Abstract
Purpose.
To investigate whether neuronal activity within the supraoculomotor area (SOA—monosynaptically connected to medial rectus motoneurons and encode vergence angle) of strabismic monkeys was correlated with the angle of horizontal misalignment and therefore helps to define the state of strabismus.
Methods.
Single-cell neural activity was recorded from SOA neurons in two monkeys with exotropia as they performed eye movement tasks during monocular viewing.
Results.
Horizontal strabismus angle varied depending on eye of fixation (dissociated horizontal deviation) and the activity of SOA cells (n = 35) varied in correlation with the angle of strabismus. Both near-response (cells that showed larger firing rates for smaller angles of exotropia) and far-response (cells that showed lower firing rates for smaller angles of exotropia) cells were identified. SOA cells showed no modulation of activity with changes in conjugate eye position as tested during smooth-pursuit, thereby verifying that the responses were related to binocular misalignment. SOA cell activity was also not correlated with change in horizontal misalignment due to A-patterns of strabismus. Comparison of SOA population activity in strabismic animals and normal monkeys (described in the literature) show that both neural thresholds and neural sensitivities are altered in the strabismic animals compared with the normal animals.
Conclusions.
SOA cell activity is important in determining the state of horizontal strabismus, possibly by altering vergence tone in extraocular muscle. The lack of correlated SOA activity with changes in misalignment due to A/V patterns suggest that circuits mediating horizontal strabismus angle and those that mediate A/V patterns are different.
The author shows that neurons in the midbrain near-response area adjacent to the oculomotor nucleus, that are known to encode vergence in normal monkeys show activity related to strabismus angle in monkeys with a sensory-induced strabismus.
Introduction
Infantile forms of strabismus occur in as much as 5% of all children.1–3 The exact cause of strabismus is often unknown.3–5 Many diverse factors, including refractive errors (anisometropia); visual acuity factors (congenital cataracts); genetic factors (congenital fibrosis of extraocular muscle, Marfan's syndrome); brainstem pathology (Duane's syndrome); and muscle pathology (dysthyroid opthalmopathy), likely trigger a cascade of events that result in misaligned eyes.6–14 Despite the generally accepted notion that most strabismus must be a “brain problem,” not much is known about how different neural structures contribute to the development and maintenance of the strabismic state. An innervational source for strabismus can be triggered by specific genetic mutations causing structural changes in cranial nerves and thereby resulting in dysinnervation and atrophy of specific extraocular muscles (generally termed as congenital cranial dysinnervation disorders), but these tend to be relatively rare.15 Pathologies that involve some sort of sensory insult during development, resulting in a cascading disruption in development of visual and oculomotor circuits and thereby misaligned eyes is more common.2,16,17
In cases of strabismus that is not due to an obvious paralytic or restrictive factor, a common feature among the different trigger factors and correspondingly the different approaches to producing animal models for strabismus is that binocular vision is disrupted in early life due to breakdown in either motor fusion (e.g., surgical strabismus models) or sensory fusion (e.g., optically induced strabismus).18–20 We have previously reported that rearing infant monkeys with daily alternating monocular occlusion (AMO) for the first several months of life results in a permanent strabismus whose properties include A/V patterns, dissociated vertical deviation (DVD), low amplitude latent nystagmus and alternating fixation, making the AMO model appropriate for studying human strabismus due to sensory disruption.21–23 It was also shown, in the AMO model, that there was a direct correlation between the responses of horizontal and vertical motoneurons and the state of horizontal or vertical misalignment.24,25 This was true for both the steady-state angle of misalignment and the eye movements associated with A-patterns of strabismus and DVD observed in these animals. These studies presented the first direct evidence that the brain was intimately involved in maintaining the strabismic state.
Although motoneurons showed correlated activity with abnormal alignment and abnormal eye movements associated with strabismus, it is unlikely that they are the source of the problem. Central structures are likely providing aberrant inputs to motoneurons. When considering sources of such aberrant input to the motoneurons, the supraoculomotor area (SOA) is implicated because of its purported role in binocular eye movements. The SOA is the area immediately adjacent to the oculomotor nucleus. Neurons in this area receive major projections from the fastigial nucleus and the posterior interposed nucleus in the cerebellum, and also project monosynaptically to the medial rectus motoneurons in the oculomotor nucleus.26 In the normal animal, neurons in the SOA show responses exclusively related to convergence or divergence eye movements and ocular accommodation.27,28 Hence, it is also referred to as the midbrain near-response region. Disruptions in this circuit could therefore produce a bias in the “vergence tone” provided by the SOA to the motoneurons, thereby resulting in strabismus. The goal of the current study was to examine the possible role of the SOA in setting the state of horizontal ocular misalignment. This study indicates that cells in the supraoculomotor area show responses that are related to strabismus angle in AMO monkeys with strabismus. Some of these data have appeared before in abstract form and in conference proceedings (Das VE. IOVS 2010;51:ARVO E-Abstract 2997).29
Methods
Subjects and Rearing Paradigms
Two strabismic juvenile rhesus (Macaca mulatta) monkeys (ages 5 and 7 years; weights 7 and 10 kg) were the subjects of the study. These were the same two animals that participated in a recently published study recording from medial rectus motoneurons.24 Strabismus was induced by disrupting development of binocular vision in infant monkeys using a daily alternating monocular occlusion (AMO) method. In this rearing paradigm, within the first 24 hours of the animals' birth, dark contact lenses are placed in front of one eye for a period of 24 hours and then switched to the fellow eye for the next 24 hours. Thereafter, the eye of occlusion is alternated daily for a period of 4 months. Other publications provide additional detail on properties of the strabismus due to AMO rearing (Das VE, et al. IOVS 2007;48:ARVO E-Abstract 5273).21–23,30
Surgical Procedures
After special rearing, the AMO animals were allowed to grow normally (unrestricted vision) until they were approximately 3–4 years of age, before behavioral and neurophysiological experiments were begun. Sterile surgical procedures performed under aseptic conditions using isoflurane anesthesia (1.25%–2.5%) were used to stereotaxically implant a head stabilization post and a recording chamber (21-mm diameter titanium cylinder; stereotaxic implant coordinates: Anterior 3-mm and lateral 1-mm with respect to ear-bar-zero; 20° tilt angle to the sagittal plane). This chamber placement allowed full access to both oculomotor nuclei and the area immediately adjacent to the oculomotor nucleus (the supraoculomotor area). During the same surgical procedure, a scleral search coil was implanted in one eye according to the method of Judge and colleagues.31 Later, in a second surgery, a second scleral search coil was implanted in the other eye. All procedures were performed in compliance with the National Institutes of Health and the Association for Research in Vision and Ophthalmology guidelines, and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Emory University.
Experimental Paradigms, Data Acquisition, and Analysis
Binocular eye position was measured using the magnetic search coil method (Primelec Industries, Regensdorf, Switzerland). Eye coil signals were calibrated by rewarding the monkey for looking within a ±2° window surrounding a 0.5° target spot that was rear projected on a tangent screen 60 cm away from the animal. Calibration of each eye was performed independently during monocular viewing. Visual stimuli were generated under computer control using the VSG2/5 stimulus generator installed in a Windows PC (Cambridge Research Systems, Cambridge, England). Single unit data was recorded using epoxy-coated tungsten electrodes (1–5 Mohm, Frederick Haer, Brunswick, ME).
Binocular eye, target, and neural data were collected as the monkeys performed fixation and horizontal or vertical sinusoidal smooth-pursuit (0.2 Hz, ± 10°) tasks under monocular viewing conditions. Eye of fixation was controlled by occluding one or the other eye using liquid crystal shutter goggles (Micron Technology Inc., Boise, ID) that were under computer control. Eye and target position feedback signals were processed with anti-aliasing filters (Krohn-Hite; Krohn-Hite Corporation, Brockton, MA) at 400 Hz before digitization at 1 kHz with a 12-bit precision (Alpha-Lab System; Alpha-Omega Engineering, Nazareth, Israel). Raw spike data was acquired at a sampling rate of 32 kHz. Spike sorting was performed offline using a template matching algorithm (Spike2 software; Cambridge Electronic Design, England). Data analysis was performed with custom software routines (MATLAB; Mathworks Inc., Natick, MA). Unit response was represented as a spike density function that was generated by convolving time stamps with a 20-ms Gaussian.32 Eye and target position data were filtered using an 80point finite impulse response digital filter with a pass band of 0 to 80 Hz prior to analysis.
The goal of the analysis was 2-fold. Study authors first wanted to establish whether changes in neuronal firing rates observed in the SOA cells corresponded to the changes in angle of misalignment observed when the eye of fixation was switched. Second, study authors wanted to investigate whether the changes in misalignment that occurred due to the presence of A-patterns were correlated with changes in SOA neuronal responses.
At the end of the experiments, the animals were perfused and brains removed for histology. For histological evaluation of the recording sites, the brain was blocked and 40-μ thick coronal plane sections were cut stereotaxically and stained for Nissl substance.
Results
Properties of Strabismus
Eye misalignment in the two animals in this study has been described before.24 Briefly, animal M1 had an exotropia of approximately 15–20° during right eye viewing and 30° during left eye viewing. Animal M2 had an exotropia of approximately 10° during right eye viewing and 15–20°deg during left eye viewing. Thus, a change in eye misalignment was brought about by simply changing the eye of fixation and is considered evidence for a dissociated horizontal deviation (DHD). In addition, the animals also presented with DVD and A-patterns. Thus, during monocular viewing horizontal and vertical smooth-pursuit, there was an inappropriate cross-axis component in the non-viewing eye that leads to the appearance of DVD and A-pattern strabismus (see Fig. 2 in Joshi and Das, 2011). Both monkeys exhibited a small latent nystagmus with frequency of <1 Hz and velocity <1°/sec for M1 and a frequency of 1.5–2 Hz and velocity of approximately 1.5°/second for M2.
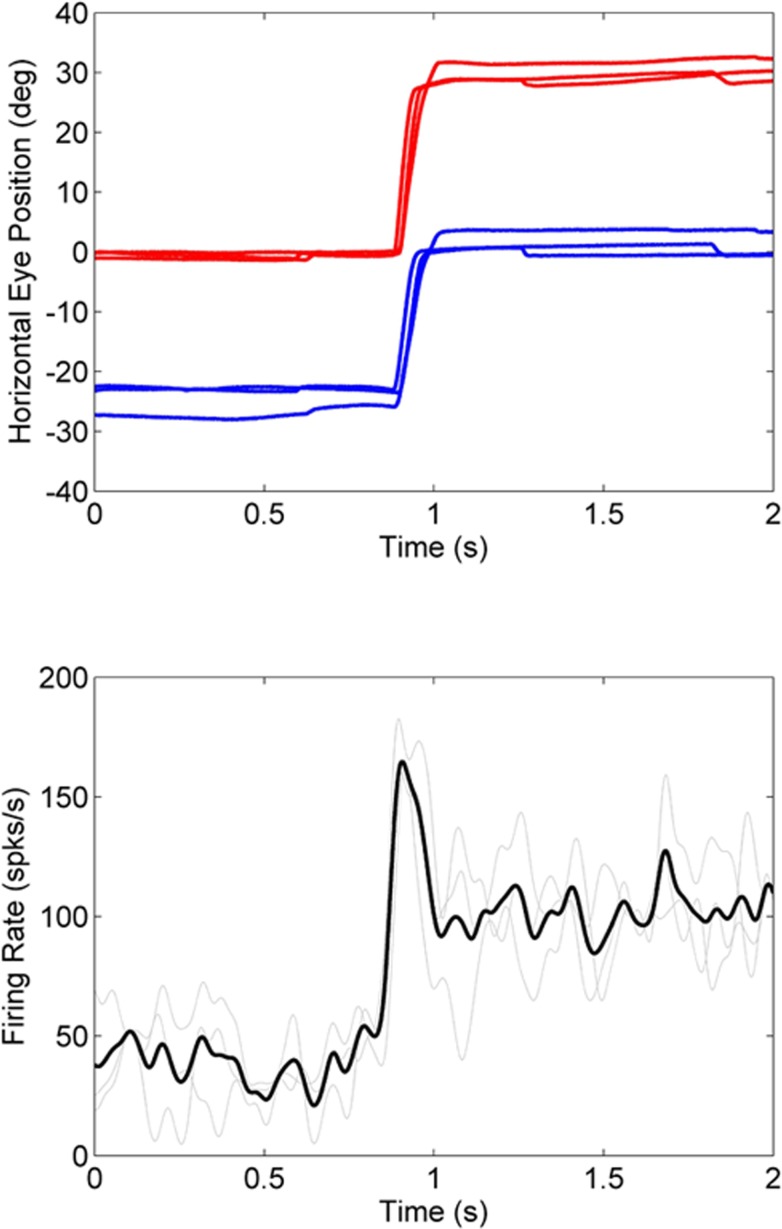
Figure 2. .
Firing rate properties of a near-response cell in animal M2. When the right eye is viewing the target, the angle of exotropia is low (∼10°) and the neural response rate is high (∼100 spks/s). When the left eye is viewing the target, the angle of exotropia is high (∼17°) and the neural response is low (∼25 spks/s). Legend is same as in Figure 1.
SOA Cell Responses during Changes in Eye Misalignment
Data were acquired from 35 cells in the SOA of the two animals (23 from M1 and 12 from M2), that were modulated as a result of change in eye misalignment. Two different types of strabismus-related cells were encountered. The more commonly encountered cells (28/35 cells; 80%) were those that showed an increased firing rate for a smaller angle of exotropia (e.g., brought about by changing the eye of fixation from left eye viewing to right eye viewing in animal M1). These cells were called the near-response cells as they increased firing rate for a ‘convergence' directed eye movement. Note that the eyes are not converged per se; rather there is a reduction in the degree of divergent misalignment (exotropia) when the eye of fixation is changed. The other cell type was far-response cells (7/35 cells, 20%), as they showed an increased firing rate for a larger angle of exotropia.
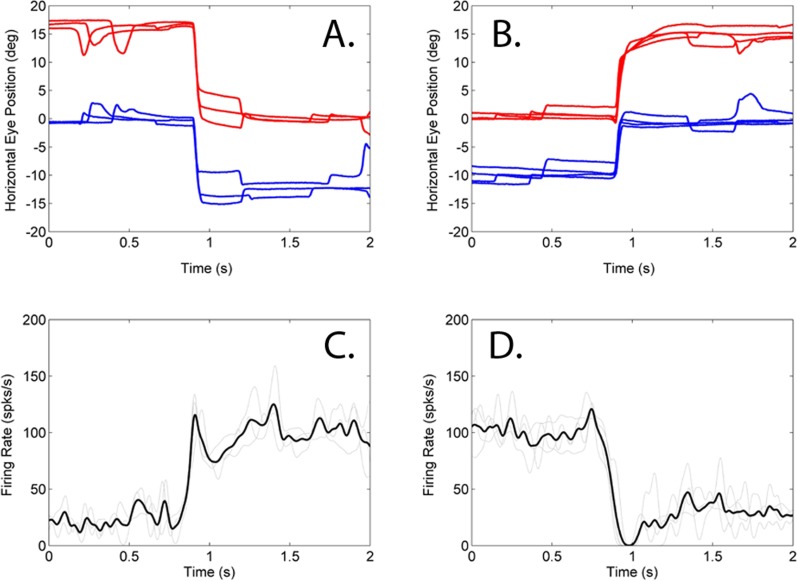
Figure 1 shows an example of a near-response cell in the SOA of animal M1 during monocular viewing of the straight-ahead fixation target (0° in top row panels of Fig. 1). The top row shows the positions of the left and right eyes for several trials in which the occluding patch was switched from the left eye to the right eye (Panel A) or from the right eye to the left eye (Panel B) during this fixation task. Note that the eye misalignment (difference in left and right eye positions) is significantly different between the right eye viewing and left eye viewing conditions. The bottom row (Panels C and D) shows the corresponding neural response rate during the switch in fixation. The data show that the cell responds with an increased firing rate during right eye fixation (smaller angle of exotropia) when compared with left eye fixation (greater angle of exotropia). For this cell, the change in neural response led the fixation switch by approximately 77 ms.
Figure 1. .
Firing rate properties of a near-response cell in animal M1. Top row shows multiple overlaid trials of fixation switch from right eye viewing to left eye viewing (A) or vice-versa (B) of a straight ahead (0°) target. Bottom row (C, D) shows the neural responses. Trials are aligned on the fixation switch. When the right eye is viewing the target (0–1 seconds in left column; 1–2 seconds in right column), the angle of exotropia is low (∼20°) and the neural response rate is high (∼125 spks/s). When the left eye is viewing the target (1–2 seconds in left column; 0–1 seconds in right column), the angle of exotropia is high (∼30°) and the neural response is low (∼20 spks/s). Legend: Right Eye – red; Left eye – blue; Individual trial firing rates – gray; average firing rate – black; rightward movements are positive and leftward movements are negative.
Figure 2 shows an example of a near-response cell from the second exotropic animal M2 with similar response properties as that of the example in Figure 1 from animal M1. Once again, a smaller angle of exotropia (right eye viewing of a straight-ahead target) is associated with a higher firing rate. For this cell, the change in firing rate led the fixation switch by approximately 51 ms.
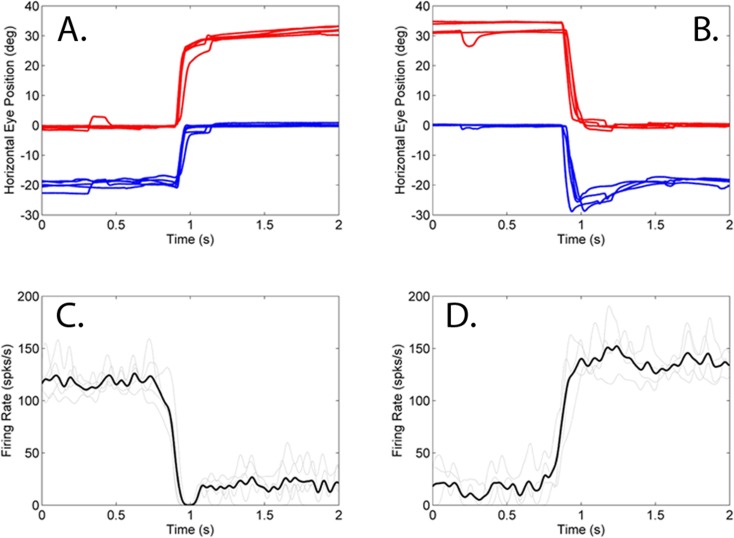
In addition to the near-response cells, a minority population of cells that showed an increased firing rate for larger angles of exotropia was also identified. Figure 3 shows an example of such a cell. In this cell, an exotropia of approximately 20° is associated with a neural response of approximately 40 spks/s. When the eye of fixation is switched from right to left, the angle of exotropia increases to approximately 30° and the neural firing rate of the cell is now approximately 100 spks/s. Similar to the near-response cells, the firing rates of the far-response cells also lead the fixation switch and for the example cell in Figure 3, the neural response lead is approximately 37 ms. This particular cell also showed a prominent burst at the time of fixation switch that was most likely related to vergence velocity.
Figure 3. .
Firing rate properties of a far-response cell in animal M1. When the right eye is viewing the target, the angle of exotropia is low (∼20°) and the neural response rate is low (∼40–50 spks/s). When the left eye is viewing the target, the angle of exotropia is high (∼30°) and the neural response is high (∼100 spks/s). Legend is same as in Figure 1.
SOA Cell Responses during Conjugate Eye Movements
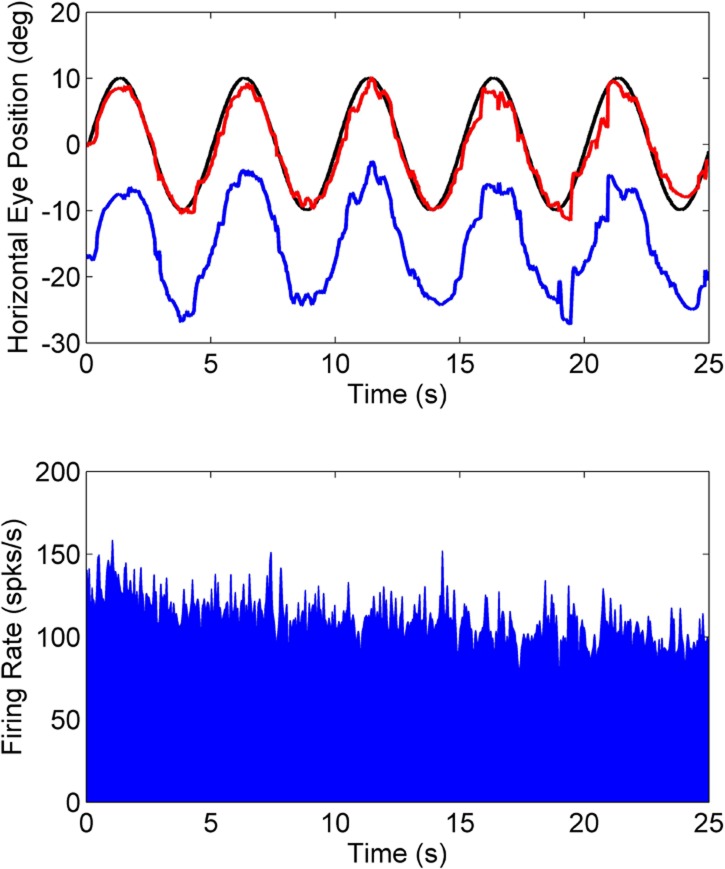
A fundamental question was whether the SOA cells recorded here indeed encoded eye misalignment and not just the position of one of the eyes. Therefore, these cells were also tested during conjugate horizontal smooth-pursuit eye movements. Figure 4 shows an example of neural response during conjugate smooth-pursuit. As clearly observed in the data plot, conjugate changes in eye position are not correlated with the SOA neural response. Since there is no change in horizontal eye misalignment during the horizontal smooth-pursuit tracking task, there is no modulation of the SOA cell response. Cells in the SOA are also known to encode ocular accommodation. Although accommodation was not measured in this study, it is possible that the gradual decay observed in the SOA cell response during the tracking task is due to change in the accommodative state of the animal. The combined response characteristics shown in Figures 1–4 are evidence that the SOA cells indeed encode eye misalignment and not eye position.
Figure 4. .
Firing rate properties of a near-response cell in animal M2 during conjugate smooth-pursuit. Animal was performing a horizontal smooth-pursuit task (0.2 Hz, ± 10°) while viewing with the right eye (top panel; right eye – red; left eye – blue; target – black). The neural response (bottom panel) shows no modulation of firing rate indicating that the SOA cell does not encode conjugate eye position.
Quantification of Eye Misalignment Sensitivities of SOA Cells
In order to quantify the relationship between the SOA cell response and the angle of eye misalignment, the neuronal sensitivity to changes in eye misalignment was calculated by performing a regression between the average firing rate (calculated over a 200-ms period of fixation) and the corresponding strabismus angle. Data obtained during several trials of fixation with each eye viewing were used to develop the fit. The slope of the regression line for each cell is a measure of the neuronal sensitivity (spikes/s/deg of misalignment) of the cell, and the threshold is a measure of the angle of exotropia at which the cell commences firing.
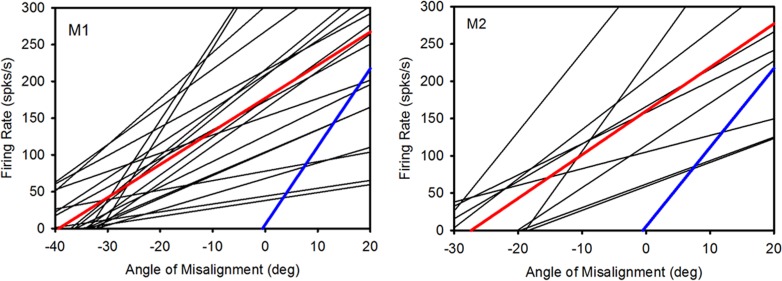
Panels in Figure 5 show the family of rate-misalignment curves for the near-response SOA cells in monkeys M1 and M2. Also shown in the plots are the population averages for each strabismic animal (red lines) and for comparison, the population average for normal animals (blue lines) as redrawn from the data published by Mays.28 Comparing the strabismic and normal animals yields two striking observations. First, the population thresholds for the strabismic animals are significantly shifted towards exotropia (divergent state) (M1: −39.3°; M2: −27.3°; Normal: −0.5°). Second, there is a significant reduction of population sensitivity in SOA cells in the strabismic animal when compared with the normal animal (M1: 4.50 ± 2.85 spks/s/deg; M2: 5.85 ± 3.39 spks/s/deg; Normals: 10.6 ± 4.08 spks/s/deg; One-way ANOVA with comparison to normal controls; P < 0.001).
Figure 5. .
Summary of response properties of SOA near-response cells in animals M1 and M2. Each black line represents the rate-misalignment curve for a single SOA cell. The x-intercept is the threshold or angle of misalignment at which the cell commences firing. The slope is a measure of the sensitivity of the cell. The red line is the population average of all the SOA near-response cells of the sample in each monkey. Shown for comparison is population average for normal monkeys (blue line; redrawn from Mays 1984).
We did not attempt a similar statistical analysis with the far-response cells since the number of cells recorded in each animal was low. However the average sensitivity for the far response cells was −4.86 spks/s/deg in M1 (n = 4) and −6.99 spks/s/deg in M2 (n = 3). For comparison, the far-response sensitivity in a normal monkey is −10.3 spks/s/deg.28
SOA Cell Responses during Cross-Axis Eye Movements
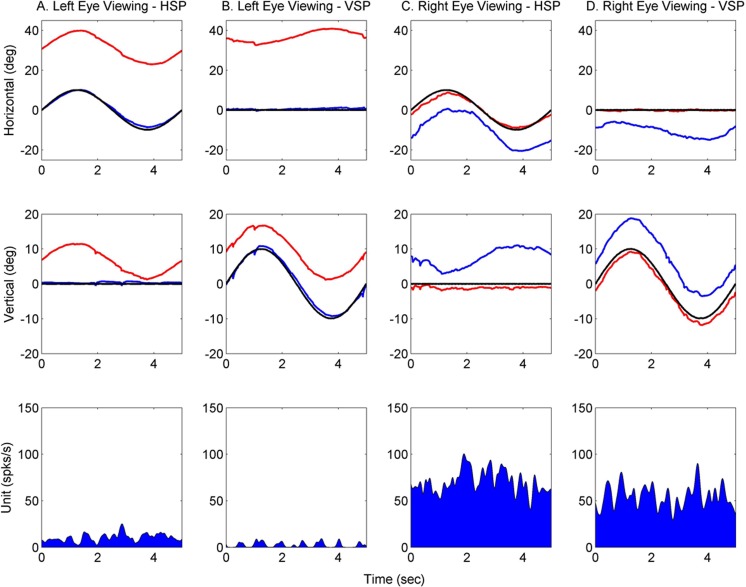
The animals under study also show A-patterns, which is effectively a change in horizontal eye misalignment with up or down-gaze (see Fig. 1 in Joshi and Das, 2011). During eye movements, the A-patterns manifest as an inappropriate horizontal eye movement in the non-fixating eye during a vertical tracking task. A question was whether there was any modulation in activity of SOA cells that was correlated with change in eye misalignment due to the inappropriate cross-axis eye movement (A-pattern). Figure 6 shows an example of neural response in a near-response cell from animal M1 during horizontal and vertical smooth-pursuit with either left or right eye viewing. Columns A and C show data obtained during horizontal smooth pursuit. As shown before in Figures 1–3, there is a shift in baseline activity of the cell due to change in eye misalignment, but there is no modulation due to change in horizontal eye position. Columns B and D show data obtained during vertical smooth pursuit with either the right or left eye viewing. The top panel in columns B and D show that there is an inappropriate horizontal eye movement in the non-viewing eye only during vertical smooth pursuit. Therefore, there is effectively a change in horizontal eye misalignment during the vertical smooth–pursuit task. However, examination of the neural response shows that the change in eye misalignment was not accompanied by any modulation of the cell activity. The same outcome was observed in all of the cells studied. This result suggests that the cross-axis eye movements that lead to A-pattern strabismus are not driven via vergence-related circuits.
Figure 6. .
SOA near-response cell firing properties during horizontal and vertical smooth-pursuit. Top row shows averaged horizontal positions of left (blue) and right (red) eyes. Middle row shows averaged vertical positions and the bottom row shows the average firing rates for each tracking condition. During left eye viewing (first two columns), the strabismus angle is large (∼30°) and the firing rate is low. During right eye viewing (last two columns), the strabismus angle is lower (∼15–20°) and the firing rate is high. During vertical smooth-pursuit (second and fourth column), there is a change in horizontal misalignment as evidenced by the cross-axis horizontal component in the covered eye. However there is no modulation of the SOA cell that is correlated with this aspect of change in eye misalignment.
Discussion
In this study, cells that appear to carry a signal related to the horizontal eye misalignment were identified for the first time. A second finding is that although these SOA cells appear to encode eye misalignment, they do not drive horizontal cross-axis eye movements leading to A-patterns in strabismus. These results, therefore, provide new insight into the disruption of neural circuits that leads to the appearance of problems of binocular eye alignment and binocular coordination of eye movements in strabismus.
Correlation of SOA Cell Activity and Eye Misalignment
The cells recorded in this study were localized to the SOA in the strabismic monkeys. Several reasons suggest that these cells are the same as those that have been reported to encode vergence angle in normal animals.28,33,34 First, the anatomical locations of these cells (1–2 mm dorsal and dorsolateral to oculomotor neurons) correspond very well to the midbrain near-response region identified before. Study authors were also able to verify the location of the recording via histological reconstruction of electrode track penetrations (Fig. 7). Second, the neuronal response characteristics correspond very well to the near-response cells of the normal animal. Near-response and far-response cells in the normal animal show modulation related to vergence (difference in position of the two eyes), but not conjugate eye movements. Similarly, cells in the study sample show responses related to strabismus angle (difference in position of the two eyes, Figs. 1–3), but not conjugate eye movements (Fig. 4). Finally, many more near-response cells were encountered than far-response cells, similar to the distribution reported in earlier studies.
Figure 7. .
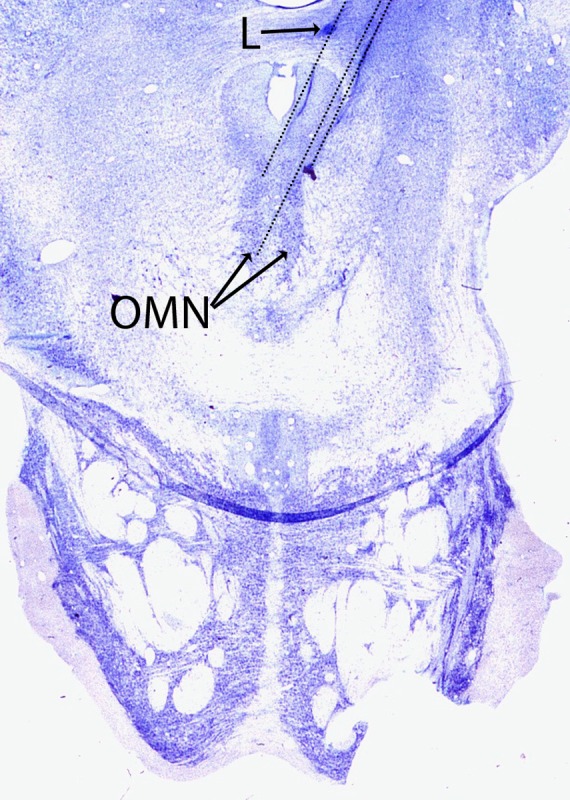
Nissl-stained coronal section at the level of the oculomotor nucleus showing the approximate recording location of SOA cells. The thin dotted lines show representative penetrations of the electrode to the oculomotor nucleus (OMN). The penetrations were at an angle of 20° to the midline. The SOA cells were generally located 1–2 mm dorsal/dorsolateral to the motoneurons in the oculomotor nucleus. A marking electrolytic lesion (L) approximately 2 mm dorsal to the SOA cells is also shown.
Comparison of the strabismic and normal animals yields significant differences in both the population threshold and the population sensitivity of the SOA cells. The threshold for normal animals is close to 0° while the threshold for the two strabismic animals was around −40° and −27°. Of note is that the SOA threshold is close to the larger of the two angles of horizontal misalignment (one for each monocular viewing condition) for each of the strabismic animals. The observation of significant levels of SOA activity even in the divergent state suggests that the SOA cells are indeed involved in maintaining the state of horizontal misalignment.
The reduced thresholds can perhaps be explained from within a recently developed framework for binocular control.35,36 Thus, King and colleagues proposed that the neural integrators encoded monocular eye position and that they provided inputs to the SOA such that SOA activity encoded the difference in position of each eye. In the normal monkey, during a conjugate eye movement, SOA activity would simply provide a DC signal to the medial rectus motoneurons that may be referred to as the “vergence tone.” During a vergence movement (again, in the normal monkey), the SOA cells provide a required disparity-driven positional command to the medial rectus motoneurons that eventually helps to adduct each eye. The attractive feature of this framework for the current data is that it provides no constraint on the threshold of the SOA cells. Since the SOA simply encodes the difference in eye position of the two monocular integrators, they could just as easily encode strabismus angle (study data) as vergence (normal monkeys). If, on the other hand, the SOA were solely a “vergence center,” the reduced thresholds that were observed might not have been expected and the prediction might have been that these cells would be shut-off in the exotropic state. Of course, this study cannot comment on whether the difference signal is arriving from monocular neural integrators, but it stands to reason that there is some representation of each eye's position upstream of the SOA.
Note that the alternate framework wherein the SOA supplies a vergence command to medial rectus motoneurons cannot be ruled out definitively. In this scenario, it would have to be hypothesized that the thresholds of the SOA cells were adaptively altered (a vergence offset) in the strabismic animals toward the divergent (exotropic) direction. Thereafter, any modulation of SOA cell activity could be the source of the vergence command that leads to observed change in eye misalignment. There is in fact some evidence that SOA cells can adapt to different levels of tonic vergence. Morley and colleagues showed that a relationship between SOA cell activity and vergence was altered in approximately 70% of cells following phoria adaptation.37 Perhaps a similar adaptive mechanism can cause drastically reduced thresholds in the strabismic monkeys.
The second difference between the normal and strabismic animals' SOA activity was the reduced sensitivities. Study findings indicate that the reduced sensitivity for vergence would result in a reduced “vergence” input provided by the SOA to the medial rectus motoneurons and, therefore, could manifest as a reduced vergence tone in extraocular muscle resulting in the monkeys maintaining an exotropic state. In support of this hypothesis, the strabismic animal with the lower sensitivity had a larger exotropia and a more reduced threshold. Note that no claims are being presented that the SOA activity is the reason that the animals developed an exotropia in the first place. Rather, it is suggested that the SOA cells are the substrate that helps maintain the divergent state.
Changes in Eye Alignment with Fixation—DHD
These experiments were able to take advantage of the fact that the strabismic animals showed changes in eye alignment depending on eye of fixation to identify and study the SOA cells. This particular strabismus phenomenon is DHD.38 Although its vertical equivalent, DVD, is the most commonly described problem associated with strabismus, DHD is also apparent in many patients with strabismus and frequently coexists with other strabismus phenomenon such as DVD and latent nystagmus.39 In a study of 28 patients with infantile esotropia who developed consecutive exotropia after strabismus surgery, DHD was observed in 50% of the patients.38 However, DHD of primary origin might be much less common.3 The observation of DHD in the AMO animals further validates its use as an appropriate animal model for sensory-induced strabismus.
One question to be asked is whether the SOA cell responses are related only to the DHD. In other words, is it possible that SOA cells only encode the change in misalignment between the right eye and left eye viewing conditions and that an underlying misalignment exists independent of SOA input? The current data cannot readily address this hypothesis. If the SOA cells are only responsible for DHD (and not the underlying misalignment), then it follows that the threshold of the SOA cells estimated earlier may not be appropriate and perhaps may not be different from the normal. However, note that the observation of reduced sensitivity of SOA cells in comparison to the normal monkeys (reduced slope of red line compared with the blue line in Fig. 5) would remain unchanged. Therefore, the SOA would still be at least partially responsible for the state of misalignment due to the reduced vergence tone input to the extraocular muscle. In the study authors' opinion, the most parsimonious explanation for SOA activity is that it is responsible for both the basic horizontal misalignment and the DHD.
Influence of Ocular Accommodation
Many SOA cells encode not only vergence angle but also ocular accommodation.27,40 Unfortunately, we did not have the technical capability to monitor or control for accommodation in our animals. Potentially, some of the misalignment sensitivity measures developed here for the SOA cells could be contaminated by sensitivity to accommodation. However, it appears highly unlikely that the observed differences in threshold and sensitivity of the SOA population of the strabismic monkeys compared with the normal animals is driven by changes in the accommodative component alone. Note also that the SOA cells project to medial rectus motoneurons and therefore changes in SOA responses that appear correlated to accommodation will also likely result in change in tone of extraocular muscles.
Lack of Correlation of SOA Cell Activity with A-Pattern Strabismus
Also interesting was the finding that the SOA cells did not show any modulation in relation to changes in horizontal eye misalignment that occurred due to A-patterns in strabismus. This result is in contrast to observations while recording from medial rectus motoneurons in the oculomotor nucleus.24 In that study, it was observed that the MRMNs were modulated depending on position of the eye that the neuron projected to, and therefore showed changes in firing for both cross-axis movements and when eye position changed due to change in eye of fixation (i.e., due to the strabismus). Based on the MRMN results and the current results from the SOA cells, a pattern appears to be emerging regarding the generation of horizontal misalignment and A/V patterns. It appears that the vergence circuits are responsible for setting the state of horizontal misalignment, while the change of misalignment with gaze position (A/V patterns) is brought about by adding a separate signal that is perhaps generated monocularly in brainstem structures responsible for generating eye movements such as saccades and pursuit. The summation of the horizontal misalignment signal and the change in misalignment due to A-patterns appears to be taking place at the level of the motoneurons.
Acknowledgments
We thank Michael Mustari for help with surgical implantation and Yoland Smith for animal histology. We also thank Anand Joshi for helpful discussions on the manuscript and Michelle Swann for technical assistance.
Footnotes
Supported by NIH Grant RO1-EY015312 (VED), UHCO core Grant P30 EY 07551, and Yerkes base Grant RR00165.
Disclosure: V.E. Das, None
References
- 1. Lorenz B. Genetics of isolated and syndromic strabismus: Facts and perspectives. Strabismus. 2002;10:147–156 [DOI] [PubMed] [Google Scholar]
- 2. Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology. 2005;112:104–108 [DOI] [PubMed] [Google Scholar]
- 3. von Noorden GK, Campos EC. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 6th ed. St. Louis, MO: Mosby; 2002. [Google Scholar]
- 4. Wright KW. Pediatric Ophthalmology and Strabismus. St. Louis, MO: Mosby; 1995. [Google Scholar]
- 5. Helveston EM. The aetiology of essential infantile (congenital) esotropia. : Lennerstrand G, Ygge J, Advances in Strabismus Research: Basic and Clinical Aspects. London, UK: Portland Press; 2000:135–152 [Google Scholar]
- 6. Schotthoefer EO, Wallace DK. Strabismus associated with thyroid eye disease. Curr Opin Ophthalmol. 2007;18:361–365 [DOI] [PubMed] [Google Scholar]
- 7. Demer JL, Ortube MC, Engle EC, Thacker N. High-resolution magnetic resonance imaging demonstrates abnormalities of motor nerves and extraocular muscles in patients with neuropathic strabismus. J Aapos. 2006;10:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyake N, Demer JL, Shaaban S, et al. Expansion of the CHN1 Strabismus Phenotype. Invest Ophthalmol Vis Sci. 2011;52:6321–6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539 [DOI] [PubMed] [Google Scholar]
- 10. Quick MW, Newbern JD, Boothe RG. Natural strabismus in monkeys: accommodative errors assessed by photorefraction and their relationship to convergence errors. Invest Ophthalmol Vis Sci. 1994;35:4069–4079 [PubMed] [Google Scholar]
- 11. Spanou N, Alexopoulos L, Manta G, Tsamadou D, Drakos H, Paikos P. Strabismus in pediatric lens disorders. J Pediatr Ophthalmol Strabismus. 2011;48:163–166 [DOI] [PubMed] [Google Scholar]
- 12. Lambert SR, Lynn M, Drews-Botsch C, et al. A comparison of grating visual acuity, strabismus, and reoperation outcomes among children with aphakia and pseudophakia after unilateral cataract surgery during the first six months of life. J Aapos. 2001;5:70–75 [DOI] [PubMed] [Google Scholar]
- 13. Wright KW. Pediatric cataracts. Curr Opin Ophthalmol. 1997;8:50–55 [PubMed] [Google Scholar]
- 14. Chiesi C, Chiesi L, Cavallini GM. Evaluation of refraction in a statistically significant sample: changes according to age and strabismus. J Pediatr Ophthalmol Strabismus. 2009;46:266–272 [DOI] [PubMed] [Google Scholar]
- 15. Oystreck DT, Engle EC, Bosley TM. Recent progress in understanding congenital cranial dysinnervation disorders. J Neuroophthalmol. 2011;31:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tollefson MM, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood hypertropia: a population-based study. Ophthalmology. 2006;113:1142–1145 [DOI] [PubMed] [Google Scholar]
- 17. Greenberg AE, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood esotropia: a population-based study. Ophthalmology. 2007;114:170–174 [DOI] [PubMed] [Google Scholar]
- 18. Crawford ML, von Noorden GK. Optically induced concomitant strabismus in monkeys. Invest Ophthalmol Vis Sci. 1980;19:1105–1109 [PubMed] [Google Scholar]
- 19. Economides JR, Adams DL, Jocson CM, Horton JC. Ocular motor behavior in macaques with surgical exotropia. J Neurophysiol. 2007;98:3411–3422 [DOI] [PubMed] [Google Scholar]
- 20. Tusa RJ, Mustari MJ, Das VE, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann N Y Acad Sci. 2002;956:346–360 [DOI] [PubMed] [Google Scholar]
- 21. Das VE. Alternating fixation and saccade behavior in nonhuman primates with alternating occlusion-induced exotropia. Invest Ophthalmol Vis Sci. 2009;50:3703–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Das VE, Fu LN, Mustari MJ, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus. 2005;13:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu L, Tusa RJ, Mustari MJ, Das VE. Horizontal saccade disconjugacy in strabismic monkeys. Invest Ophthalmol Vis Sci. 2007;48:3107–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joshi AC, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2011;52:6697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Das VE, Mustari MJ. Correlation of cross-axis eye movements and motoneuron activity in non-human primates with “A” pattern strabismus. Invest Ophthalmol Vis Sci. 2007;48:665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. May PJ, Porter JD, Gamlin PD. Interconnections between the primate cerebellum and midbrain near-response regions. J Comp Neurol. 1992;315:98–116 [DOI] [PubMed] [Google Scholar]
- 27. Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. J Neurophysiol. 1986;55:915–930 [DOI] [PubMed] [Google Scholar]
- 28. Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol. 1984;51:1091–1108 [DOI] [PubMed] [Google Scholar]
- 29. Das VE. Cells in the supraoculomotor area in monkeys with strabismus show activity related to the strabismus angle. Ann N Y Acad Sci. 2011;1233:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Das VE, Ono S, Tusa RJ, Mustari MJ. Conjugate adaptation of saccadic gain in non-human primates with strabismus. J Neurophysiol. 2004;91:1078–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: An improved method. Vision Research. 1980;20:535–538 [DOI] [PubMed] [Google Scholar]
- 32. Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol. 1987;57:147–161 [DOI] [PubMed] [Google Scholar]
- 33. Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986;56:1007–1021 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Gamlin PD, Mays LE. Antidromic identification of midbrain near response cells projecting to the oculomotor nucleus. Exp Brain Res. 1991;84:525–528 [DOI] [PubMed] [Google Scholar]
- 35. King WM, Zhou W. New ideas about binocular coordination of eye movements: is there a chameleon in the primate family tree? Anatomical Record. 2000;261:153–161 [DOI] [PubMed] [Google Scholar]
- 36. King WM, Zhou W. Neural basis of disjunctive eye movements. Ann N Y Acad Sci. 2002;956:273–283 [DOI] [PubMed] [Google Scholar]
- 37. Morley JW, Judge SJ, Lindsey JW. Role of monkey midbrain near-response neurons in phoria adaptation. J Neurophysiol. 1992;67:1475–1492 [DOI] [PubMed] [Google Scholar]
- 38. Brodsky MC. Dissociated horizontal deviation: clinical spectrum, pathogenesis, evolutionary underpinnings, diagnosis, treatment, and potential role in the development of infantile esotropia (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007;105:272–293 [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson ME, McClatchey SK. Dissociated horizontal deviation. J Pediatr Ophthalmol Strabismus. 1991;28:90–95 [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Mays LE, Gamlin PD. Characteristics of near response cells projecting to the oculomotor nucleus. J Neurophysiol. 1992;67:944–960 [DOI] [PubMed] [Google Scholar]