firmly linked with the cardio-renal physiology [reviewed in:6,7], with phototransduction [reviewed in: 2,8,9]; and with the ODORANT signaling [reviewed in: 10,11].
An original finding is that ROS-GC1 is a bimodal Ca2+-transduction switch controlled in opposed fashions by GCAPs and CD-GCAPs (Ca2+-dependent GCAP) [12]. Two such CD-GCAPs are S100B and neurocalcin δ. The former is present in the presynaptic ends of photoreceptors [13], the latter in the retinal bipolar and ganglion neurons [14]. Both are controlled by the physiological range of [Ca2+]i with nearly equal K1/2 values between 200 nM to 800 nM. A rise of 50 to 100 nM [Ca2+]i turns “OFF” and 200 to 800 nM turns “ON” the ROS-GC1 transduction switch.
The photoreceptor ROS-GC1 operation is solely controlled by two GCAPs. Its almost exclusive presence in rods [15] and total exclusiveness in cones [16] indicate that it is the dominant driving force of the phototransduction machinery. Specific mutations in its gene cause Leber's congenital amaurosis and multiple cone rod dystrophies [reviewed in: 17].
Being also present outside the retina -- in bovine pineolocytes [18], rat olfactory bulb [19], bovine spermatogenic cells [20] and with the finding that through its catalytic domain it is also modulated by bicarbonate [21] -- the developing picture is that ROS-GC1 transduction system is multifunctional, multimodular and is globally present in sensory and sensory-linked neurons. It is a general transducer of the Ca2+signals, yet occasionally it may transduce other signals such as that of bicarbonate. Its central function is to generate cyclic GMP, a second messenger of the multiple neuron-specific functions. A universal theoretical neuronal signaling scheme has been proposed. Its key components are the [Ca2+]I, its bimodal ROS-GC1 transduction switch and the CNG-gated channel. Ca2+ pulse in a given neuron is sensed by a GCAP or a CD-GCAP, it decelerates or accelerates the ROS-GC transduction system and through its second messenger cyclic GMP closes or opens the nearby CNG channel causing hyper- or depolarization of the neuronal plasma membrane [Fig.2 in: 1].
The present investigation focuses on the photoreceptor ROS-GC1. It provides biochemical explanation of the means by which ROS-GC1 senses the divergent LIGHT-dependent [Ca2+]i signals, transmits them to a Ca2+ transduction switch, and sends the information to the catalytic module for final translation into the production of the second messenger, cyclic GMP. The findings disclose a new LIGHT-dependent signal transduction model of the membrane guanylate cyclase family.
Materials and Methods
Mutagenesis
Deletion and point mutants of ROS-GC1 were constructed using Quick-change mutagenesis kit (Stratagene) and appropriate mutagenic primers. The mutations were verified by sequencing.
Expression and guanylate cyclase activity assay
ROS-GC1 and its mutants were expressed in COS cells. Their membrane fraction was assayed for guanylate cyclase activity [12,13,18]. The cyclic GMP formed was quantified by radioimmunoassay [22].
YFP-tagged GCAP1 and GCAP2
Recombinant YFP-GCAP1 was constructed as in [23]. YFP-GCAP2 was obtained identically, except that 5′- TCAGATCTCGAGCTCAAGCTTGCCAGGATGG-GGCAGCAGTT-3′ forward primer and 5′-CGTCGACTGCAGAATTCGACAGAACATGGCACTT-TTCCGCC-3′ reverse primer were used for amplification of GCAP2 coding sequence. The amplified sequence was inserted into pZsYellow1-N1 vector.
Immunocytochemistry
YFP-GCAP1 and YFP-GCAP2 were expressed individually or together with ROS-GC1 or its Δ657WTAPELL663 mutant in COS cells grown in coverslip chambers in DMEM medium supplemented with 10% fetal bovine serum. 72 hr after transfection the cells were viewed directly or fixed in 4% paraformaldehyde in Tris-buffered saline (TBS) for 15 min at room temperature. The fixed cells were washed with TBS, blocked in 10% preimmune donkey serum in TBS/0.5% Triton X-100 (TTBS) for 1hr at room temperature, washed with TTBS, incubated with ROS-GC1 antibody (diluted 50:1) in blocking solution overnight at 4°C, washed with TTBS and then incubated with DyLight 649-conjugated donkey anti-rabbit antibody (200:1) for 1 hr and washed with TTBS. Images were acquired using an inverted Olympus IX81 microscope/FV1000 Spectral laser confocal system, and analyzed using Olympus FluoView FV10-ASW software. Digital images were processed using Adobe Photoshop software.
Expression of GCAP1 and GCAP2 was as described previously [23,24].
Co-immunoprecipitation
Membranes of COS cells expressing ROS-GC1 or its Δ657WTAPELL663 mutant were isolated and solubilized in a buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100 and 2 mM PMSF. The solubilized membranes were incubated overnight at 4°C with GCAP1 or GCAP2 bound to their respective affinity purified antibodies coupled to AminoLink®. The AminoLink-antibody-antigen complexes were washed several times with the 20 mM Tris-HCl/150 mM NaCl buffer pH 7.5 containing 1 mM EGTA. Bound antigens were eluted using SDS-sample buffer, separated through SDS-PAGE, transferred to nitrocellulose membrane, and probed with antibodies against ROS-GC1.
Results
Based on the ANF-RGC signaling template that the WTAPELL motif is critical for its regulatory catalytic activity [25] and the fact that this motif is conserved in all membrane guanylate cyclases, the question was raised: would the 657WTAPELL663 motif be also critical in ROS-GC1 signaling? What follows is the systematic analysis of this issue.
To assess the role of the 657WTAPELL663 motif in ROS-GC1 signaling a mutant with this segment deleted (Δ657WTAPELL663) was constructed and analyzed for Ca2+-modulated activity.
The 657WTAPELL663 motif has no role in basal ROS-GC1 activity
Before using the mutant for the study designed, it was verified that the deletion did not affect membrane targeting and/or is not lethal for the enzymatic integrity of the expressed protein.
The wt-ROS-GC1 and the Δ657WTAPELL663 mutant were expressed in COS cells and their guanylate cyclase activities determined. They were practically identical, 117 and 123 pmol cyclic GMP formed minute−1 mg−1 protein for the wt and the mutant protein, respectively. Mock transfected membranes showed very low activity, 0.3 pmol cyclic GMP formed minute−1 mg−1 protein. Thus, the deletion had no role on the basal structural integrity of the guanylate cyclase. This conclusion was validated by assessing the mutant's substrate characteristics. The Km value for GTP was 605 μM, a value comparable to 614 μM of wt-ROS-GC1.
657WTAPELL663 motif is critical for the Ca2+ signaling of ROS-GC1
To assess if the 657WTAPELL663 motif plays a role in the Ca2+-modulated signaling processes of its sensors, GCAP1 and GCAP2, the membranes of COS cells expressing wt-ROS-GC1 or the Δ657WTAPELL663 mutant were analyzed for GCAP1- and GCAP2-dependent activity.
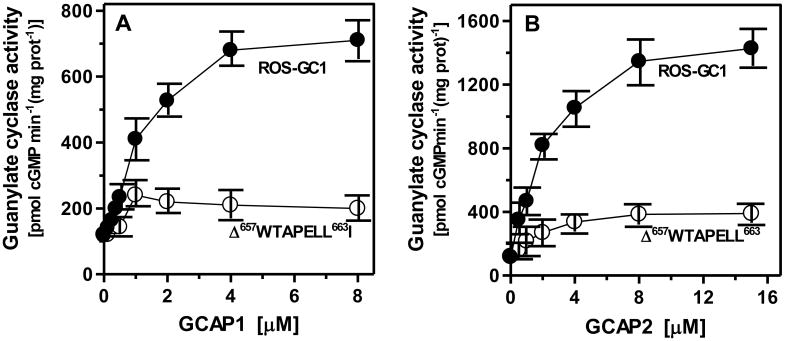
Ca2+-free GCAP1 stimulated ROS-GC1 in a dose dependent fashion with an EC50 of ∼0.6 μM and the maximal activation of about 6-fold above the basal value (Fig. 1A: closed circles). GCAP1 was, however, without almost any effect on the activity of the deletion mutant (Fig. 1A: open circles). The observed stimulation of ∼0.5-fold (10%) above the basal activity was negligible, within the experimental error. Therefore, the 657WTAPELL663 motif is absolutely critical for the GCAP1-modulated Ca2+ signaling activity of ROS-GC1.
Figure 1. Role of the 657WTAPELL663 motif in GCAP signaling of ROS-GC1 activity.
Wt-ROS-GC1 and the Δ657WTAPELL663 mutant were individually expressed in COS cells and their membranes were analyzed for GCAP1 (A) and GCAP2 (B) -dependent cyclase activity. The experiment was done in triplicate and repeated three times. The results presented (mean ± SD) are from these experiments.
Similar to GCAP1, Ca2+-free GCAP2 stimulated wt-ROS-GC1 activity in a dose dependent fashion with an EC50 of 2 μM and Vmax of ∼1400 pmol cyclic GMP formed min−1 mg−1 protein (Fig. 1B: closed circles). Under the same conditions the Δ657WTAPELL663 mutant's stimulation was ∼75% lower (Fig. 1B: open circles). Thus, like GCAP1, the 657WTAPELL663 motif is also critical for the GCAP2-modulated Ca2+ signaling activity of ROS-GC1. Therefore, it is concluded that the motif controls the total Ca2+-signaling activity of ROS-GC1.
The deletion of the 657WTAPELL663 motif does not affect GCAPs binding to ROS-GC1
Two possible mechanisms by which the 657WTAPELL663 motif could control the Ca2+-modulated ROS-GC1 activity were considered: ONE, through its direct binding to GCAPs; TWO, through being a common transducer of the GCAPs-mediated Ca2+ signal. Both mechanisms were novel and intriguing. Consider ONE: The GCAPs binding domains in ROS-GC1 have been determined. They are L503-I522 for GCAP1 [26] and Y965-Y981 for GCAP2 [24]. Then: Is the WTAPELL domain an additional and common for GCAP1 and GCAP2 binding domain? Consider TWO. Is the WTAPELL motif critical for transmitting the binding signals of both GCAPs?
These possibilities were analyzed in vivo by monitoring the interaction in co-transfected cells and in vitro by co-immunoprecipitation. In both types of analyses wt-ROS-GC1 served as positive control.
Interaction in co-transfected cells
GCAP1 and GCAP2 were tagged with yellow fluorescence protein (YFP) and expressed alone or together with wt-ROS-GC1 or its Δ657WTAPELL663 mutant in COS cells. The expression of GCAP1 and GCAP2 was monitored by YFP fluorescence; and of ROS-GC1 or the mutant, through immunostaining with ROS-GC1 antibody.
Expressed alone GCAP1-YFP and GCAP2-YFP were evenly scattered in all cellular compartments including nucleus (Fig. 2A and 2F). Identical pattern of expression has been observed earlier for GCAP1 [23,27]. When co-expressed with ROS-GC1 or its deletion mutant the YFP-GCAPs fluorescence migrated to the membranes (Fig. 2B, 2D for GCAP1 and 2G, 2I for GCAP2) and followed the pattern of ROS-GC1 or the Δ657WTAPELL663 mutant's expression in these cells (compare figure 2B with 2C, 2D with 2E, 2G with 2H, and 2I with 2J). These results demonstrate that the WTAPELL motif does not control the binding characteristics of any GCAP to its ROS-GC1 domain. This conclusion was validated by the co-immunoprecipitation experiments.
Figure 2. GCAP1 and GCAP2 bind ROS-GC1 and its 657WTAPELL663 deletion mutant in COS-cells.
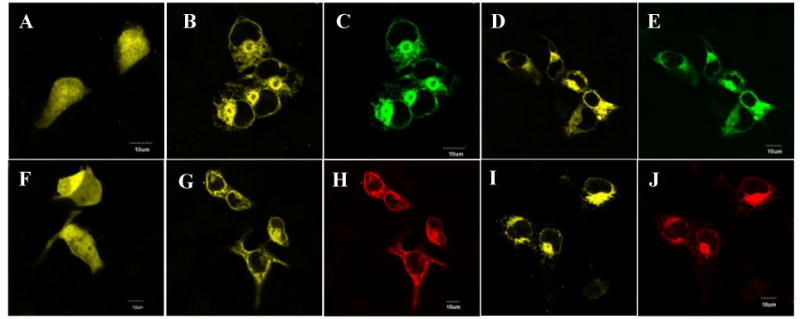
COS-cells were transfected with GCAP1-YFP, GCAP2-YFP or/and wt-ROS-GC1 or the Δ657WTAPELL663 mutant cDNAs. 72 hr after transfection the cells were viewed directly (laser excitation at 515 nm for YFP) or fixed and incubated with ROS-GC1 antibody followed by incubation with secondary antibodies conjugated with DyLight 649 (excitation at 643 nM). The cells were viewed using an inverted Olympus IX81 microscope/FV1000 Spectral laser confocal system. (A) GCAP1-YFP. (B) GCAP1-YFP co-expressed with ROS-GC1. (C) ROS-GC1. (D) GCAP1-YFP co-expressed with Δ657WTAPELL663 mutant. (E) Δ657WTAPELL663 mutant. (F) GCAP2-YFP. (G) GCAP2-YFP co-expressed with ROS-GC1. (H) ROS-GC1. (I) GCAP2-YFP co-expressed with Δ657WTAPELL663 mutant. (J) Δ657WTAPELL663 mutant. In figures (C) and (D) red color was digitally converted to green.
Co-immunoprecipitation
The COS cells membranes expressing wtROS-GC1 or the Δ657WTAPELL663 mutant were solubilized and incubated in the presence of 1 mM EGTA with GCAP1 and affinity purified GCAP1 antibody coupled to AminoLink® coupling gel or GCAP2 and its AminoLink®- coupled antibody. The immunoprecipitated complexes were separated from the coupling gel and analyzed by Western blotting using anti ROS-GC1 antibody. A single ROS-GC1 immunoreactive band was present in complexes precipitated with GCAP1 and GCAP2 antibody regardless whether ROS-GC1 or the Δ657WTAPELL663 mutant was present in the reaction mixture (Supplemental figure 1).
These results establish the validity of mechanism TWO, which is that 657WTAPELL663 is a common transduction motif of both GCAPs in the Ca2+ signaling of ROS-GC1.
W657 of the motif is the key [Ca2+]i signaling node of ROS-GC1
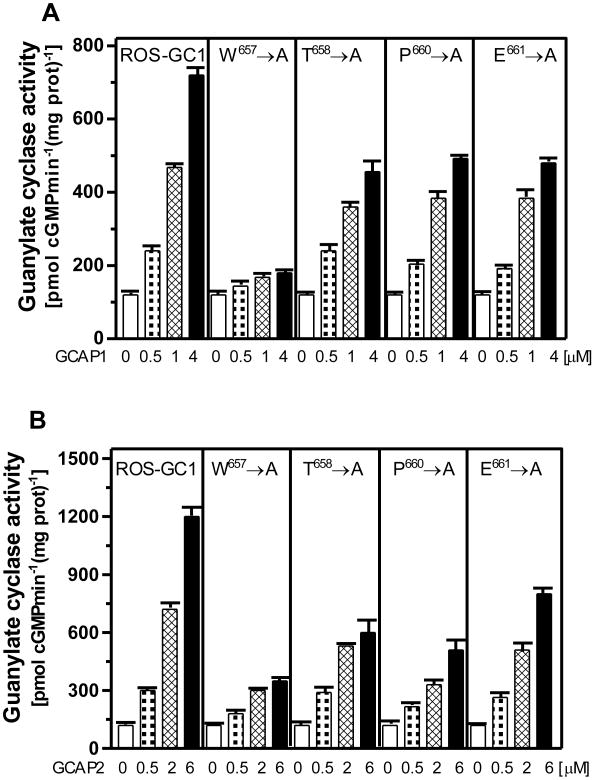
To assess the signaling role of the individual residues in the 657WTAPELL663 motif, alanine scanning was performed. The W657A, T658A, P670A and E671A mutants were individually expressed in COS cells and analyzed for their GCAP1- and GCAP2-dependent Ca2+ signaling activities.
The basal activities of the mutants ranged between 110 and 120 pmol cyclic GMP formed min− 1mg−1protein and were comparable with the activity wt-ROS-GC1 (117 pmol cyclic GMP formed/min/mg protein). Thus, these mutants were properly and equally targeted to cell plasma membrane and were suitable for the comparative analysis.
In the presence of Ca2+-free GCAP1 all the mutants exhibited lower than wt-ROS-GC1 maximal stimulated activity (Fig. 3A). The W657A mutant was the least responsive. Compared to the wt ROS-GC1, its maximal response to GCAP1 was only 27%, indicating that it controlled 73% of the total GCAP1-mediated Ca2+ signaling catalytic activity of ROS-GC1 (Fig. 3A). Each of the other mutants--T658A, P660A, and E661A -- responded with about 60% of the wt ROS-GC activity (Fig. 3A), indicating that their contribution to the total ROS-GC activity was ∼40%.
Figure 3. Alanine scanning of the 657WTAPELL663 motif reveals the importance of the W657 residue in GCAP signaling of ROS-GC1 activity.
The ROS-GC1 W657A, T658A, P670A, and E671A mutants were expressed in COS cells which membranes were analyzed for GCAP1 (A) and GCAP2 (B) -dependent cyclase activity. Wt-ROS-GC1 served as control. The experiment was done in triplicate and repeated three times. The results presented (mean ± SD) are from these experiments.
Similar signaling pattern was observed for GCAP2 (Fig. 3B): The W657A mutant was the least responsive, achieving about 30% of the wt ROS-GC1 saturated activity; the other mutants responded with 55-65% of the maximal catalytic activity.
It is therefore concluded that all the four residues of the 657WTAPELL663 motif are important for the Ca2+-modulated signaling activities of the GCAPs, yet, the most critical is the W657 residue accounting for about 70% of the total signaling activity. Is its aromatic character the cause of this significance?
To test this possibility, W657 residue was mutated to phenylalanine and the GCAP1- and GCAP2-dependent activities of the W657F mutant were assessed. The mutant was stimulated in a dose-dependent fashion and mimicked the wt-ROS-GC1 response to both GCAPs (Supplemental figure 2). These results establish that the 657WTAPELL663 motif is the common Ca2+ transmitter switch (Ca2+TS) of the GCAPs in ROS-GC1.
Discussion
Cued by the findings on the signaling mechanism of the prototype membrane guanylate cyclase ANF-RGC [25], the presented study demonstrates that the 657WTAPELL663 motif of the photoreceptor ROS-GC1 is also vital for its regulatory activity. This finding has a Core Impact on the membrane guanylate cyclase field; and illuminates a basic principle of the Phototransduction machinery by which it controls different shades of the LIGHT signal.
Core Impact
The study exposes a common structural characteristic between the two guanylate cyclases, yet they are linked to totally diverse functions. One, ANF-RGC is a surface receptor membrane guanylate cyclase that is a key regulator of the physiological processes of blood pressure, fluid secretion and smooth muscle relaxation. ROS-GC1 appears to be a general intracellular Ca2+ signal transducer in many sensory neurons, specifically linked with operation of the phototransduction machinery. Two, ANF-RGC is a single component transduction system; ROS-GC subfamily is the two component system. Three, post transmembrane signaling events of ANF-RGC are tightly controlled by ATP; of ROS-GC1 by [Ca2+]i, through its two Ca2+ sensors: GCAP1 and GCAP2. Four, the signal transmission in ANF-RGC is unidirectional flowing downward to the catalytic domain; in ROS-GC1, it is bidirectional: downward for GCAP1 and upward for GCAP2 (Fig. 4). Five, the orientation of the WTAPELL motif in two guanylate cyclases: in ANF-RGC it is located before the catalytic domain; in ROS-GC1 it is wedged between two GCAP sites.
Figure 4. 657WTAPELL663 motif of ROS-GC1 is a common Ca2+ transduction switch (Ca2+TS) of the two GCAP pathways.
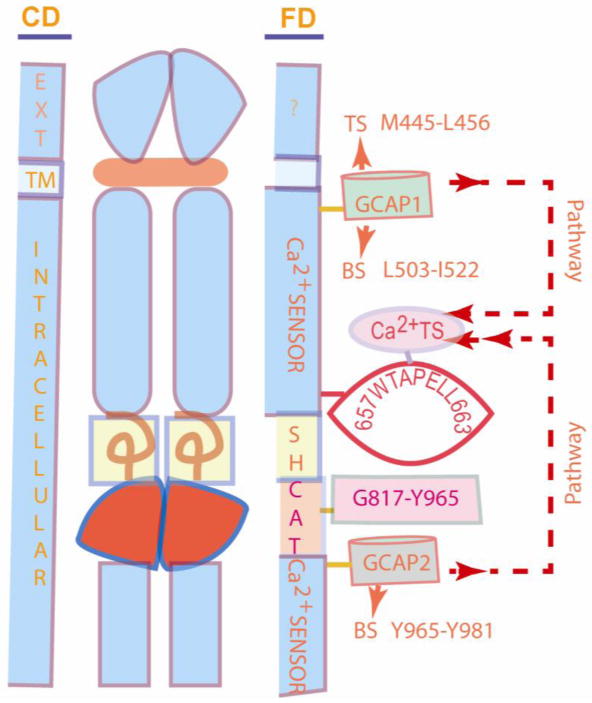
Middle panel is the topographical sketch of ROS-GC1. CD (left panel) names the cellular domains: EXT, extracellular; TM, transmembrane; Intracellular. FD (right panel) names the functional domains: The domains of Ca2+-sensors: GCAP1- Transduction site (TS), M445-L-; Binding site (BS), L503-I522; GCAP2- BS, Y965-Y981, are at the opposite sides of the catalytic domain (CAT) G817-Y965. SH represents the Signaling Helix domain. Ca2+TS domain is represented by the 657WTAPELL663 motif. The CD and the FD boundaries are not represented proportionately. The two GCAP pathways run in opposite directions, shown by the arrows with dashed lines. These pathways converge on Ca2+TS, which serves as their receiver, processor and transmitter to CAT domain. There, they are translated into the LIGHT signal's second messenger, cyclic GMP. It is predicted that the pathways never overlap because they are temporally distinct.
Phototransduction Machinery
Photoreceptor ROS-GC1 transduction system is the driving force of phototransduction. Its absence disables and its malfunction creates abnormalities in the perception of LIGHT. Its operation is well understood. Under Dark Current, cyclic GMP generated through ROS-GC1 basal activity keeps a fraction of CNG channels of the rods and cones open. This allows the influx of Na2+ and Ca2+. The LIGHT signal triggers activation of cyclic GMP-specific phosphodiesterase and hydrolyzes cyclic GMP. The decrease in cyclic GMP causes the channels to close preventing influx of Na+ and Ca2+. Because the extrusion of Ca2+ continues through the Na2+/Ca2+,K+-exchanger, the [Ca2+]i declines from 250 nM to about 25 nM [28]. The decline hyperpolarizes the rod and cone plasma membranes, converts the LIGHT signal into the electric signal; and concomitantly, signals activation of ROS-GC1 to restore cyclic GMP to its Dark Current level. It appears that GCAP1 undergoes a transition from being a sensor of [Ca2+]i in the Dark current state to being a sensor of Mg2+ in the illuminated state [reviewed in: 29].
This general phototransduction model has now been upgraded to a new “Ca2+-relay model” of ROS-GC1 activation (Fig. 1 in [8] and Fig. 2 in [1]). The key advancement in this model is that the LIGHT signal-induced fall of [Ca2+]i switches ROS-GC1 from a GCAP1 mode to a GCAP2 mode; in this manner it calibrates the production of cyclic GMP thus, the perception of luminosity. In the Dark state both GCAPs are bound to the ROS-GC1 dimer. The ROS-GC1 activity is sufficient to maintain the Dark concentration of cyclic GMP. Illumination of rods or cones results in the decreased level of cyclic GMP and, in turn, to a light-intensity- dependent fall in [Ca2+]i. At the intermediate fall level, only GCAP1 is an activator of ROS-GC1. Stronger light-intensity-dependent further fall of [Ca2+]i switches GCAP2 into an activator. In this manner gradation in light intensity level oscillates ROS-GC1 from a “GCAP1 mode” to a GCAP2 mode” or vice versa.
Because the domains of two GCAPs are far apart, on the opposite sides of the catalytic domain (Fig. 4), and they perform specific non-overlapping tasks (vide supra), the natural wisdom would be that the signaling pathways of these two GCAPs are separate, processed through distinct mechanisms. The core of the present finding is that this is not the case. Both pathways use the common transduction motif 657WTAPELL663 as the receiver, processor and transmitter of the divergent Ca2+ signals to the catalytic domain, G817-Y965, where they are translated into the production of cyclic GMP. This 657WTAPELL663 motif is named Ca2+ TS (Ca2+ Transmitter Switch).
The key node of Ca2+TS is W657. It controls almost 80% of GCAP1-modulated and 70% of the GCAP2-modulated Ca2+ signaling activity of ROS-GC1. Other nodes—T658, P660 and E661—to a lesser degree also influence the Ca2+TS activity. However, all these nodes of the SWITCH are needed for its full signaling activity.
The discovery of Ca2+TS and the finding that it is the transducer of both GCAP-mediated Ca2+ signaling pathways brings the phototransduction field to the next level and paves the way for the next question: How do the two GCAP-modulated divergent pathways show functional specificity in adjusting the LIGHT signal luminosity?
The answer may lie in the fact that the two pathways never occur at the same moment. GCAP1 pathway occurs only to sense the bright and intermediate state of the luminosity and the GCAP2 pathway only for sensing the dim light (Fig. 1 in [2]). The functional discrimination exists in their binding characteristics and their migratory pathways to the destination of Ca2+TS (Fig. 4). GCAP1 binding to its domain, L503-I522, occurs at one-order magnitude higher [Ca2+]i than that of GCAP2 to its domain, Y965-Y981. And, then, the GCAP1 pathway to Ca2+TS passes through the segment between the residues 523 to 656 and of GCAP2 through the segment between the residues 964 to 664. These provide specificity to their functionality. Migration from Ca2+TS to the core catalytic domain of two GCAP pathways is identical. In this manner the two GCAPs and ROS-GC1 are locked to sense and process the light-dependent Ca2+ signals and translate them into adequate levels of cyclic GMP. The migration patterns of the two Ca2+ pathways are depicted in figure 4.
Supplementary Material
Highlights.
Documents identity of an intriguing transduction mechanism of the [Ca2+]i signals by the photoreceptor ROS-GC1.
Mechanism has a Core and Specific Impact on the signal transduction field.
Core Impact. It exposes a common structural transduction switch through which all cyclases function.
Specific Impact. Provides a function-specific mechanism for the switch in photoreceptor machinery.
Defines a model how the switch senses and processes different shades of light.
Acknowledgments
This work was supported by Public Health Service grants HL084584 and HL084584S and an equipment grant from the Lions of Pennsylvania Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma RK. Membrane guanylate cyclase is a beautiful signal transduction machine: overview. Mol Cell Biochem. 2010;334:3–36. doi: 10.1007/s11010-009-0336-6. [DOI] [PubMed] [Google Scholar]
- 2.Koch KW, Duda T, Sharma RK. Ca(2+)-modulated vision-linked ROS-GC guanylate cyclase transduction machinery. Mol Cell Biochem. 2010;334:105–115. doi: 10.1007/s11010-009-0330-z. [DOI] [PubMed] [Google Scholar]
- 3.Duda T, Jankowska A, Venkataraman V, Nagele RG, Sharma RK. A novel calcium-regulated membrane guanylate cyclase transduction system in the olfactory neuroepithelium. Biochemistry. 2001;40:12067–12077. doi: 10.1021/bi0108406. [DOI] [PubMed] [Google Scholar]
- 4.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duda T, Sharma RK. Ca2+-modulated ONE-GC odorant signal transduction. FEBS Lett. 2009;583:1327–1330. doi: 10.1016/j.febslet.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 8.Koch KW, Duda T, Sharma RK. Ca(2+)-modulated vision-linked ROS-GC guanylate cyclase transduction machinery. Mol Cell Biochem. 2010;334:105–115. doi: 10.1007/s11010-009-0330-z. [DOI] [PubMed] [Google Scholar]
- 9.Detwiler P. Open the loop: dissecting feedback regulation of a second messenger transduction cascade. Neuron. 2002;36:3–4. doi: 10.1016/s0896-6273(02)00940-6. [DOI] [PubMed] [Google Scholar]
- 10.Sharma RK, Duda T. Odorant-linked ROS-GC subfamily membrane guanylate cyclase transduction system. Mol Cell Biochem. 2010;334:181–189. doi: 10.1007/s11010-009-0333-9. [DOI] [PubMed] [Google Scholar]
- 11.Zufall F, Munger SD. Receptor guanylyl cyclases in mammalian olfactory function. Mol Cell Biochem. 2010;334:191–197. doi: 10.1007/s11010-009-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pozdnyakov N, Yoshida A, Cooper NG, Margulis A, Duda T, Sharma RK, Sitaramayya A. A novel calcium-dependent activator of retinal rod outer segment membrane guanylate cyclase. Biochemistry. 1995;34:14279–14283. doi: 10.1021/bi00044a002. [DOI] [PubMed] [Google Scholar]
- 13.Duda T, Koch KW, Venkataraman V, Lange C, Beyermann M, Sharma RK. Ca(2+) sensor S100beta-modulated sites of membrane guanylate cyclase in the photoreceptor-bipolar synapse. EMBO J. 2002;21:2547–2556. doi: 10.1093/emboj/21.11.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan A, Venkataraman V, Fik-Rymarkiewicz E, Duda T, Sharma RK. Structural, biochemical, and functional characterization of the calcium sensor neurocalcin delta in the inner retinal neurons and its linkage with the rod outer segment membrane guanylate cyclase transduction system. Biochemistry. 2004;43:2708–2723. doi: 10.1021/bi035631v. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JY, Lange C, Helten A, Höppner-Heitmann D, Duda T, Sharma RK, Koch KW. Regulatory modes of rod outer segment membrane guanylate cyclase differ in catalytic efficiency and Ca(2+)-sensitivity. Eur J Biochem. 2003;270:3814–3821. doi: 10.1046/j.1432-1033.2003.03770.x. [DOI] [PubMed] [Google Scholar]
- 16.Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–4887. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt DM, Buch P, Michaelides M. Guanylate cyclases and associated activator proteins in retinal disease. Mol Cell Biochem. 2010;334:157–168. doi: 10.1007/s11010-009-0331-y. [DOI] [PubMed] [Google Scholar]
- 18.Venkataraman V, Nagele R, Duda T, Sharma RK. Rod outer segment membrane guanylate cyclase type 1-linked stimulatory and inhibitory calcium signaling systems in the pineal gland: biochemical, molecular, and immunohistochemical evidence. Biochemistry. 2000;39:6042–6052. doi: 10.1021/bi9929960. [DOI] [PubMed] [Google Scholar]
- 19.Duda T, Venkataraman V, Krishnan A, Nagele RG, Sharma RK. Negatively calcium-modulated membrane guanylate cyclase signaling system in the rat olfactory bulb. Biochemistry. 2001;40:4654–4662. doi: 10.1021/bi0027985. [DOI] [PubMed] [Google Scholar]
- 20.Jankowska A, Burczynska B, Duda T, Warchol JB, Sharma RK. Calcium-modulated rod outer segment membrane guanylate cyclase type 1 transduction machinery in the testes. J Androl. 2007;28:50–58. doi: 10.2164/jandrol.106.000182. [DOI] [PubMed] [Google Scholar]
- 21.Duda T, Sharma RK. Distinct ONE-GC transduction modes and motifs of the odorants: Uroguanylin and CO(2) Biochem Biophys Res Commun. 2010;391:1379–1384. doi: 10.1016/j.bbrc.2009.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nambi P, Aiyar NV, Sharma RK. Adrenocorticotropin-dependent particulate guanylate cyclase in rat adrenal and adrenocortical carcinoma: comparison of its properties with soluble guanylate cyclase and its relationship with ACTH-induced steroidogenesis. Arch Biochem Biophys. 1982;217:638–646. doi: 10.1016/0003-9861(82)90545-8. [DOI] [PubMed] [Google Scholar]
- 23.Pertzev A, Duda T, Sharma RK. Ca(2+) sensor GCAP1: A constitutive element of the ONE-GC-modulated odorant signal transduction pathway. Biochemistry. 2010;49:7303–7313. doi: 10.1021/bi101001v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duda T, Fik-Rymarkiewicz E, Venkataraman V, Krishnan R, Koch KW, Sharma RK. The calcium-sensor guanylate cyclase activating protein type 2 specific site in rod outer segment membrane guanylate cyclase type 1. Biochemistry. 2005;44:7336–7345. doi: 10.1021/bi050068x. [DOI] [PubMed] [Google Scholar]
- 25.Duda T, Bharill S, Wojtas I, Yadav P, Gryczynski I, Gryczynski Z, Sharma RK. Atrial natriuretic factor receptor guanylate cyclase signaling: new ATP-regulated transduction motif. Mol Cell Biochem. 2009;324:39–53. doi: 10.1007/s11010-008-9983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange C, Duda T, Beyermann M, Sharma RK, Koch KW. Regions in vertebrate photoreceptor guanylyl cyclase ROS-GC1 involved in Ca(2+)-dependent regulation by guanylyl cyclase-activating protein GCAP-1. FEBS Lett. 1999;460:27–31. doi: 10.1016/s0014-5793(99)01312-5. [DOI] [PubMed] [Google Scholar]
- 27.Peshenko IV, Olshevskaya EV, Dizhoor AM. Binding of guanylyl cyclase activating protein 1 (GCAP1) to retinal guanylyl cyclase (RetGC1). The role of individual EF-hands. J Biol Chem. 2008;283:21747–2157. doi: 10.1074/jbc.M801899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dizhoor AM, Olshevskaya EV, Peshenko IV. Mg2+/Ca2+ cation binding cycle of guanylyl cyclase activating proteins (GCAPs): role in regulation of photoreceptor guanylyl cyclase. Mol Cell Biochem. 2010;334:117–124. doi: 10.1007/s11010-009-0328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.