Abstract
Background
There is a clinical association between asthma and chronic rhinosinusitis (CRS). This study was designed to determine whether severity of coexistent asthma affects the clinical presentation of CRS.
Methods
Cross-sectional analysis was performed of prospectively collected data in 187 patients with CRS who were evaluated in a large, tertiary academic nasal and sinus center. Patients were stratified into three groups based on asthma status using National Institutes of Health criteria: (1) nonasthmatic, (2) intermittent/mild asthma, (3) or moderate/severe asthma.
Results
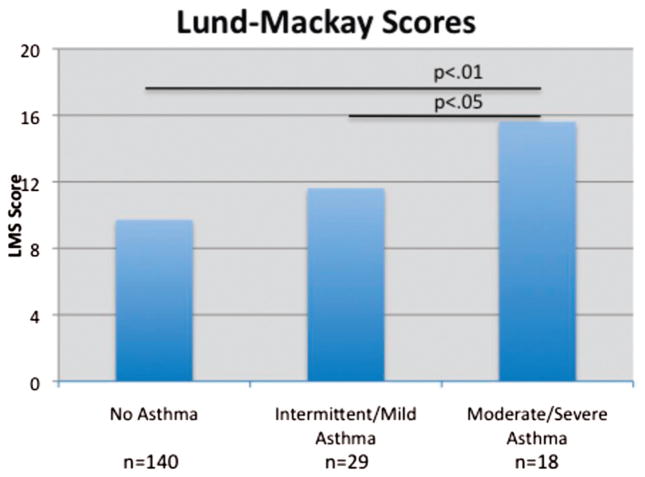
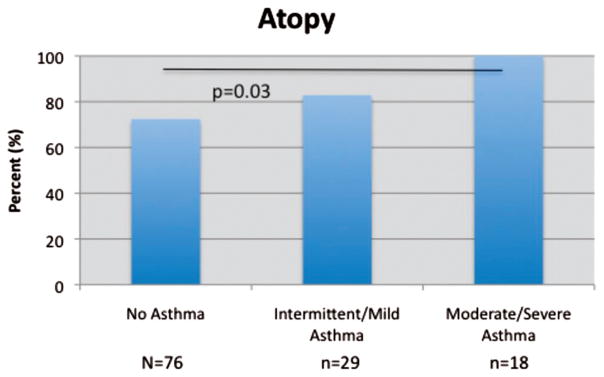
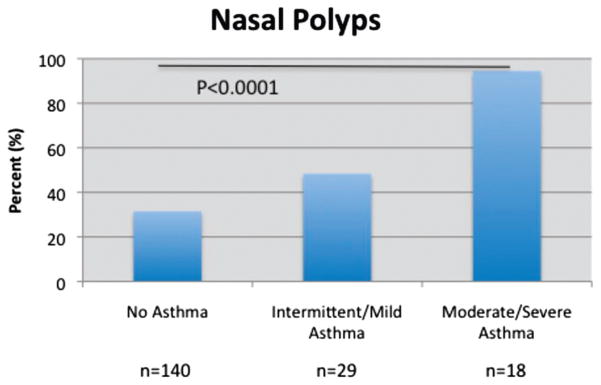
Mean Lund-Mackay scores were 9.7, 11.6, and 15.6, respectively. ANOVA testing with post-hoc Tukey analysis revealed that Lund-MacKay scores were significantly greater in group 3 than either group 1 (p < 0.05) or group 2 (p < 0.01). The prevalence of allergic sensitization was 72.4, 82.8, and 100% in groups 1, 2, and 3, respectively (p < 0.03). The prevalence of nasal polyposis was 31.4% in group 1, 48.3% in group 2, and 94.4% in group 3 (p < 0.0001). No differences were observed regarding demographic factors or the incidence of the triad of aspirin sensitivity, asthma, and nasal polyposis among those with different severities of asthma.
Conclusion
Increasing severity of asthma is associated with advancing radiological severity of CRS and a greater prevalence of allergic sensitization and nasal polyposis. This large adult series shows that asthma severity may have a significant correlation with the presentation of CRS. This study adds to the growing support for the unified airway theory.
Chronic rhinosinusitis (CRS) is one of the most prevalent chronic conditions in the United States. According to the National Health and Nutrition Examination Survey, ~14% of Americans have CRS, and these patients account for 91.2 million health care visits annually.1,2 Furthermore, because of the refractory nature of this disease, patients who fail conventional medical treatment account for 200,000 sinus surgeries annually.3
The association between CRS and asthma has been clearly established, although the exact mechanism is still controversial.4–6 Approximately 20–33% of patients with CRS also manifest asthma, a prevalence of approximately four times greater than that of the general population.7,8 The associations between these two diseases have clear clinical implications, and other authors have suggested that the severity of asthma correlates with the extent of CRS as measured by CT scans and symptom scores.9
In the present study, we sought to investigate the relationship between asthma severity and the clinical presentation of CRS including Lund-Mackay score (LMS), nasal polyposis, atopy, and aspirin sensitivity triad (acetylsalicylic acid [ASA] triad). We hypothesize that asthma severity positively correlates with CRS severity as measured by LMSs, as well as the presence of polyposis, atopy, and ASA triad.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Review Board of Northwestern University, and all patients gave informed consent for participation and use of clinical data for these purposes. The study population included 224 consecutive CRS patients, prospectively recruited into a tissue banking study at the time of endoscopic sinus surgery. All patients were evaluated and had their surgery performed at a tertiary academic sinus and allergy center at Northwestern University Feinberg School of Medicine between January 2007 and August 2009. The data were reviewed retrospectively. All patients met the following inclusion criteria: (1) diagnosis of CRS by criteria set by the American Academy of Otolaryngology–Head and Neck Surgery Task Force on Rhinosinusitis10; (2) failure of maximal medical therapy including antibiotics, oral corticosteroids, and nasal corticosteroids and thus qualification for functional endoscopic sinus surgery (FESS); (3) baseline CT scan of paranasal sinuses establishing sinusitis. The CT was typically done on the date of initial presentation unless the patient was having an acute exacerbation, in which case the patients were treated with standard medical therapy and scanned when they reached their baseline symptoms. All patients underwent nasal endoscopy to document inflammation and/or nasal polyposis. Exclusion criteria included antrochoanal polyposis, cystic fibrosis, immune deficiency, or neoplasm affecting the sinonasal tract. After taking these criteria into account, 187 patients remained in the study. Patients were divided into three groups: (1) nonasthmatic, (2) intermittent/mild asthma, or (3) moderate/severe asthma, based on 2007 National Heart, Lung, and Blood Institute criteria.11 Criteria guidelines were based on symptom frequency, nighttime awakenings, β-agonist use, interference with normal activity, pulmonary function tests, and exacerbations requiring corticosteroids. Physicians in Northwestern University’s Department of Allergy and Immunology performed the classification.
LMS was calculated by two independent observers blinded to the subjects’ asthma status, using CT scans of paranasal sinuses obtained before sinus surgery. Each sinus was assigned a score between 0 (completely aerated) and 2 (completely opacified). The osteomeatal complex was scored with either 0 or 2 based on a similar methodology. The possible tabulated score ranged from 0 to 24 for each patient.
Patients underwent allergy testing using a skin-prick panel of 24 inhaled aeroallergens from 8 classes (dog, cat, dust mite, grass, tree, ragweed, mold, and cockroach). A positive histamine control and a negative saline control were included as well. A wheal diameter of >5 mm with flare at 20 minutes was considered a positive result, and patients were designated as atopic in the presence of a positive reaction to any allergen. Note that analysis of atopy as an independent variable was only undertaken for patients who completed skin testing at the Northwestern University Sinus and Allergy Center. Patients with asthma were routinely tested, but 64 of 140 patients from the “no asthma” group also had no symptoms of atopy (itchy/watery eyes, nasal pruritis, and sneezing) and allergy testing was deferred. These patients were excluded from the statistical analysis of this variable because they did not undergo skin testing.
Data were analyzed to determine the effect of asthma severity on LMS, nasal polyposis, atopy, and ASA triad. LMS scores were analyzed by ANOVA testing with post hoc Tukey analysis. Nasal polyposis, atopy, ASA triad, age, and gender were analyzed using chi-square tests. A value of p < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism software (La Jolla, CA).
RESULTS
The data were analyzed by comparison of the three groups based on asthma status and severity: (1) no asthma, (2) intermittent/mild asthma, or (3) moderate/severe asthma. There were 140, 29, and 18 patients in groups 1, 2, and 3, respectively. The demographics of all three groups were comparable, and there were no statistical differences in gender, age, or ethnicities represented (Table 1).
Table 1.
Demographic and clinical characteristics by group
| No Asthma | Intermittent/Mild Asthma | Moderate/Severe Asthma | p Value | |
|---|---|---|---|---|
| Patients | 140 | 29 | 18 | |
| M/F | 80:60; 1.33 | 11:18; 0.61 | 7:11; 1.57 | 0.14 |
| Age (yr) | 40.7, SD = 11.5 | 40.4, SD = 9.8 | 46.1, SD = 15.2 | NS |
| Ethnicity | NS | |||
| White (%) | 99 (70.7) | 17 (58.6) | 13 (72.2) | |
| Black (%) | 15 (10.7) | 4 (13.8) | 3 (16.7) | |
| Other (%) | 26 (18.6) | 8 (27.6) | 2 (11.1) | |
| Atopy* (%) | 55 (72.4) | 24 (82.8) | 18 (100) | <0.05 |
| Polyps (%) | 44 (31.4) | 14 (48.3) | 17 (94.4) | <0.0001 |
| ASA triad (%) | N/A# | 4 (13.8) | 5 (27.8) | 0.24 |
Atopic status was collected only for patients who completed skin testing at Northwestern University Sinus and Allergy Center. Seventy-six of 140 patients from “no asthma” group included in statistical analysis.
ASA Triad status for “no asthma” group was not taken into consideration for statistical analysis.
ASA = Acetylsalicylic acid.
The mean LMS was 9.7, 11.6, and 15.6 for groups 1, 2, and 3, respectively (p < 0.0001, ANOVA; Fig. 1). Furthermore, post hoc analysis revealed LMS was significantly greater in group 3 than either group 1 (p < 0.05) or group 2 (p < 0.01). Group 2 exhibited a greater LMS than group 1, but this did not reach statistical significance in post-hoc testing. The prevalence of atopy was 72.4, 82.8, and 100% for groups 1, 2, and 3, respectively (p = 0.03; Fig. 2). The prevalence of nasal polyposis was 31.4, 48.3, and 94.4% for groups 1, 2, and 3, respectively (p < 0.0001; Fig. 3). The prevalence of ASA triad was 13.8% for group 2 and 27.8% for group 3, but this difference did not reach statistical significance. Note that group 1 was not included in the statistical analysis for ASA triad because of the absence of asthma in these patients.
Figure 1.
Asthma severity and Lund-Mackay score.
Figure 2.
Asthma severity and atopy percentage.
Figure 3.
Asthma severity and nasal polyposis percentage.
DISCUSSION
A mounting body of literature has supported the association between sinusitis and asthma. Epidemiologically, it has been suggested that up to 38% of patients with allergic rhinitis have asthma, and, conversely, up to 50% of asthmatic patients also have allergic rhinitis.12 In another population of patients, up to 80% of asthmatic patients had some radiological evidence of sinusitis.13
The present study sought to further elucidate this relationship in a sample of CRS patients who failed maximal medical therapy and underwent sinus surgery. Our observation that radiological severity of CRS (LMS) increases with asthma severity is consistent with previously published reports.14–16 The present data also corroborate a previous study from our institution revealing that asthmatic patients had higher LMS than nonasthmatic patients (p < 0.0001).8 Bresciani et al.9 also stratified asthmatic patients into severe and moderate-to-severe based on daily oral corticosteroid dependence. The severe asthma group had increased CT severity scores compared with the moderate-to-severe asthma group (p < 0.0005). It should be noted, however, that the sample of patients studied in that report was not restricted to those with CRS, and that asthma classification system was based on corticosteroid use. Unlike previous studies, our experimental design used classification of asthma severity using National Institutes of Health guidelines in a group of CRS patients who had failed maximal medical therapy. The present series illustrates that, even using these strict criteria in patients with recalcitrant CRS, radiological disease severity does correlate with asthma status. It is notable that prior studies have shown that radiological severity, assessed by LMS, is weakly correlated with CRS severity as measured by patient-based symptom questionnaires.17 Although this observation presents a limitation in quantifying severity of CRS, radiological severity is still useful in that it is objective in nature. Thus, we can conclude that asthma severity and CRS (radiological) severity are correlated in patients with medically refractive disease necessitating surgery. It deserves further study as to whether this also applies to the entire population of CRS patients. Advancing asthma was also associated with increasing prevalence of polyposis and atopy, supporting our original hypothesis. When the present study population was stratified by polyp status, we observed no difference in radiological severity between nonasthmatic and asthmatic patients. This suggests that increasing radiological severity with worsening asthma is attributable to the increasing prevalence of polyposis. Finally, although we did not find any statistically significant associations, it is possible that samples with a larger subpopulation of ASA triad patients may elucidate an impact of this variable.
There is also growing evidence that aggressive treatment of rhinosinusitis can subsequently lead to an improvement of asthma severity.18–20 In a study of 30 patients who had FESS, 90% reported asthma improvement, 74.1% had reduced numbers of asthma attacks, 46% indicated less inhaler usage, and 65% reported decreased steroid requirement.21 Upper airway management alleviated lower airway severity as shown using multiple benchmarks, suggesting the need for clinicians to take both comorbid conditions into consideration when addressing the management of these respiratory diseases. The present study design precluded conclusions about the effect of FESS in altering severity of asthma as measured by objective lung function. Regarding this issue, there is conflicting evidence in the literature as to whether there are significant differences in symptoms, medications, and pulmonary function tests after FESS.22
Although the association linking upper airways disease and lower airway disease is compelling; the mechanism that underlies this connection is still under debate.23 Marney et al.24 discusses proposed mechanisms that could explain the unified airway hypothesis including aspiration of inflamed sinus secretions into the lower airways, enhanced vagal stimulation in the infected sinus leading to bronchospasm, excessive drying of the lower airways by mouth breathing as a consequence of nasal obstruction, production of bacterial toxins that induce partial B-blockade, and production in the infected sinus of cytokines and bronchoconstrictive mediators. The most recent evidence seems to support the latter, which suggests a mechanism of a systemic inflammation. Joe et al.7 emphasize the role of eosinophils as the primary effector cell in the pathophysiology of both upper airway sinusitis and lower airway asthma.
One limitation of the present study is that not all patients in the “no asthma” group underwent skin testing. These patients were excluded from analysis of atopy as an independent variable. Although this may have skewed the prevalence of atopy in that group, it is almost certain that routine testing of the entire nonasthmatic group would have yielded an even lower incidence of atopy in that arm, because testing was only performed in the nonasthmatic patients with allergy symptoms. This possibility is corroborated by observations of others, revealing a background incidence of atopy at 53.4%.25 Thus, the present series may have overestimated the prevalence of atopy in patients without asthma; however, the data still showed that, by comparison, asthma status positively correlated with prevalence of atopy. Another limitation of this report is that although it indicates a clear association between severity of CRS and asthma, it does not elucidate any causal relationship between the two disorders. It remains possible, and perhaps likely, that these conditions are both manifestations of a global airway inflammatory process. Although some patients may exhibit greater disease burden in one part of the airway than another, this series does illustrate that among recalcitrant CRS patients, advancing asthma status correlates with advanced radiological sinus disease. Further directions for this research will be to prospectively determine whether these findings will be observed in broader populations of CRS patients and to determine whether intervention for CRS impacts the course of lower respiratory comorbidities.
CONCLUSION
This data illustrate a strong relationship between increasing severity of asthma with advancing radiological severity of CRS. Asthma stratification also correlates with a greater prevalence of both allergic rhinitis and nasal polyposis. This large adult series shows that asthma severity may have a significant correlation with the presentation of CRS.
Acknowledgments
Funded by Studies of Human Airway Disease including those supported by NIH Grant 1R01 HL078860 Role of Epithelium in Immunity, Inflammation and Disease
Footnotes
Presented at 2010 annual meeting of the American Academy of Otolaryngology—Head and Neck Surgery, Boston, Massachusetts, September 28, 2009
The authors have no conflicts to declare pertaining to this article
References
- 1.Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Vital Health Stat. 1999;10:200. [PubMed] [Google Scholar]
- 2.Smith WM, Davidson TM, Murphy C. Regional variations in chronic rhinosinusitis, 2003–2006. Otolaryngol Head Neck Surg. 2009;141:347–352. doi: 10.1016/j.otohns.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemmerdinger SA, Jacobs JB, Lebowitz RA. Accuracy and cost analysis of image-guided sinus surgery. Otolaryngol Clin North Am. 2005;38:453–460. doi: 10.1016/j.otc.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Dixon AE. Rhinosinusitis and asthma: The missing link. Curr Opin Pulm Med. 2009;15:19–24. doi: 10.1097/MCP.0b013e32831da87e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seybt MW, McMains KC, Kountakis SE. The prevalence and effect of asthma on adults with chronic rhinosinusitis. Ear Nose Throat J. 2008;86:409–411. [PubMed] [Google Scholar]
- 6.Slavin RG. The upper and lower airways: The epidemiological and pathophysiological connection. Allergy Asthma Proc. 2008;29:553–556. doi: 10.2500/aap.2008.29.3169. [DOI] [PubMed] [Google Scholar]
- 7.Joe SA, Thakkar K. Chronic rhinosinusitis and asthma. Otolaryngol Clin North Am. 2008;41:297–309. doi: 10.1016/j.otc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol. 2009;23:145–148. doi: 10.2500/ajra.2009.23.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresciani Megon, Paradis L, Des Roches A, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 11.National Heart, Lung, and Blood Institute. Expert Report Panel 3. National Heart, Lung, and Blood Institute; Bethesda, MD: 2007. Guidelines for the diagnosis and management of asthma. [Google Scholar]
- 12.Maesano A. Epidemiological evidence of the occurrence of rhinitis and sinusitis in asthmatics. Allergy. 1999;54:7–13. doi: 10.1111/j.1398-9995.1999.tb04401.x. [DOI] [PubMed] [Google Scholar]
- 13.Slavin RG. Asthma and sinusitis. J Allergy Clin Immunol. 1992;90:534–537. doi: 10.1016/0091-6749(92)90180-a. [DOI] [PubMed] [Google Scholar]
- 14.Dhong HJ, Kim HY, Chung YJ, et al. Computed tomographic assessment of chronic rhinosinusitis with asthma. Am J of Rhinol. 2006;20:450–452. doi: 10.2500/ajr.2006.20.2929. [DOI] [PubMed] [Google Scholar]
- 15.Ramadan HH, Fornell R, Ortiz AO, et al. Correlation of Allergy and Severity of Sinus Disease. Am J of Rhinol. 1999;13:345–347. doi: 10.2500/105065899781367500. [DOI] [PubMed] [Google Scholar]
- 16.Kountakis SE, Bradley DT. Effect of asthma on sinus computed tomography grade and symptom scores in patients undergoing revision functional endoscopic sinus surgery. Am J Rhinol. 2003;17:215–219. [PubMed] [Google Scholar]
- 17.Zheng Y, Zhao Y, Lv D, et al. Correlation between computed tomography staging and quality of life instruments in patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:249–254. doi: 10.2500/ajra.2010.24.3430. [DOI] [PubMed] [Google Scholar]
- 18.Dhong HJ, Jung YS, Chung SK, et al. Effect of endoscopic sinus surgery on asthmatic patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2001;124:99–104. doi: 10.1067/mhn.2001.111596. [DOI] [PubMed] [Google Scholar]
- 19.Dunlop G, Scadding GK, Lund VJ. The effect of endoscopic sinus surgery on asthma: management of patients with chronic rhinosinusitis, nasal polyposis, and asthma. Am J Rhinol. 1999;13:261–265. doi: 10.2500/105065899782102809. [DOI] [PubMed] [Google Scholar]
- 20.Awad OG, Fasano MB, Lee JH, et al. Asthma outcomes after endoscopic sinus surgery in aspirin-tolerant versus aspirin-induced asthmatic patients. Am J Rhinol. 2008;22:197–203. doi: 10.2500/ajr.2008.22.3148. [DOI] [PubMed] [Google Scholar]
- 21.Senior BA, Kennedy DW, Tanabodee J, et al. Long-term impact of functional endoscopic sinus surgery on asthma. Otolaryngol Head Neck Surg. 1999;121:66–68. doi: 10.1016/S0194-5998(99)70127-0. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein MF, Grundfast SK, Dunsky EH, et al. Effect of functional endoscopic sinus surgery on bronchial asthma outcomes. Arch Otolaryngol Head Neck Surg. 1999;125:314–319. doi: 10.1001/archotol.125.3.314. [DOI] [PubMed] [Google Scholar]
- 23.Marple BF. Allergic rhinitis and inflammatory airway disease: Interactions within the unified airspace. Am J Rhinol Allergy. 2010;24:249–254. doi: 10.2500/ajra.2010.24.3499. [DOI] [PubMed] [Google Scholar]
- 24.Marney SR. Pathophysiology of reactive airway disease and sinusitis. Ann Otol Rhinol Laryngol. 1996;105:98–100. doi: 10.1177/000348949610500203. [DOI] [PubMed] [Google Scholar]
- 25.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]