Abstract
Our recent studies showed that total body irradiation (TBI) induces long-term bone marrow (BM) suppression in part by induction of hematopoietic stem cell (HSC) senescence through the reactive oxygen species (ROS). In the present study, we examined if Mn (III) meso-tetrakis (N-ethylpyridinium-2-yl) porphyrin (MnTE), a superoxide dismutase mimetic and potent antioxidant, can mitigate TBI-induced long-term BM injury in a mouse model. Our results showed that post-TBI treatment with MnTE significantly inhibited the increases in ROS production and DNA damage in HSCs and reduction in HSC frequency and clonogenic function induced by TBI. In fact, the clonogenic function of HSCs from irradiated mice after MnTE treatment was comparable to that of HSCs from normal controls on a per HSC basis, suggesting that MnTE treatment inhibited the induction of HSC senescence by TBI. This suggestion is supported by the finding that MnTE treatment also reduced the expression of p16Ink4a (p16) mRNA in HSCs induced by TBI and improved the long-term and multi-lineage engraftment of irradiated HSCs after transplantation. Therefore, the results from this study demonstrated that MnTE has the potential to be used as a therapeutic agent to mitigate TBI-induced long-term BM suppression by inhibiting IR-induced HSC senescence through the ROS-p16 pathway.
Keywords: Ionizing radiation, Oxidative stress, Hematopoietic stem/progenitor cells, Radioprotection, Manganese (III) meso-tetrakis (N-ethylpyridinium-2-yl) porphyrin (MnTE), SOD mimetic
INTRODUCTION
Bone marrow (BM) suppression is the most common dose-limiting side effect of conventional cancer therapy using ionizing radiation (IR) and/or certain chemotherapeutic agents and the primary cause of death after accidental or intentional exposure to a moderate or high dose of total body irradiation (TBI) [1–4]. Acute and residual (or long-term) BM injury not only limits the success of cancer treatment but also adversely affects the quality of life of cancer patients. It has been well established that acute myelosuppression occurs due to induction of apoptosis in the rapidly proliferating hematopoietic progenitor cells (HPCs) and to a lesser degree in the relatively quiescent hematopoietic stem cells (HSCs) after exposure to IR and chemotherapy [1, 5]. Acute BM suppression is an immediate medical concern, because it could cause high mortality and morbidity and worsen the outcome of cancer treatment. However, the management of acute myelosuppression has been significantly improved in recent years by the use of various hematopoietic growth factors (HGFs) such as granulocyte-colony stimulating factor, granulocyte/macrophage-colony stimulating factor, or erythropoietin, which have the ability to promote the recovery of BM hematopoietic function primarily by stimulating HSC and HPC proliferation and differentiation.
However, a large portion of the patients and the victims of nuclear events also develop residual BM injury manifested by a decrease in HSC reserves and impairment in HSC selfrenewal after recovering from acute myelosuppression [1, 2, 4]. Unlike acute myelosuppression, residual BM damage is latent. Patients and animals with residual BM injury usually have normal blood cell counts under normal homeostatic conditions in spite of a decrease in HSC reserves [6–9]. Because of this latency, the clinical implications of residual BM injury have been largely overlooked. Moreover, the importance of long-term BM damage is further obscured by the seemingly complete recovery of peripheral blood cell counts, BM cellularity and the number of colony-forming units (CFUs), especially after the use of HGFs. In fact, the use of HGFs may worsen chemotherapy- and IR-induced residual BM damage by promoting HSC and HPC proliferation and differentiation at the expense of HSC self-renewal [10–12]. This could lead to an accelerated exhaustion of HSCs and further compromise the long-term recovery of BM hematopoietic function. Although residual BM damage is latent, it is long lasting and shows little tendency for recovery, and can lead to the development of hypoplastic anemia or a meylodysplastic syndrome at later times or following additional hematopoietic stress such as subsequent cycles of consolidation cancer treatment or BM transplantation [2–4]. Therefore, effective treatments that can mitigate chemotherapy and irradiation-induced long-term BM suppression are needed.
Using a mouse model, we and others have shown that IR and chemotherapy induce residual BM injury primarily via induction of HSC senescence [6–9], which is an irreversible loss of the proliferation capacity of HSCs. More recently, our studies have demonstrated that exposure of mice to TBI induces persistent oxidative stress selectively in HSCs in part via up-regulation of NADPH oxidase 4 (NOX4) [13]. Oxidative stress can induce HSC senescence by causing sustained DNA damage and/or inducing p16Ink4a (p16) expression through the p38 MAPK pathway [14–18]. For example, several recent studies showed that induction of oxidative stress was also primarily responsible for the loss of HSC self-renewal and premature exhaustion of HSCs in mice with mutations in the ATM [15, 19] and certain Fanconi anemia (FA) genes [14] and deletion of FoxO3(s) [16–18]. Furthermore, prolonged treatment of these mice with an antioxidant can rescue the defect [15–19]. These new findings prompted us to examine if antioxidant treatment can mitigate TBI-induced long-term BM suppression by inhibiting TBI-induced oxidative stress and senescence in HSCs.
Mn (III) meso-tetrakis (N-ethylpyridinium-2-yl) porphyrin (MnTE) is one of the most potent synthetic superoxide dismutase (SOD) mimetics and an effective peroxynitrite scavenger [20, 21]. Its remarkable therapeutic effects in numerous oxidative stress-related diseases have been reported and summarized in recent reviews [20, 21]. For example, administration of MnTE prior to TBI substantially protected mice against radiation-induced lethality [22]. Furthermore, MnTE has been used successfully as an effective radioprotectant to prevent and mitigate pulmonary injury induced by IR [23]. In addition, it was reported recently that MnTE treatment reduced eye injury upon radiation [24]. However, MnTE provides no observable protection against the induction of apoptosis in various tumor cells by radiation and chemotherapeutic agents [20, 21]. Moreover, the antitumor effects of radiation [25] and certain chemotherapeutic agents [26] were greatly enhanced when they were combined with MnTE. Due to the remarkable therapeutic potential of MnTE, we examined its effects on TBI-induced long-term BM suppression in our well established and characterized mouse model. The data from these studies showed that post TBI treatment with MnTE significantly inhibited TBI-induced increases in ROS production, oxidative DNA damage, and DNA double strand breaks (DSBs) in HSCs. The reduction of oxidative stress was associated with a significant increase in HSC frequency and clonogenic function. These findings suggest that MnTE treatment may inhibit IR-induced HSC senescence. This suggestion is supported by the finding that MnTE treatment reduced IR-induced expression of p16Ink4a (p16) mRNA in HSCs and improved the long-term and multi-lineage engraftment of irradiated HSCs after transplantation. Therefore, the results from this study demonstrated that MnTE has the potential to be used as a therapeutic agent to mitigate IR-and chemotherapy-induced residual BM injury.
MATERIALS AND METHODS
Reagents
PE-conjugated anti-Sca-1 (Clone E13-161.7); APC-conjugated anti-c-kit (Clone 2B8); FITC-conjugated CD45.2 (Clone 104); PE- or biotin-conjugated anti-CD3e (Clone 145-2C11), anti-CD45R/B220 (Clone RA3-6B2), anti-Gr-1 (Clone RB6-8C5), anti-CD11b (Clone M1/70), and anti-Ter-119 (Clone Ter-119); purified rat anti-CD16/CD32 (Clone 2.4G2, Fcγ blocker); and FITC-conjugated streptavidin were purchased from BD-Pharmingen (San Diego, CA). APC-conjugated anti-B220 (Clone RA3-6B2) and anti-Thy1.2 (Clone 53-2.1) antibodies were obtained from eBioscience (San Diego, CA). Anti-phospho-Histone H2AX (Ser139) (γH2AX) (clone JBW301) and anti-8-Hydroxy-2’-deoxyguanosine (8-OH-dG) (clone N45.1) were purchased from Millipore (Billerica, MA) and Cosmo Bio Co., Ltd (Japan), respectively. Hoechst-33342 was obtained from Sigma-Aldrich (St. Louis, MO). MnTE was synthesized and purified as previously described [27]. 2', 7’-dichlorofluorescin diacetate (DCFDA) was obtained from Invitrogen (Carlsbad, CA).
Mice
Male C57BL/6 mice were purchased from The Charles River Laboratories through the National Cancer Institute. Mice were housed 4 to a cage at the Medical University of South Carolina (MUSC) and University of Arkansas for Medical Sciences (UAMS) AAALAC-certified animal facilities. Animals received food and water ad libitum. All mice were used at approximately 8–10 weeks-of-age. The Institutional Animal Care and Use Committees of MUSC and UAMS approved all experimental procedures used in this study.
Total body irradiation (TBI) and MnTE treatment
Mice were exposed to a sublethal dose (6.5 Gy) of IR in a JL Shepherd Model 143 137Cesium γ-irradiator (JL Shepherd, Glendale, CA) at a rate of 2.4 Gy/min. Mice were irradiated on a rotating platform. For MnTE treatment, six hours after TBI, mice were treated with MnTE (6 mg/kg, 0.1 ml) by s.c. injection and then the injection was repeated every day for 30 days. As a control, mice were irradiated or not irradiated but received s.c. injection of the same volume of vehicle (0.1 ml PBS).
Isolation of bone marrow mononuclear cells (BM-MNCs), lineage-negative hematopoietic cells (Lin−), HPCs (Lin− c-kit+ Sca1− cells) and HSCs (Lin− c-kit+ Sca1+ cells)
BM-MNCs and Lin− cells were isolated as described previously [8, 9]. HPCs and HSCs were sorted after pre-incubation of Lin− cells with anti-CD16/32 antibody to block the Fcγ receptors and then labeled with streptavidin-FITC, and anti-Sca-1-PE and anti-c-Kit-APC antibodies as we previously reported [8, 9].
Analysis of the levels of intracellular reactive oxygen species (ROS)
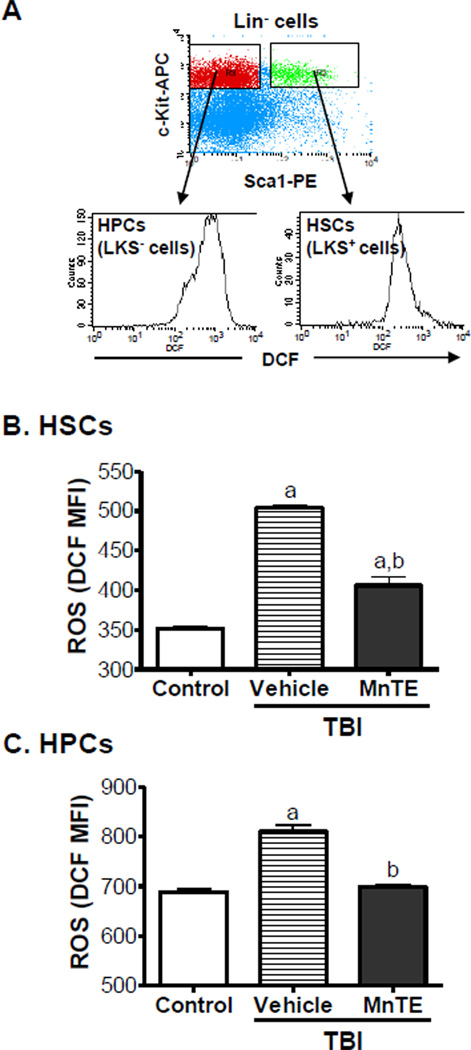
Lin− cells (1 × 106/ml) stained with anti-Sca-1-PE and anti-c-Kit-APC antibodies were suspended in PBS supplemented with 5 mM glucose, 1 mM CaCl2, 0.5 mM MgSO4 and 5 mg/ml BSA and then incubated with DCFDA (10 µM) for 30 minutes at 37 °C. The levels of ROS in HPCs and HSCs were analyzed by measuring the mean fluorescence intensity (MFI) of 2', 7’-dichlorofluorescein (DCF) using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA). For each sample, a minimum of 200,000 Lin− cells was acquired and the data were analyzed using CellQuest software (Becton-Dickinson). In all experiments, PE and APC isotype controls and other positive and negative controls were included as appropriate. Examples of the analysis of ROS production in HPCs and HSCs by flow cytometry are presented in Fig. 1.
Fig. 1. MnTE inhibits TBI-induced increase in ROS production in HSCs and HPCs.
A. A representative analysis of ROS production in HPCs and HSCs by flow cytometry. B. The levels of intracellular ROS in HSCs and HPCs were measured 30 days after mice were exposed to a sublethal dose (6.5 Gy) of TBI and then received daily s.c. injections of vehicle or MnTE (6 mg/kg). Cells from un-irradiated mice were included in the study as controls. The data are presented as mean ± SE (N = 3 independent assays) of mean fluorescent intensity (MFI) of DCF. a, p<0.05 vs. control; and b, p<0.05 vs. TBI with vehicle.
Immunofluorescent 8-OH-dG and γH2AX staining
Approximately 4,000 sorted HPCs and HSCs were cytospun onto slides and fixed in 4% paraformaldehyde solution for 10 min at room temperature. Cells were permeabilized with 0.2% Triton X-100 on ice and blocked with 5% fetal bovine serum prior to incubation with anti-8-OH-dG (1:1000) or anti-γH2AX (1:500) for 2 hours at room temperature. Cells were then treated with Alexa Fluor-568 conjugated anti-mouse IgG antibody (1:500) in 5% FBS solution for 1 hr at room temperature. Nuclei were counterstained with Hoechst-33342. Slides were finally mounted in Vectashield (from Vecta, Burlingame, CA). Images were acquired using a fluorescent microscopy as described below. Approximately 200 nuclei images were acquired for each sample using a fluorescent microscope and the number of γH2AX foci for each cell was accounted, averaged and expressed as γH2AX foci/cell.
Immunofluorescent microscopy
Cells were viewed and acquired by a Zeiss Axio Observer.Z1 (Carl Zeiss, Germany) with an Apo 60X/1.4 oil DICIII objective. The images were captured using AxioVision (4.7.1.0) software (Carl Zeiss). The collected images were then displayed with the Adobe Photoshop V6.0.
Analysis of HSC and HPC frequencies by flow cytometry
BM-MNCs were pre-incubated with biotin-conjugated anti-CD3e, anti-CD45R/B220, anti-Gr-1, anti-CD11b, and anti-Ter-119 antibodies and with anti-CD16/32 antibody to block the Fcγ receptors and then labeled with streptavidin-FITC and anti-Sca-1-PE and anti-c-Kit-APC antibodies as we previously reported [8, 9]. The frequencies of HSCs and HPCs were analyzed by flow cytometry using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA). For each sample, a minimum of 200,000 BM-MNCs was acquired and the data were analyzed using CellQuest software (Becton-Dickinson). The numbers of HSCs and HPCs in each mouse were calculated by multiplication of the total numbers of BM-MNCs harvested from the two hind legs of each mouse with the frequencies of HSCs and HPCs in BM-MNCs.
Colony-forming cell (CFC) assay and cobblestone area-forming cell (CAFC) assay
The CFC and CAFC assays were performed as described elsewhere [8, 9].
Analysis of p16 and p53 mRNA expression
The expression of p16 and p53 mRNA was determined by real-time RT-PCR as we previously reported [8, 9].
Competitive repopulation assay (CRA)
One million of BM-MNCs pooled from five irradiated C57BL/6 (CD45.2) mice (4 weeks after TBI) with or without MnTE treatment as described above were mixed with 2 × 105 competitive BM-MNCs pooled from three normal C57BL/6 congenic mice, e.g. B6.SJL-PtprcaPep3b/BoyJ or C57BL/6-Ly-5.1 (CD45.1). They were transplanted into lethally irradiated (9.5 Gy TBI) C57BL/6-Ly-5.1 mice (5–6 recipients/group) by tail vein injection. Donor cell engraftment was determined at 1 and 2 months after transplantation by immunostaining of the cells in the recipient peripheral blood with FITC-conjugated anti-CD45.2, PE-conjugated anti-B220, CD11b and Gr-1, and APC-conjugated anti-B220 and Thy1.2 antibodies and analyzed by flow cytometry.
Statistical Analysis
The data were analyzed by analysis of variance (ANOVA). In the event that ANOVA justified post hoc comparisons between group means, these were conducted using the Student-Newman-Keuls test for multiple comparisons. For experiments in which only single experimental and control groups were used, group differences were examined by unpaired Student's t test. Differences were considered significant at p < 0.05. All of these analyses were done using GraphPad Prism from GraphPad Software (San Diego, CA).
RESULTS
MnTE inhibits TBI-induced persistent oxidative stress in HSCs and HPCs
Our recent studies have shown that exposure of mice to a sublethal dose of TBI causes long-term BM suppression in part by induction of persistent hematopoietic oxidative stress and HSC senescence [13]. MnTE is a potent antioxidant that has the ability to reduce oxidative tissue damage in various pathological conditions by scavenging ROS [20, 21]. Therefore, we assumed that MnTE can be used as a therapeutic agent to effectively mitigate TBI-induced long-term BM suppression via inhibition of ROS production. To test this hypothesis, we exposed mice to a sublethal dose (6.5 Gy) of TBI. Six hours after irradiation, the mice were treated with vehicle or MnTE daily via s.c. injection for 30 days. ROS production in HSCs and HPCs was analyzed by flow cytometry after the last MnTE treatment. As shown in Fig.1, the production of ROS was significantly elevated in HSCs and HPCs even 30 days after TBI, demonstrating that TBI can cause persistent hematopoietic oxidative stress. The elevation of ROS production induced by TBI was greater in HSCs (1.43-fold) than that in HPCs (1.18-fold) as shown in our previous report [13]. MnTE treatment attenuated the elevation in ROS production in HSCs and HPCs, indicating that MnTE can effectively inhibit TBI-induced persistent oxidative stress in the hematopoietic system.
MnTE inhibits TBI-induced persistent DNA damage and increase in DSBs in HSCs and HPCs
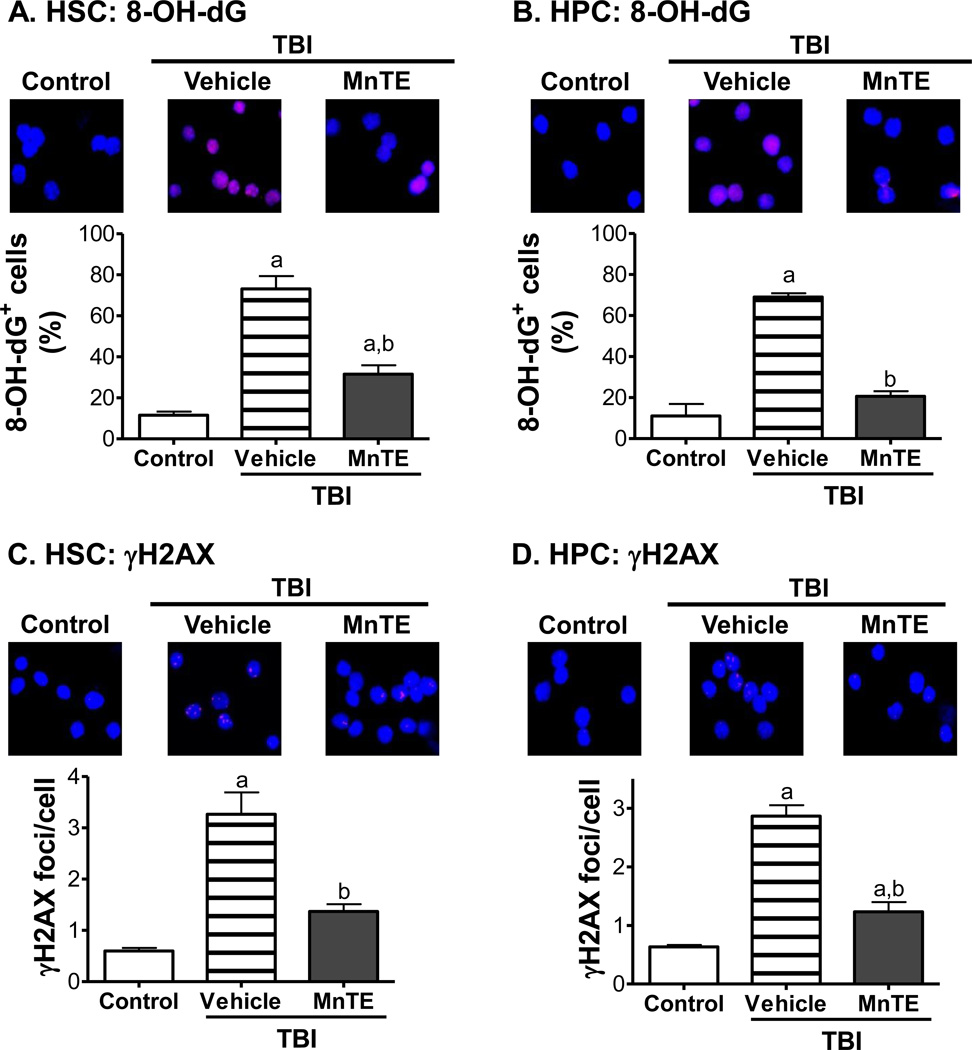
Since MnTE can inhibit TBI-induced ROS production in HSCs and HPCs, next we examined if MnTE can reduce TBI-induced DNA damage in HSCs and HPCs. This was achieved by immunostaining of sorted HSCs and HPCs with antibodies against 8-OH-dG and γH2AX to detect oxidative DNA damage and DSBs, respectively [28, 29]. As shown in Fig. 2, HSCs and HPCs from un-irradiated mice exhibited minimal 8-OH-dG immunostaining and had very few γH2AX foci. Thirty days after exposure to TBI, significant increases in 8-OH-dG immunostaining and numbers of γH2AX foci were observed in HSCs and HPCs from the irradiated mice without MnTE treatment. Treatment with MnTE significantly reduced the increases in 8-OH-dG immunostaining and number of γH2AX foci in these cells after TBI, indicating that MnTE treatment can inhibit TBI-induced sustained oxidative DNA damage and DSBs in HSCs and HPCs.
Fig. 2. MnTE reduces TBI-induced oxidative DNA damage and DSBs in HSCs and HPCs.
Mice were un-irradiated as controls or irradiated and then treated with vehicle or MnTE as described above. HSCs and HPCs were isolated from the mice 30 days after TBI by cell sorting and then immunostained with antibodies against 8-OH-dG and γH2AX to detect oxidative DNA damage and DSBs, respectively. A & B. Representative photomicrographs of HSC and HPC 8-OH-dG immunofluorescent staining (Red) and nucleic counterstaining with Hoechst-33342 (Blue) is shown in the upper panel. Percentages of 8-OH-dG positive HSCs and HPCs are presented in the lower panel as mean ± SE of 3 independent experiments. a, p<0.05 vs. control; and b, p<0.05 vs. TBI with vehicle. C & D. Representative photomicrographs of HSC and HPC γH2AX immunofluorescent staining (Red) and nucleic counterstaining with Hoechst-33342 (Blue) is shown in the upper panel. The average numbers of γH2AX foci per cell in HSCs and HPCs are presented in the lower panel as mean ± SE of 3 independent experiments. a, p<0.05 vs. control; and b, p<0.05 vs. TBI with vehicle.
MnTE attenuates TBI-induced residual BM injury in part via inhibition of HSC senescence
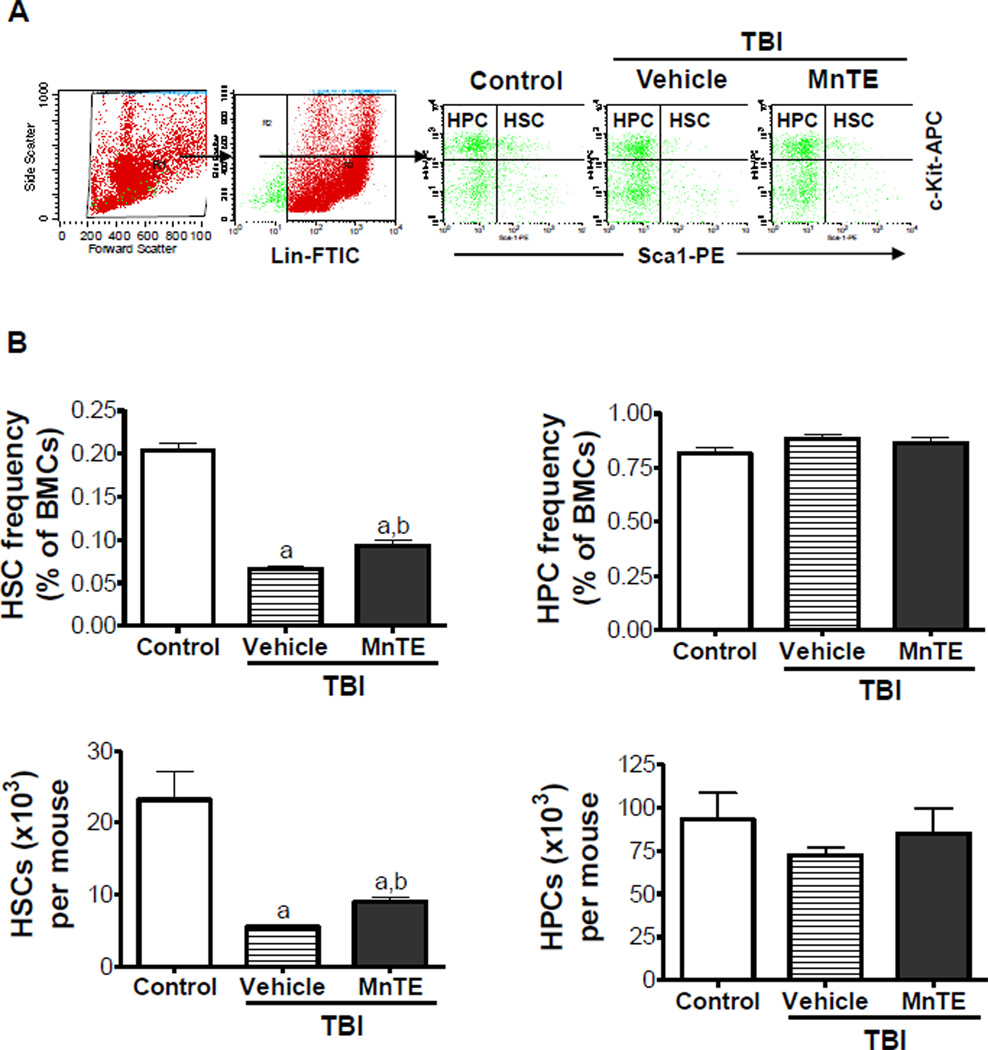
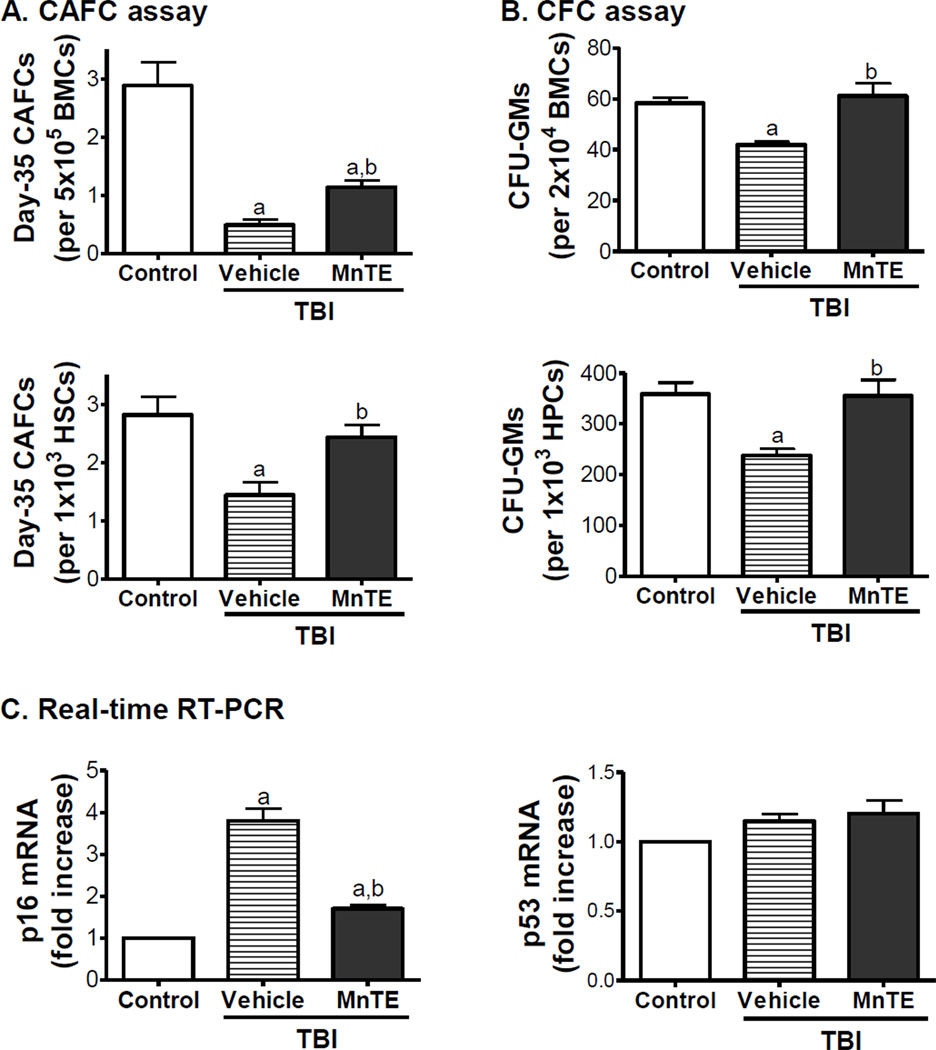
Our previous studies have shown that exposure of mice to TBI induces sustained hematopoietic oxidative stress and DNA damage in HSCs, which lead to induction of HSC senescence and long-term BM suppression [13]. This was confirmed in the present study as irradiated mice without MnTE treatment exhibited a substantial decrease in HSCs (Fig. 3) and a significant reduction of day-35 CAFCs and CFU-GMs that measure HSC and HPC clonogenic function (Figs.4A&B), respectively, while the levels of various blood cells and total BM MNC counts in the irradiated mice were relatively unchanged compared to un-irradiated animals (data not shown). The long-term BM suppression induced by TBI is likely attributable to the induction of HSC senescence as shown in our and others’ previous studies [6–9, 13]. This suggestion is supported by the findings that HSCs from irradiated mice expressed increased levels of p16 mRNA (Fig.4C), a widely used senescence biomarker and an important mediator of cellular senescence induction [30, 31]. There was no change in p53 mRNA expression in irradiated HSCs because the expression of p53 is regulated primarily at the level of post transcription (Fig.4C). However, after treatment with MnTE the irradiated mice showed a significant recovery in the frequency and number of HSCs and dramatic improvement in HSC and HPC function (Figs.3&4). In fact, the clonogenic function of HSCs from irradiated mice after MnTE treatment was comparable to that of HSCs from normal controls on a per HSC basis, suggesting that MnTE treatment inhibited IR-induced HSC senescence. This suggestion is supported by the finding that MnTE treatment also reduced IR-induced expression of p16 mRNA in HSCs (Fig.4C). These findings indicate that treatment with MnTE can attenuate TBI-induced residual BM injury at least in part via inhibition of HSC senescence.
Fig. 3. MnTE attenuates TBI-induced reduction of HSCs.
Mice were un-irradiated as controls or irradiated and then treated with vehicle or MnTE as described above. A. A representative gating strategy of HSC and HPC analysis by flow cytometry. B. The frequencies of HSCs and HPCs in BM-MNCs and absolute numbers of HSCs and HPCs per mouse are presented as mean ± SE of 3 independent experiments. a, p<0.05 vs. control; and b, p<0.05 vs. TBI with vehicle.
Fig. 4. MnTE mitigates TBI-induced residual BM injury in part by inhibition of HSC senescence.
Mice were un-irradiated as controls or irradiated and then treated with vehicle or MnTE as described above. A & B. The clonogenic function of HSCs and HPCs in BM-MNCs was measured by day-35 CAFC assay and CFC assay, respectively. The number of total day-35 CAFCs and CFU-GMs is also expressed as a function of HSCs and HPCs according to the frequencies of HSCs and HPCs in BM-MNCs quantified by flow cytometry. This is achieved by calculating the number of CAFCs and CFU-GMs in 1000 HSCs (Lin− c-kit+ Sca1+ cells) and HPCs (Lin− c-kit+ Sca1− cells), respectively, according to the formula: number of CAFCs or CFU-GMs / total number of HSCs or HPCs in 500,000 BM-MNCs × 1000. Data are presented as mean ± SE (N = 3). C. Analysis of p16 and p53 mRNA expression in HSCs by real-time RT-PCR. The data are presented as fold-change from controls (mean ± SE). a, p<0.05 vs. control; and b, p<0.05 vs. TBI with vehicle.
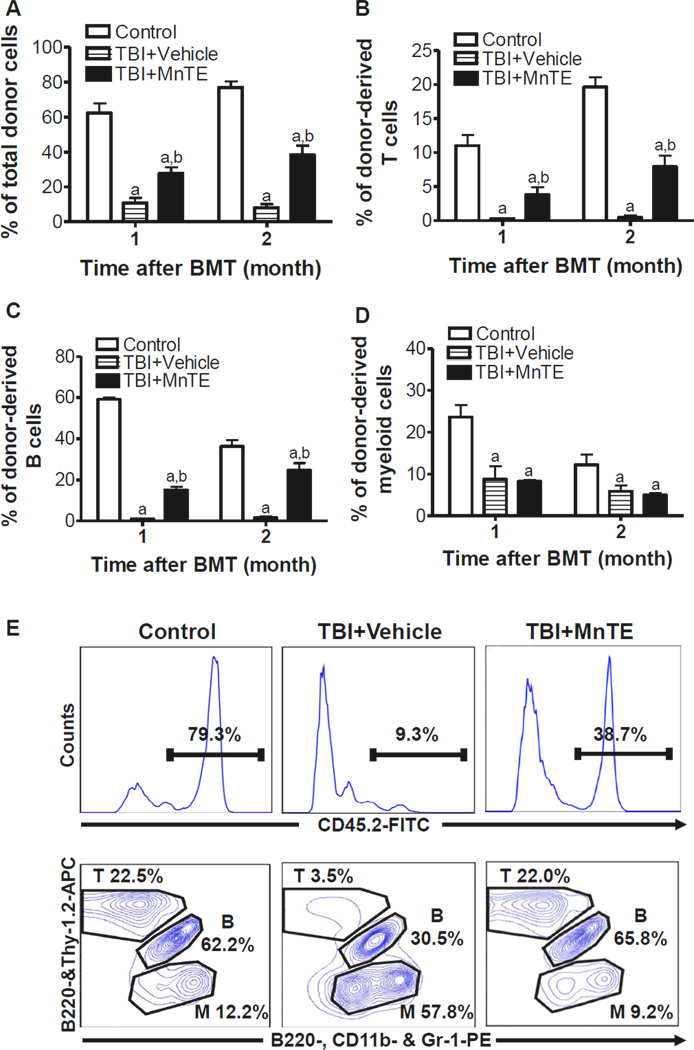
MnTE enhances long-term and multi-lineage engraftment of irradiated HSCs after BM transplantation
Since long-term and multi-lineage engraftment is the only gold standard to measure HSC function, we performed a competitive repopulation assay to validate whether inhibition of TBI-induced HSC senescence by MnTE treatment could improve HSC function. Mice receiving 106 BM-MNCs from un-irradiated mice along with 2 × 105 competitive BM-MNCs exhibited more than 62% of donor cell engraftment 1 month after transplantation (Fig.5A). The donor cell engraftment increased slightly up to about 76% at 2 months (Fig.5A), with 19.6% of T cells, 33.6% of B cells and 12.2% of myeloid cells derived from the donor cells (Fig.5B–D). As shown in Fig.5A–D, mice receiving the donor cells from irradiated mice with vehicle treatment showed a substantial decrease in donor cell engraftment in all the lineages examined at both time points with a skewing toward more myeloid cell lineage and less lymphoid cell differentiation (Fig.5E). The decrease was significantly reduced when the irradiated donor mice were treated with MnTE after TBI (Fig.5A–D). Moreover, the myeloid cell skewing seen in the cells from the irradiated mice treated with vehicle was also corrected by MnTE treatment as MnTE treatment significantly improved the lymphoid reconstitution after transplantation (Fig.5B, C & E). These findings indicate that MnTE treatment indeed preserved the function of HSCs after TBI, resulting in enhanced long-term and multi-lineage engraftment after BM transplantation.
Fig. 5. MnTE enhances long-term and multi-lineage engraftment of irradiated HSCs after BM transplantation.
Donor cell engraftment was determined at 1 and 2 months in lethally irradiated recipients after transplantation of BM-MNCs from control or TBI mice treated with vehicle or MnTE as described above in a competitive repopulating assay. The percentage of donor-derived peripheral blood leukocytes (CD45.2+ cells) (A), T cells (CD45.2+Thy-1.2+ cells) (B), B cells (CD45.2+B220+ cells) (C), and myeloid (CD45.2+CD11b+ and/or Gr-1+ granulocyte-monocyte-macrophage) (D) are presented as mean ± SE (5–6 mice/group). a, p<0.05 vs. control; and b, p<0.05 vs. TBI with vehicle. E & F. Representative analyses of donor cell engraftment by flow cytometry. Upper panel: total donor (CD45.2+) cell engraftment; Lower panel: engraftment of donor-derived T (CD45.2+Thy-1.2+ cells), B (CD45.2+B220+ cells), and M (myeloid, CD45.2+CD11b+ and/or Gr-1+) cells.
DISCUSSION
Currently, there are more than 11 million cancer survivors in the United States and most of them are expected to survive another 5 years or more after diagnosed with cancer. Unfortunately, long-term cancer survivors are at increasing risks to develop treatment-related late effects, such as residual BM injury [1–4]. The mechanisms by which IR and chemotherapy cause late normal tissue injury have not been fully elucidated. However, an increasing body of evidence suggests that the late effects are caused, at least in part, by the induction of chronic oxidative stress [32–34]. This suggestion is in agreement with our finding which demonstrates that IR induces long-term BM suppression via induction of persistent hematopoietic oxidative stress that leads to sustained DNA damage in HSCs and induction of HSC senescence. As such, we assumed that IR-induced residual BM injury may be treatable with a potent antioxidant.
To test this hypothesis, we examined the effect of MnTE on TBI-induced long-term BM injury in a mouse model, because MnTE has been proven efficacious in numerous in vitro and in vivo models of oxidative stress injuries, such as disorders of central nervous system, diabetes, and ischemia/reperfusion injury [20, 21]. In addition, it is very efficacious in preventing and mitigating IR-induced damage to various normal tissues [22–24]. While reducing radiation damage to normal tissues, MnTE does not protect tumor cells against the induction of apoptosis by ionizing radiation and chemotherapeutic agents [25, 26, 35]. Moreover, it acts as an anticancer agent in its own right and enhances the therapeutic efficacy of chemotherapy and radiotherapy [20, 25, 26, 35, 36]. The later effect has been assigned to its anti-angiogenic mode of action by suppressing HIF-1α activation and VEGF expression [21, 36]. Therefore, MnTE has the potential to increase the therapeutic efficacy of chemotherapy and radiotherapy by not only reducing normal tissue injury but also inhibiting tumor growth directly and indirectly via antiangiogenesis.
The results presented in this study provide the proof-of-principle that post TBI treatment with MnTE can effectively mitigate IR-induced residual BM injury. This is likely achieved by inhibition of TBI-induced chronic oxidative stress in HSCs, because MnTE is a potent antioxidant. It has been well established that oxidative stress can cause cellular senescence in a variety of cells including HSCs by induction of DNA damage such as DSBs [15, 19, 37, 38]. DSBs can initiate DNA damage response via sequential activation of ATM, Chk2, and p53 [39]. Activation of p53 induces p53 down-stream targets, including the cell cycle inhibitor p21Cip1/Waf1 (p21) to induce cell cycle arrest. Alternatively, ROS can also activate p38 through a mechanism remained to be determined [15]. Activation of both of these pathways can converge at p16 and increase in p16 expression can eventually lead to permanent cell cycle arrest or induction of cellular senescence [40–42]. By inhibiting ROS production, we assume that MnTE may prevent HSCs from undergoing senescence to mitigate TBI-induced long-term BM suppression. This assumption is supported by the findings that MnTE treatment inhibited TBI-induced persistent oxidative DNA damage and DSBs; and reduced the expression of p16 in HSCs after TBI, which led to a significant improvement of HSC clonogenic function and long-term repopulating activity after transplantation.
However, MnTE has many other functions and biological activities [20, 21]. Particularly, it can affect the expression of many genes by regulating cellular redox-based transcriptional activity of various transcriptional factors [21]. For example, MnTE can suppress HIF-1α activation to prevent the induction of TGF-β expression by IR, which may contribute to its pulmonary radioprotection in a rodent model of radiation-induced lung fibrosis [21, 23]. TGF-β is also a hematopoietic suppressive factor that plays an important role in the pathogenesis of BM suppression in patients with myelodysplastic syndromes and chronic anemia [43, 44]. It has yet to be determined whether inhibition of TGF-β production by MnTE can also contribute to the reduction of TBI-induced residual BM injury.
In addition, it has been suggested that induction of oxidative DNA damage is also probably responsible for the induction of hematopoietic genetic instability by IR and chemotherapy [45, 46]. Induction of hematopoietic genetic instability can lead to the development of leukemia and lymphoma in the victims of nuclear events and cancer patients after cancer treatment. Therefore, it will be interesting to determine if MnTE can also be used to inhibit IR- and chemotherapy-induced hematopoietic genetic instability for the prevention of cancer therapy-induced leukemia and lymphoma.
Furthermore, there is an increasing body of evidence demonstrating that oxidative stress is a common cause for the induction of premature exhaustion of HSCs and hematopoietic genetic instability under various pathological conditions, including aging and mutations in the ATM and certain Fanconi anemia (FA) genes [15–19]. Therefore, MnTE may have the potential to be used as a therapeutics for other oxidative stress-related hematopoietic pathology.
Acknowledgments
The authors thank Mrs. Aimin Yang for her excellent technical assistance and Mr. Richard Peppler and Dr. Haiqun Zeng at the Hollings Cancer Center Flow Cytometry & Cell Sorting Shared Resource for the flow cytometric analysis and cell sorting. This study was supported in part by grants from the National Institute of Allergy and Infectious Diseases, National Cancer Institute, and National Natural Science Foundation of China, the Winthrop W. Rockefeller Endowment for Leukemia Research, and the Arkansas Research Alliance Scholarship from the Arkansas Science & Technology Authority.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 2.Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985;5:101–110. [PubMed] [Google Scholar]
- 3.Gale RP. Myelosuppressive effects of antineoplastic chemotherapy. In: Testa NG, Gale RP, editors. Hematopoiesis: Long-term effects of chemotherapy and radiation. New York: Marcel Dekker; 1988. pp. 63–73. [Google Scholar]
- 4.Lohrmann HPE, Schreml W. Long-term hematopoietic damage after cytotoxic drug therapy for solid tumors. In: Testa N, Gale RP, editors. Hematopoiesis: Long-term effects of chemotherapy and radiation. New York: Marcel Dekker; 1988. pp. 325–337. [Google Scholar]
- 5.Meng A, Wang Y, Brown SA, Van ZG, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and - independent mechanisms. Exp Hematol. 2003;31:1348–1356. doi: 10.1016/j.exphem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Hellman S, Botnick LE. Stem cell depletion: an explanation of the late effects of cytotoxins. Int J Radiat Oncol Biol Phys. 1977;2:181–184. doi: 10.1016/0360-3016(77)90028-1. [DOI] [PubMed] [Google Scholar]
- 7.Mauch P, Rosenblatt M, Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood. 1988;72:1193–1196. [PubMed] [Google Scholar]
- 8.Meng A, Wang Y, Van ZG, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–5419. [PubMed] [Google Scholar]
- 9.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner RV, Begue R, McKinnon E. The effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on primitive hematopoietic stem cell (PHSC) function and numbers, after chemotherapy. Exp Hematol. 2001;29:1053–1059. doi: 10.1016/s0301-472x(01)00685-3. [DOI] [PubMed] [Google Scholar]
- 11.van OR, Robinson S, Sheridan T, Mislow JM, Dawes D, Mauch PM. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood. 1998;92:1950–1956. [PubMed] [Google Scholar]
- 12.van OR, Robinson S, Sheridan T, Mauch PM. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000;18:120–127. doi: 10.1634/stemcells.18-2-120. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal. 2008;10:1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283:25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 20.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide Dismutase Mimics: Chemistry, Pharmacology, and Therapeutic Potential. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batinic-Haberle I, Spasojevic I, Tse HM, Tovmasyan A, Rajic Z, St Clair DK, et al. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids. 2010 doi: 10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Park JW. A manganese porphyrin complex is a novel radiation protector. Free Radic Biol Med. 2004;37:272–283. doi: 10.1016/j.freeradbiomed.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao XW, Crapo JD, Mekonnen T, Lindsey N, Martinez P, Gridley DS, et al. Radioprotective effect of a metalloporphyrin compound in rat eye model. Curr Eye Res. 2009;34:62–72. doi: 10.1080/02713680802546948. [DOI] [PubMed] [Google Scholar]
- 25.Moeller BJ, Batinic-Haberle I, Spasojevic I, Rabbani ZN, Anscher MS, Vujaskovic Z, et al. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Jaramillo MC, Frye JB, Crapo JD, Briehl MM, Tome ME. Increased manganese superoxide dismutase expression or treatment with manganese porphyrin potentiates dexamethasone-induced apoptosis in lymphoma cells. Cancer Res. 2009;69:5450–5457. doi: 10.1158/0008-5472.CAN-08-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batinic-Haberle I. Manganese porphyrins and related compounds as mimics of superoxide dismutase. Methods Enzymol. 2002;349:223–233. doi: 10.1016/s0076-6879(02)49337-8. [DOI] [PubMed] [Google Scholar]
- 28.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyokuni S, Iwasa Y, Kondo S, Tanaka T, Ochi H, Hiai H. Intranuclear distribution of 8-hydroxy-2'-deoxyguanosine. An immunocytochemical study. J Histochem Cytochem. 1999;47:833–836. doi: 10.1177/002215549904700613. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol. 2004;39:1751–1759. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Gius D, Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal. 2006;8:1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 33.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(Spec No 1):S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 35.Jackson IL, G.-F.B.B.-H.I.P.S.Z.Y.D.M.a.V.Z Hyperthermia enhances the antiangiogenic effect of metalloporphyrin mimetic of superoxide dismutase. 24th Annual Meeting of the European Society for Hyperthermic Oncology; Prague, Czech. 2007. Republic. Ref Type: Conference Proceeding. [Google Scholar]
- 36.Rabbani ZN, Spasojevic I, Zhang X, Moeller BJ, Haberle S, Vasquez-Vivar J, et al. Antiangiogenic action of redox-modulating Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin, MnTE-2-PyP(5+), via suppression of oxidative stress in a mouse model of breast tumor. Free Radic Biol Med. 2009;47:992–1004. doi: 10.1016/j.freeradbiomed.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 38.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 40.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- 43.Allampallam K, Shetty V, Mundle S, Dutt D, Kravitz H, Reddy PL, et al. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. Int J Hematol. 2002;75:289–297. doi: 10.1007/BF02982044. [DOI] [PubMed] [Google Scholar]
- 44.Zorat F, Shetty V, Dutt D, Lisak L, Nascimben F, Allampallam K, et al. The clinical and biological effects of thalidomide in patients with myelodysplastic syndromes. Br J Haematol. 2001;115:881–894. doi: 10.1046/j.1365-2141.2001.03204.x. [DOI] [PubMed] [Google Scholar]
- 45.Huang L, Snyder AR, Morgan WF. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene. 2003;22:5848–5854. doi: 10.1038/sj.onc.1206697. [DOI] [PubMed] [Google Scholar]
- 46.Wright EG. Radiation-induced genomic instability in haemopoietic cells. Int J Radiat Biol. 1998;74:681–687. doi: 10.1080/095530098140943. [DOI] [PubMed] [Google Scholar]