Abstract
The purpose of this study was to evaluate risk factors, protective factors, and outcomes associated with Clostridium difficile-associated disease (CDAD) in allogeneic hematopoietic stem cell transplant (HSCT) recipients. A case-control study was performed with 37 CDAD cases and 67 controls. In the multivariable logistic regression analysis, receipt of a 3rd or 4th generation cephalosporin was associated with increased risk of CDAD (OR=4.6, 95% CI 1.6 – 13.1). Receipt of growth factors was associated with decreased risk of CDAD (OR=0.1, 95% CI 0.02 – 0.3). Cases were more likely to develop a blood stream infection after CDAD than were controls at any point before discharge (p<0.001). CDAD cases were more likely than controls to develop new onset GVHD (p<0.001), new onset severe GVHD (p<0.001), or new onset gut GVHD (p=0.007) after CDAD/discharge. Severe CDAD was a risk factor for death at 180 days in multivariable Cox proportional hazards regression (HR=2.6, 95% CI 1.1 – 6.2). CDAD is a significant cause of morbidity and mortality in allogeneic HSCT patients, but modifiable risk factors exist. Further study is needed to determine the best methods of decreasing patients’ risk of CDAD.
Keywords: Clostridium difficile, hematopoetic stem cell transplant, epidemiology
INTRODUCTION
Clostridium difficile-associated disease (CDAD) is the most common infectious cause of hospital-associated diarrhea, and the spectrum of illness caused by CDAD ranges from mild diarrhea to life-threatening toxic megacolon, colon perforation, and death. Patients undergoing hematopoietic stem cell transplantation (HSCT) have many of the primary risk factors for CDAD, including antibiotic exposure and high severity of illness. However, there are relatively few studies of CDAD in HSCT recipients, and most studies include both allogeneic and autologous transplant recipients (1–11). The largest published study in these patients reported 41 CDAD episodes in 351 transplant patients; however, the purpose of that study was to evaluate all nosocomial infections after transplantation, and specific risk factors for CDAD were not evaluated (5). The largest study of CDAD in allogeneic transplant recipients included only 10 CDAD episodes (4).
Risk factors for CDAD in hematopoietic transplant recipients are poorly understood, and most studies have been unable to identify even a single risk factor for CDAD (3;4;9;11). One study reported chemotherapy as a risk factor for CDAD (12); however, this study was performed during a CDAD outbreak on a medical oncology floor, and the results may not be applicable to endemic settings. The difficulties in identifying unique risk factors for CDAD in HSCT recipients may arise from the ubiquity of traditional risk factors for CDAD in this population or from the small sample sizes of previous studies.
Previous studies evaluating outcomes associated with CDAD in hematopoietic transplant recipients have produced conflicting results. Increased frequency of graft-versus-host disease (GVHD) and viral infections, higher overall mortality, shorter median survival (4), and severe enterocolitis (6) have been reported in allogeneic transplant recipients and leukemia patients. However, in other studies, CDAD appears to be a mild, self-limited illness that responds to traditional antibiotic therapy (3;6;9;11).
We performed a case-control study on a large HSCT unit to risk factors for and outcomes associated with CDAD in allogeneic stem cell transplants.
PATIENTS AND METHODS
This study was performed at Barnes-Jewish Hospital (BJH), a 1250-bed academic hospital in St. Louis, Missouri. The hospital’s medical informatics database was queried to identify all patients from who received an allogeneic HSCT from August 1, 2001 through July 31, 2003 and to identify all allogeneic HSCT recipients who had a stool sample positive for C. difficile during the study period. Patients with a past history of CDAD were excluded. Thirty-seven allogeneic transplant recipients developed CDAD during the study period, either during their transplant hospitalization or a subsequent hospitalization. If a patient had >1 CDAD episode during the study period, the hospitalization of the first CDAD episode was used as the index hospitalization. Controls were randomly selected from the remaining allogeneic transplant recipients for a 1:2 case-control study. If a control had multiple admissions during the study period, one admission was randomly selected as the index hospitalization. Several controls were excluded because it was subsequently identified they had not received an allogeneic HSCT until after the study period. All patients included in the study were hospitalized on the hematopoietic transplant unit, which consists of 27 private, positive pressure, HEPA-filtered rooms.
CDAD rates on the HSCT ward were the highest in the hospital during the study period, but no CDAD outbreaks occurred during that time. It is not known if the CDAD outbreak strain (NAP1/B1/O27) was present on the HSCT ward during the study period.
Patients’ medical charts were reviewed for demographics, underlying disease, symptoms, infections, complications, medications received (including antibiotics and antineoplastic agents), and outcomes. In order to determine outcomes and adverse events, cases were followed 180 days from their CDAD diagnosis date and controls were followed for180 days from the discharge date of their index hospitalization. For CDAD cases, only outcomes that occurred after CDAD onset were included in analyses. A standardized data collection tool was used. All data collectors were trained in the use of the tool, and results were checked for accuracy by an infectious disease physician. Data were double entered to ensure accuracy. The Washington University Human Subjects Committee approved this study.
Definitions
A case of CDAD was defined as a patient with a positive stool toxin assay for C. difficile. The toxin assay used by the hospital’s laboratory at the time of the study was the Tech Lab C. difficile tox A/B II toxin assay (Tech Lab, Blacksburg, VA). The decision to order a C. difficile toxin assay was made by the patient’s treating physician based on the patient’s symptoms. The microbiology laboratory will not test formed stool for C. difficile. Thus, all positive toxin assays were considered cases of CDAD.
Infections were defined according to the Centers for Disease Control and Prevention (CDC) National Nosocomial Infections Surveillance (NNIS) definitions (13). Fever was defined as any single temperature ≥38.3°C or temperatures ≥38.0°C for greater than an hour. Hypothermia was defined as any single temperature ≤35.6°C or temperatures ≤35.9°C for greater than an hour. Hypotension was defined as a systolic blood pressure ≤90 mmHG or a drop >40 from baseline systolic blood pressure, plus medical intervention (intravenous fluids or vasopressors). Acute renal failure was defined as a new increase of serum creatinine to ≥2.0 mg/dl. Acute liver dysfunction was defined as a new increase of total serum bilirubin ≥4.0 mg/dl. Signs and symptoms of CDAD, including ileus, abdominal tenderness or distension, and peritoneal signs, were determined based on chart review. Radiology and surgery notes were also reviewed for evidence of toxic megacolon, colon perforation, bowel necrosis, bowel ischemia, or endoscopy findings. Graft-versus-host disease (GVHD) severity was determined from physicians’ notes and pathology reports and graded according to standard criteria (14).
Diarrhea was defined as ≥3 loose bowel movements for ≥48 hours. However, frequency and severity of diarrhea can be difficult to determine retrospectively from physicians’ notes. The nursing notes for the transplant ward include an intake/output assessment, and because intestinal output volume is recorded only if diarrhea is present, total daily intestinal output was included in the determination of CDAD severity. CDAD severity was determined based on symptoms present within 24 hours of diagnosis; cases were divided into mild/moderate and severe CDAD. Further details on the methods used for classifying CDAD severity have been described elsewhere (15).
Analysis
Analyses were performed with SPSS, version 14.0 (SPSS, Chicago, IL). Univariate analyses were performed with chi-square tests for categorical data, Mann-Whitney U tests for continuous data, and Kaplan-Meier for survival. Hierarchical loglinear modeling was used to further evaluate the relationships between CDAD, growth factors, and neutropenia. Loglinear modeling describes patterns of associations without specifying roles of the variables (i.e., no dependent variable). Starting with a saturated model, higher order interactions were removed one at a time until the most parsimonious model that accounts for all of the variability of the saturated model was identified. The coefficients of the higher order interaction terms in the loglinear models were converted to odds ratios. Multivariable analyses were performed with logistic regression and Cox proportional hazards. Because of sample size restrictions, all of the variables could not be modeled simultaneously. Multivariable models were built in a stepwise fashion using variables significant on univariate analysis, variables with biological plausibility, and variables that were potential confounders. Models were compared with −2 log likelihood comparisons and the most parsimonious model that best fit the data was chosen as the final model. A P≤0.05 was considered significant.
RESULTS
Variables associated with risk of or protection from CDAD
The study population included 37 CDAD cases and 67 controls. Demographics of the study population are given in Table 1. Sixteen (43%) of the cases were classified as having mild to moderate CDAD and 21 (57%) as having severe CDAD (15). Gender, race, underlying diagnosis (acute leukemia/blast crisis, chronic leukemia, myelodysplastic syndrome, lymphoma/multiple myeloma), disease status (complete remission, partial remission, relapse/refractory), and reason for admission were similar between cases and controls. Although the difference was not statistically significant, 57% of cases versus 39% of controls had been hospitalized in the 60 days prior to their index admission (OR=2.1; 0.9 – 4.7).
Table 1.
Demographics of Study Population
| Characteristic | Controls (n=67) | Cases (n=37) | OR (95% CI) |
|---|---|---|---|
| Female | 30 (45) | 12 (32) | 0.6 (0.3–1.4) |
| White | 61 (91) | 35 (95) | 0.6 (0.1–3.0) |
| Age | 46 (21–66) | 48 (19–65) | |
| Hospitalized in previous 60 days | 26 (39) | 21 (57) | 2.1 (0.9–4.7) |
| Underlying Diagnosis | |||
| Acute leukemia/blast crisis | 32 (49) | 18 (49) | Reference |
| Chronic leukemia | 8 (12) | 8 (22) | 1.8 (0.6 – 5.6) |
| Myelodysplastic syndrome | 7 (11) | 6 (16) | 1.5 (0.4 – 5.2) |
| Lymphoma/Multiple myeloma | 19 (29) | 5(14) | 0.5 (0.2 – 1.5) |
| Disease status | |||
| Complete remission | 37 (55) | 18 (49) | Reference |
| Partial remission | 4 (6) | 4 (11) | 2.1 (0.5 – 9.2) |
| Relapse/Refractory | 26 (39) | 15 (41) | 1.2 (0.5 – 2.8) |
| Reason for admission | |||
| Transplant | 33 (49) | 17 (46) | Reference |
| Infection | 16 (24) | 12 (32) | 1.5 (0.6 – 3.8) |
| GVHD | 3 (5) | 4 (11) | 2.6 (0.5 – 12.9) |
| Other* | 15 (22) | 4 (11) | 0.5 (0.2 – 1.8) |
| Unrelated donor | 33 (50) | 20 (54) | 1.2 (0.5 – 2.6) |
Other reasons for admission include GI bleed, pulmonary embolism, syncope, altered metal status, chemotherapy, acute renal failure, arthritis, hyperglycemia, double vision, and rash.
Results of the univariate analysis of variables associated with risk of or protection against CDAD are given in Table 2. For all variables listed, significance was ≤0.10. Comorbid conditions associated with increased risk of CDAD included diabetes (35% of cases vs. 15% of controls; OR=3.1; 95% CI 1.2 – 8.0) and lung disease (11% of cases vs. 2% of controls; OR=8.0; 95% CI 0.9 – 74.5). Antibiotics associated with an increased risk of CDAD included receipt of >7 days of a 3rd or 4th generation cephalosporin (32% vs. 6%; OR=8.3; 95% CI 2.3 – 30.4). Several variables were associated with lower risk of CDAD. Only 43% of cases, compared to 72% of controls, were neutropenic during admission (OR=0.3; 95% CI 0.1 – 0.7). Cases were less likely than controls to have received a growth factor during the hospitalization (24% vs. 60%; OR=0.2; 95% CI 0.1 –0.5). There were no significant differences between cases and controls in diagnosis of either gut GVHD or any type of GVHD before CDAD (for cases) or discharge (for controls).
Table 2.
Potential Variables Associated with CDAD on Univariate Analysis (P≤0.10)
| Characteristic | Controls (n=67) | Cases (n=37) | OR (95% CI) |
|---|---|---|---|
| Diabetes* | 10 (15) | 13 (35) | 3.1 (1.2 – 8.0) |
| Lung disease | 1 (2) | 4 (11) | 8.0 (0.9 – 74.5) |
| Neutropenia | 48 (72) | 16 (43) | 0.3 (0.1 – 0.7) |
| Receipt of a growth factor during admission | 40 (60) | 9 (24) | 0.2 (0.1 – 0.5) |
| Receipt of a 3rd/4th gen. cephalosporin during admission | |||
| None | 36 (54) | 13 (35) | Reference |
| >0–7 days | 27 (40) | 12 (32) | 1.2 (0.5 – 3.1) |
| >7 days | 4 (6) | 12 (32) | 8.3 (2.3 – 30.4) |
| Receipt of carbepenems during admission | 23 (34) | 6 (16) | 0.4 (0.1 – 1.0) |
Includes steroid-induced diabetes
Loglinear modeling was used to further assess the associations between CDAD, growth factors, and neutropenia. The most parsimonious model included the following interaction terms: CDAD*growth factors and neutropenia*growth factors. Patients who received growth factors were 0.2 times (95% CI 0.1 – 0.5) as likely to develop CDAD as patients who did not receive growth factors. Patients who were neutropenic were 31.5 times (95% CI 8.3 – 115.2) as likely to receive growth factors as patients who were not neutropenic. There was no significant interaction between CDAD and neutropenia when controlling for exposure to growth factors.
The results of the multivariable regression are given in Table 3. The final model included receipt of growth factors, receipt of 3rd or 4th generation cephalosporins, diabetes and pre-engraftment period. Other variables assessed but not included in the final model included neutropenia, receipt of chemotherapy, and receipt of carbepenems. Neutropenia was not associated with CDAD in any of the models; whereas growth factors remained associated with decreased risk of developing CDAD in all models. Receipt of a 3rd or 4th generation cephalosporin, diabetes, and being in the pre-engraftment phase were all significantly associated with risk of CDAD.
Table 3.
Multivariable Analysis of Variables Associated with CDAD on Logistic Regression
| Variable | OR (95% CI) |
|---|---|
| Growth factor | 0.1 (0.02, 0.3) |
| 3rd/4th gen. cephalosporin | 4.6 (1.6, 13.1) |
| Diabetes | 5.0 (1.5, 16.5) |
| Pre-engraftment period | 4.0 (1.2, 13.1) |
Outcomes of CDAD
CDAD cases were no more likely than controls to requireTPN, ICU care, vasopressors, or develop acute liver or renal failure. Compared to controls, CDAD cases were more likely to develop GVHD, severe GVHD, and gut GVHD. CDAD cases were also more likely to develop a BSI, and more likely to die. Overall, 16 (24%) of controls, 7 (44%) of mild/moderate CDAD cases, and 15 (71%) of severe CDAD cases had onset of GVHD after CDAD (after discharge from index hospitalization for controls) (p<0.001). Severe GVHD developed after CDAD or discharge in 9 (13%) of controls, 5 (31%) of mild/moderate CDAD cases, and 12 (57%) of severe CDAD cases (p<0.001). New onset gut GVHD developed after CDAD or discharge in 3 (4%) of controls, 3 (18%) of mild/moderate CDAD cases, and 6 (29%) of severe CDAD cases (p=0.007).
Overall, 6 (9%) of controls developed a BSI after discharge, whereas 8 (50%) of mild/moderate CDAD cases and 12 (57%) of severe CDAD cases developed BSIs after CDAD (p<0.001). None of the controls developed >1 BSI episode after discharge, but 6 (16%) cases had >1 BSI after CDAD (p<0.001). The BSI rate among controls was 0.8 per 1000 patient-days, compared to 5.7 for mild/moderate CDAD cases and 11.6 for severe CDAD cases. Compared to controls, the relative risk of BSI among mild/moderate CDAD cases was 7.3 times higher (95% CI 3.2 – 16.7) and the relative risk among severe CDAD cases was 14.7 times higher (95% CI 7.3 – 29.6).
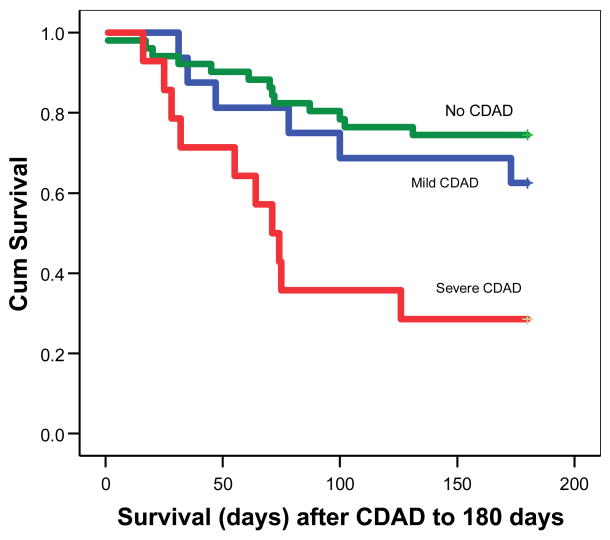
Kaplan-Meier analysis was performed to compare survival at 180 days between controls, mild/moderate CDAD cases, and severe CDAD cases (Figure 1). Median survival was >180 days for both controls and mild/moderate CDAD cases but was 71 days for severe CDAD cases (Log-rank p = 0.001). Table 4 presents variables significantly associated with death at 180 days on univariate analysis (p<0.05): severe CDAD, having a BSI after CDAD (or discharge for controls), relapsed or refractory underlying disease, younger age, late post-engraftment transplant phase, GVHD after CDAD (or discharge for controls), and severe GVHD after CDAD (or discharge for controls).
Figure 1.
Table 4.
Variables Associated with Death at 180 Days
| Variable | Unadjusted HR | Adjusted HR |
|---|---|---|
| Age (continuous) | 0.9 (0.9–1.0) | |
| Severe CDAD | 3.7 (1.7, 8.1) | 2.6 (1.1, 6.2) |
| Bloodstream infection* | 2.6 (1.3, 5.6) | |
| Relapse/refractory disease | 3.2 (1.6, 6.8) | 3.7 (1.7, 7.8) |
| Late post-engraftment period | 2.4 (1.2, 5.0) | |
| GVHD* | 2.5 (1.2, 5.3) | |
| Severe GVHD* | 3.4 (1.6, 7.1) | 2.7 (1.2, 6.1) |
onset after CDAD (after discharge from index hospitalization for controls)
Univariate analysis indicated there was no difference in survival for controls and mild/moderate CDAD cases; therefore, to maximize the degrees of freedom available, these two groups were combined and compared with severe CDAD cases in multivariable Cox models. In the final Cox proportional hazards model, severe CDAD remained significantly associated with death after adjustment for severe GVHD (onset after CDAD) and relapsed/refractory underlying disease (HR=2.6; 95% CI 1.1 – 6.2) (Table 5).
DISCUSSION
CDAD poses a unique challenge in HSCT recipients. Patients receiving these transplants have ubiquitous risk factors for CDAD; most, if not all, receive antibiotics, and all are severely ill and immunocompromised. Because of this, studies of CDAD in general hospitalized patients are not specific enough for HSCT patients and do not help practitioners determine which allogeneic transplant recipients are at increased risk for CDAD. Previous research has indicated HSCT patients may be at increased risk for severe outcomes of CDAD (4;6), so an understanding of modifiable risk and protective factors in this population is important to prevent severe disease due to CDAD. This study is the largest to date to evaluate variables associated with risk of and protection from CDAD in allogeneic HSCT patients. One important finding of this study was that, among all classes of antibiotics studied, receipt of a third or fourth generation cephalosporin was significantly associated with risk of CDAD in allogeneic HSCT patients. This has been reported in CDAD studies in general patient populations but has not been reported previously in the allogeneic HSCT population.
This study is the first to report that receipt of growth factors was associated with reduced risk of CDAD. This finding has biologically plausibility because growth factors stimulate growth of epithelial cells in the colon and are immunostimulatory beyond just promoting production of granulocytes. Neutropenia also appeared to be protective against CDAD on univariate analysis. A potential rationale for this finding also exists. Toxin A is very potent at inducing a neutrophilic inflammatory response, and research indicates this inflammatory response is responsible for the pathophysiology of CDAD (16). Therefore, it is possible that patients may be less likely to develop CDAD if neutrophils are not present. However, subsequent analyses identified neutropenia to be a marker of growth factor exposure and not protective against CDAD. When modeled with the “pre-engraftment transplant phase” variable, the odds ratio for growth factors actually decreased (i.e., became more protective). Patients often receive growth factors during this phase of the transplant process, so it appears that the “pre-engraftment phase” variable was actually masking some of the protective effect of growth factors. In summary, growth factor exposure is a complicated variable that impacts numerous aspects of the transplant course, but our analysis indicates it may have an important and previously unrecognized protective impact on CDAD. This is an intriguing finding that could decrease patients’ risk of CDAD, but additional studies with larger sample sizes are needed.
The results of the outcomes analyses indicated that CDAD cases, whether classified as mild/moderate or severe, are at increased risk for GVHD, severe GVHD and gut GVHD. While previous studies have indicated a higher frequency of GVHD among CDAD cases (4), the increased risk of gut GVHD after CDAD has not been reported previously. C. difficile toxins A and B damage the colonic mucosa, possibly exposing host intestinal and gut flora antigens. Mouse models have found mice with more severe intestinal mucosal damage are at increased risk of gut GVHD, and mice with suppressed gut flora are lower risk for developing GVHD (14;17). CDAD cases were also at higher risk for developing BSIs in the outcomes analyses. This confirms a relationship initially seen during preliminary analysis in this population (15). CDAD has also been identified as a risk factor for VRE BSI in leukemic patients (18). The damage to the colonic mucosa caused by toxins A and B may provide a portal of entry for gut flora to enter the bloodstream in these highly immunocompromised patients. Again, the relationships between CDAD and increased risk of gut GVHD and BSI reported here require confirmation with further studies. GVHD and BSI are two of the leading causes of morbidity and mortality after allogeneic HSCT, and if confirmed, this finding would provide further justification for enhanced CDAD prevention efforts among allogeneic HSCT patients.
The finding that only patients with severe CDAD, not mild/moderate CDAD, are at increased risk for death at 180 days is intriguing and warrants further study. This finding may explain why some studies have found CDAD to be a mild illness in HSCT recipients (3;9;11), but others have found CDAD to be associated with an increased risk of adverse events (4;6). The CDAD severity grading scale used here was designed to classify CDAD severity based on symptoms present early in the clinical course, and it may become extremely useful in identifying CDAD cases at increased risk for death early in their illness. Vancomycin has been shown to be associated with a more rapid response the therapy compared to metronidazole in severely ill patients with CDAD (19), and patients classified in the “severe CDAD” category could be treated with vancomycin, perhaps preventing some of the deaths in these cases.
There are several limitations to this study. The first is a relatively small sample size. Because of the small sample size, only 3–4 variables could be included in any given multivariable model. However, this study is still the largest study of CDAD in the allogeneic transplant population. The second limitation is the retrospective case-control study design. Because of the long period of time (2 years) required to compile even the 37 CDAD cases presented here, the case-control design provided a time-efficient method of studying risk factors and outcomes associated with CDAD. Nevertheless, a prospective cohort study is needed to confirm the findings presented here. Previously, the only risk factor for CDAD identified in the hematopoietic transplant population was chemotherapy (4). Chemotherapy is a necessary part of the treatment for these patients’ underlying diseases and is only marginally modifiable. The risk factor (receipt of a 3rd/4th generation cephalosporin) and protective factor (receipt of growth factors) identified here are far more modifiable than receipt of chemotherapy, and additional studies to determine the impact of these findings to clinical practice are necessary to help identify methods to decrease the frequency of CDAD infections. Reducing CDAD could improve the short and long-term prognosis of patients undergoing allogeneic transplants.
Supplementary Material
Footnotes
Preliminary data was presented in part at the 45th Annual Meeting of the Infectious Diseases Society of America, San Diego, CA (October 4-7, 2007).
Reference List
- 1.Altclas J, Requejo A, Jaimovich G, Milovic V, Feldman L. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis. 2002;34:723. doi: 10.1086/338721. [DOI] [PubMed] [Google Scholar]
- 2.Avery R, Pohlman B, Adal K, Bolwell B, Goldman M, Kalaycio M, et al. High prevalence of diarrhea but infrequency of documented Clostridium difficile in autologous peripheral blood progenitor cell transplant recipients. Bone Marrow Transplant. 2000;25:67–69. doi: 10.1038/sj.bmt.1702086. [DOI] [PubMed] [Google Scholar]
- 3.Bilgrami S, Feingold JM, Dorsky D, Edwards RL, Bona RD, Khan AM, et al. Incidence and outcome of Clostridium difficile infection following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;23:1039–1042. doi: 10.1038/sj.bmt.1701773. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti S, Lees A, Jones SG, Milligan DW. Clostridium difficile infection in allogeneic stem cell transplant recipients is associated with severe graft-versus-host disease and non-relapse mortality. Bone Marrow Transplant. 2000;26:871–876. doi: 10.1038/sj.bmt.1702627. [DOI] [PubMed] [Google Scholar]
- 5.Dettenkofer M, Ebner W, Bertz H, Babikir R, Finke J, Frank U, et al. Surveillance of nosocomial infections in adult recipients of allogeneic and autologous bone marrow and peripheral blood stem-cell transplantation. Bone Marrow Transplant. 2003;31:795–801. doi: 10.1038/sj.bmt.1703920. [DOI] [PubMed] [Google Scholar]
- 6.Gorschluter M, Glasmacher A, Hahn C, Schakowski F, Ziske C, Molitor E, et al. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis. 2001;33:786–791. doi: 10.1086/322616. [DOI] [PubMed] [Google Scholar]
- 7.Hanna H, Raad I, Gonzalez V, Umphrey J, Tarrand J, Neumann J, et al. Control of nosocomial Clostridium difficile transmission in bone marrow transplant patients. Infect Control Hosp Epidemiol. 2000;21:226–228. doi: 10.1086/501751. [DOI] [PubMed] [Google Scholar]
- 8.Kavan P, Sochor M, Nyc O, Lochmann O, Koutecky J, Skala PJ, et al. Pseudomembraneous clostridium after autologous bone marrow transplantation. Bone Marrow Transplant. 1998;21:521–523. doi: 10.1038/sj.bmt.1701117. [DOI] [PubMed] [Google Scholar]
- 9.Tomblyn M, Gordon L, Singhal S, Tallman M, Williams S, Winter J, et al. Rarity of toxigenic Clostridium difficile infections after hematopoietic stem cell transplantation: implications for symptomatic management of diarrhea. Bone Marrow Transplant. 2002;30:517–519. doi: 10.1038/sj.bmt.1703703. [DOI] [PubMed] [Google Scholar]
- 10.van Kraaij MG, Dekker AW, Verdonck LF, van Loon AM, Vinje J, Koopmans MP, et al. Infectious gastro-enteritis: an uncommon cause of diarrhoea in adult allogeneic and autologous stem cell transplant recipients. Bone Marrow Transplant. 2000;26:299–303. doi: 10.1038/sj.bmt.1702484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen KY, Woo PC, Liang RH, Chiu EK, Chen FF, Wong SS, et al. Clinical significance of alimentary tract microbes in bone marrow transplant recipients. Diagn Microbiol Infect Dis. 1998;30:75–81. doi: 10.1016/s0732-8893(97)00213-7. [DOI] [PubMed] [Google Scholar]
- 12.Blot E, Escande MC, Besson D, Barbut F, Granpeix C, Asselain B, et al. Outbreak of Clostridium difficile-related diarrhoea in an adult oncology unit: risk factors and microbiological characteristics. J Hosp Infect. 2003;53:187–192. doi: 10.1053/jhin.2002.1356. [DOI] [PubMed] [Google Scholar]
- 13.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 14.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Dubberke ER, Sadhu J, Gatti R, Reske KA, DiPersio JF, Devine SM, et al. Severity of Clostridium difficile-associated disease (CDAD) in allogeneic stem cell transplant recipients: evaluation of a CDAD severity grading system. Infect Control Hosp Epidemiol. 2007;28:208–211. doi: 10.1086/511792. [DOI] [PubMed] [Google Scholar]
- 16.Kyne L, Farrell RJ, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am. 2001;30:753–75x. doi: 10.1016/s0889-8553(05)70209-0. [DOI] [PubMed] [Google Scholar]
- 17.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 18.Roghmann MC, McCarter RJ, Jr, Brewrink J, Cross AS, Morris JG., Jr Clostridium difficile infection is a risk factor for bacteremia due to vancomycin-resistant enterococci (VRE) in VRE-colonized patients with acute leukemia. Clin Infect Dis. 1997;25:1056–1059. doi: 10.1086/516112. [DOI] [PubMed] [Google Scholar]
- 19.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.