Abstract
Purpose.
To determine whether retrobulbar blood flow (RBF) velocities are predictive of conversion to glaucoma.
Methods.
A total of 262 glaucoma suspects were prospectively selected. Participants had normal visual field, increased intraocular pressure, and glaucomatous optic disc appearance at baseline. Topographic analysis of the optic nerve head was performed using a confocal laser scanning ophthalmoscope and the blood flow velocity of retrobulbar vessels was measured by color Doppler imaging. Conversion to glaucoma was assessed according to the changes in the color-coded Moorfields Regression Analysis (MRA) classification of the confocal laser scanning system during a 48-month follow-up period. Survival curves and hazard ratios (HRs) for the association between RBF parameters and conversion to glaucoma were calculated.
Results.
End-diastolic velocity and mean velocity in the ophthalmic artery were reduced in subjects that converted to glaucoma based on MRA (36 individuals, 13.7%), while resistivity (RI) and pulsatility indices were increased in the same vessel. Patients with RI values lower than 0.75 in the ophthalmic artery had a survival rate (MRA-converters versus nonconverters) of 93.9%, whereas individuals with RI values greater than 0.75 had a survival rate of 81.7% (HR = 3.306; P = 0.002).
Conclusions.
Abnormal RBF velocities measured by color Doppler ultrasound may be a risk factor for conversion to glaucoma. An RI value higher than 0.75 in the ophthalmic artery was associated with the development of glaucoma.
Significant abnormalities of blood flow velocities in the ophthalmic artery may increase the risk of developing a glaucomatous optic neuropathy.
Introduction
Glaucoma, a major cause of blindness worldwide,1 is a progressive multifactorial optic neuropathy characterized by the loss of ganglion cells and their axons in the retina.2 Elevated intraocular pressure (IOP) is a well-known major risk factor for glaucoma. Nevertheless, in a large proportion of patients, glaucoma progresses independent of therapeutic IOP reduction. Based on the results of the Early Manifest Glaucoma Trial, Heijl et al.3 reported that 45% of glaucoma patients progressed despite an average IOP reduction of 25% at the 6-year follow up. Leske et al.4 observed that 67% of patients progressed over 11 years of follow-up despite IOP reduction.
Accumulating evidence over the past two decades suggests that vascular factors play a role in glaucoma pathogenesis. Vascular dysregulation, or the failure of the blood perfusion system to adjust to the blood flow requirements of the optic nerve head (ONH), may lead to unstable or low ocular perfusion.5–7 Although the mechanisms underlying the vasodilator and vasoconstrictor responses to changes in ocular perfusion pressure remain unknown, a breakdown in the autoregulation of the ONH blood flow is proposed as a mechanism in glaucoma pathogenesis.6 Retrobulbar blood flow (RBF) regulation is strongly dependent on ocular perfusion pressure as well as on IOP itself. Thus, glaucoma may involve both mechanical and vascular factors, either independently or by their influence on each other.
Previous studies suggested a relationship between systemic circulatory disorders, reduced flow in the retrobulbar and ocular vessels, and glaucoma progression or severity.7–10 Evidence for decreased optic nerve blood flow in relation to visual field damage has been reported in glaucoma patients.11–12 Nevertheless, based on available data, this is the first study to evaluate the relationship between RBF velocities and the development of glaucoma based on structural changes of the ONH over a long time period.
Methods
The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of the study hospital. Patients with suspected glaucoma were recruited as part of ongoing studies within the Glaucoma Service of the Miguel Servet University Hospital at Zaragoza (Spain). All individuals from December 2004 to July 2005 who met the inclusion criteria were consecutively preselected for the present study.
A glaucoma suspect was defined as having a glaucomatous optic disc appearance (as determined by clinical assessment, see definition below), elevated IOP (≥21 mmHg), and normal standard automated perimetry (SAP). Inclusion criteria were: best-corrected visual acuity of 20/40 or better; refractive error of less than 5 spherical diopters (D) and 2 D cylinder; transparent ocular media (nuclear color/opalescence, cortical, or posterior subcapsular lens opacity <1) according to the Lens Opacities Classification System III system13; and open anterior chamber angle. The exclusion criteria were intraocular surgery within 3 months before inclusion in the study or through the follow-up period, diabetes, history of ocular or neurologic disease, and current use of a medication that could affect visual field sensitivity.
A total of 290 eyes of 290 glaucoma suspects were prospectively pre-enrolled. All the baseline examinations were performed within 6 weeks of the subject's date of enrollment into the study. When both eyes fulfilled the inclusion criteria, only one eye per subject was randomly chosen for the study.
Study Protocol
Participants underwent a comprehensive ophthalmologic examination: clinical history, visual acuity, biomicroscopy of the anterior segment using a slit-lamp, gonioscopy, Goldmann applanation tonometry, ultrasonic pachymetry, and ophthalmoscopy of the posterior segment. Additionally, a fasting blood sample was obtained from an arm vein to determine triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and plasma glucose levels.
At least one reliable SAP test per year was performed using an automated visual field perimeter (Humphrey 750i Visual Field Analyzer; Carl Zeiss Meditec, Dublin, CA) with the SITA Standard 24-2 program. Near addition was added to the subject's refractive correction. If fixation losses were greater than 20% or false positive or false negative rates were greater than 15%, the test was repeated. Participants completed the perimetry tests prior to any clinical examination or structural test. A normal SAP was defined as visual field indices (mean deviation and pattern standard deviation) within 95% confidence limits, with fewer than three non-edge contiguous points within the same hemifield identified as significant (P < 0.05) in the pattern deviation plot, and glaucomatous hemifield test results within normal limits. Visual field progression was evaluated with a linear regression analysis between mean deviation of SAP and age,14 and progression was defined as a significant negative trend detected at the 48-month follow-up visit with respect to baseline.
Clinical assessment of the ONH was performed after mydriasis (0.5% tropicamide; Alcon Laboratories Inc., Fort Worth, TX) by evaluating stereophotographs of the optic disc (Canon CF-60UV fundus camera; Canon Inc., Tokyo, Japan). The photographs were evaluated by two glaucoma specialists (AF and LP) blinded to the patients' identity and clinical history. Glaucomatous optic disc morphology was defined as diffuse neuroretinal rim narrowing with concentric enlargement of the optic cup, localized notching, or both.15 Any disagreement was resolved by consensus.
Topographic analysis of the ONH was performed using a confocal scanning laser ophthalmoscope (Heidelberg retina tomograph [HRT3]; Heidelberg Engineering, Heidelberg, Germany). Only scans with “acceptable,” “good,” or “very good” image quality scores were included in the study. The HRT3 software displays several windows in which the topographic results are detailed: stereometric parameters, the Moorfields Regression Analysis (MRA) classification, the Glaucoma Probability Score (GPS) classification, and interactive measurements.16–19 The MRA17 compares a subject's rim area with the predicted rim area for a given disc area and age, based on confidence limits of a regression analysis derived from an internal database. The optic disc is divided into six color-coded sectors, and each sector is classified as “within normal limits” if the percentage of the rim falls within the 95% confidence interval (CI; colored green); “borderline” if the percentage of the rim is between the 95% and 99.9% CI (colored yellow); and “outside normal limits” if the result is greater than the 99.9% CI (colored red).
Blood flow velocities of retrobulbar vessels of each eye were measured by color Doppler imaging (CDI) using a 7.5-MHz linear phased-array transducer (Sonoline Sienna; Siemens, Erlangen, Germany). IOP, and systolic and diastolic blood pressure readings were acquired immediately before performing CDI measurements. Although CDI cannot measure volumetric blood flow, it is a noninvasive technique for measuring blood velocities,20 such as peak systolic velocity (PSV), ending diastolic velocity (EDV), and mean flow velocity (MV) in the ophthalmic artery (OA), the central retinal artery (CRA), and the temporal and nasal short posterior ciliary arteries (TPCA and NPCA, respectively). Additionally, some indices can be obtained from these main variables: Pourcelot's resistivity index (RI = [PSV–EDV]/PSV); the pulsatility index (PI = [PSV–EDV]/MV), and the systolic/diastolic ratio (S/D).21,22 The CDI transducer was gently placed on the closed upper eyelid using coupling gel, taking care to minimize pressure on the globe. All subjects were in the supine position during the examination. The same trained radiologist (PS) performed all tests following standard procedures.20 PSV, EDV, MV, RI, PI, and S/D were measured from the OA, CRA, TPCA, and NPCA. Maximum velocity (Vmax), minimum velocity (Vmin), MV, and PI were also obtained from the central retinal vein (CRV).
Follow-Up and Definition of Study Endpoints
CDI was performed only during the baseline examination and patients were required to have at least a 48-month follow-up, with a minimum of a once yearly reliable HRT examination, unless they reached the criteria for conversion to glaucoma.
In this study, conversion to glaucoma was defined by a change of at least three sectors in the color-coded MRA classification at any moment during the 48-month follow-up.17 A change in the MRA classification (from within normal limits to borderline, from borderline to outside normal limits, or from normal to outside normal limits) in any of the following four sectors, nasal superior, temporal superior, nasal inferior, or temporal inferior, was considered to be a sector change. The temporal and nasal sectors of HRT3 were excluded from the statistical analysis because the superior and inferior sectors of the optic disc are the most sensitive for the detection of early glaucomatous changes.19,23–29 Thus, the sample was divided into two groups: subjects who converted to glaucoma (MRA-converters), which included participants with worsening in at least three MRA sectors during the follow-up period,30–32 and subjects who did not convert to glaucoma (nonconverters), comprised of participants who did not meet the previous condition.
Among MRA-converters, the follow-up time was defined as the period between the HRT baseline visit and the date of the three-sector deterioration of the MRA (the study endpoint). Among nonconverters, the follow-up time was defined as the time between the HRT baseline visit and the date of the last available HRT test result. Participants were not treated during the follow-up period unless they met the MRA-conversion criterion (end of follow-up period). They were then treated at the discretion of the attending ophthalmologist.
Statistical Analysis
All statistical analyses were performed using IBM's statistical software (SPSS version 19.0; IBM Corporation, Somers, NY).
OPP was calculated as the difference between the arterial blood pressure and the IOP (which is considered a substitute for venous pressure), according to the following formula: OPP = (1/3 systolic blood pressure + 2/3 diastolic blood pressure) × 2/3 − IOP.
CDI measurement reproducibility was determined by calculating the coefficient of variation (COV) between three consecutive PSV, EDV, MV, and RI measurements of the OA, CRA, TPCA, and NPCA. Each COV was calculated as the relevant standard deviation divided by the mean of the measurement values expressed as a percentage.
The main statistical analysis performed in this study was a survival analysis, which is the name for time-to-event analysis. The point of survival analysis is to follow subjects over time and observe the time point at which they experience the event of interest. The HR is the probability that an individual experiences an event at a determined time while that individual is at risk for an event. In this study, an event was considered to be the worsening of at least three MRA sectors (excluding nasal and temporal sectors) with respect to the baseline exam, as described above. Censoring occurred when three MRA sectors changed or at the end of the study period (48 months).
Survival analysis was performed to evaluate the conversion to glaucoma based on MRA results, and compare the development of glaucoma depending on baseline RI (cut-off point of 0.75).33 Differences in the Kaplan-Meier survival plots were calculated by the log-rank test. HRs and 95% CI for associations between RBF velocities and MRA conversion were determined using Cox regression models. Only the raw flow velocities in the studied vessels (PSV, EDV, MV, Vmax, and Vmin) were included in the model and the RBF parameters calculated from the raw flow velocities (RI, PI, and S/D) were excluded from the analysis. Nevertheless, HRs of conversion to glaucoma based on an RI in the OA over and below 0.75 and 0.78 were calculated. For all analyses, P < 0.05 was considered statistically significant. Nevertheless, when multiple comparisons were performed, the Bonferroni correction was applied to make the level of significance P < 0.002.
Results
From the initial group of 290 glaucoma suspects who fulfilled the inclusion criteria, 2 did not provide informed consent, 20 did not complete all of the required tests, and 6 were unable to perform at least one of the tests included in the study protocol. These 28 cases were excluded and finally 262 eyes of 262 patients of Caucasian origin were included in the statistical analysis. The images obtained with the HRT3 for all preselected individuals had at least “acceptable” quality. The Kolmogorov-Smirnov test confirmed that all variables analyzed in this study followed a normal distribution.
The COVs in the OA were 7.4%, 8.5%, 8.2%, and 1.3% for PSV, EDV, MV, and RI, respectively. In the CRA, the COVs for PSV, EDV, MV, and RI were 9.1%, 10.3%, 10.0%, and 1.8%, respectively. In the TPCA, the COVs for PSV, EDV, MV, and RI were 15.5%, 15.7%, 15.3%, and 2.5%, respectively. In the NPCA, the COVs for PSV, EDV, MV, and RI were 15.2%, 16.6%, 16.4%, and 2.9%, respectively.
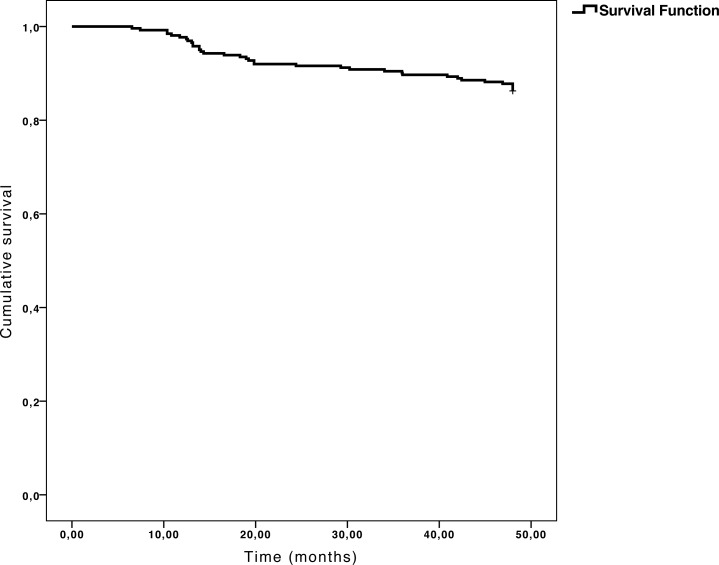
The study sample included 122 males and 140 females. Mean age was 51.5 ± 11.0 years, mean IOP was 23.56 ± 2.4 mmHg, and mean deviation of SAP was −0.49 ± 1.4 decibels. As defined above, according to the changes in the MRA over the 48-month follow-up, the sample was divided into two groups: 36 MRA-converters (13.7%) and 226 nonconverters (86.3%, Fig. 1). Table 1 shows the clinical characteristics of each group included in the study at baseline. There were no differences in age, best-corrected visual acuity (BCVA), IOP, central corneal thickness, mean deviation of SAP, pattern standard deviation of SAP, systolic and diastolic blood pressure, OPP, plasma glucose, and lipid profile between MRA-converters and nonconverters. Nevertheless, MRA-converters had a higher vertical-cup-to-disc ratio in stereophotographs than the nonconverter group. No significant changes in IOP were detected in any of the groups during the follow-up period.
Figure 1. .
Kaplan-Meier survival plot (conversion to glaucoma curve). Thirty-six (13.7%) patients had at least three MRA sectors changing from within normal limits to borderline (or outside normal limits) or from borderline to outside normal limits. Nasal and temporal sectors were excluded from the analysis.
Table 1. .
Demographic and Clinical Characteristics of Both Study Groups at Baseline
|
Mean ± SD |
P |
||
|
Nonconverter Group |
MRA-Converter Group |
||
| Age (years) | 51.96 ± 10.8 | 48.94 ± 11.9 | 0.13* |
| BCVA (Snellen) | 0.88 ± 0.1 | 0.87 ± 0.1 | 0.58* |
| IOP (mmHg) | 23.36 ± 2.1 | 23.75 ± 2.7 | 0.32* |
| Pachymetry (μm) | 562.15 ± 38.6 | 543.94 ± 40.2 | 0.009* |
| C/D | 0.49 ± 0.2 | 0.61 ± 0.1 | <0.001* |
| MD of SAP (dB) | −0.47 ± 1.4 | −0.60 ± 1.3 | 0.61* |
| PSD of SAP | 1.56 ± 0.5 | 1.40 ± 0.5 | 0.08* |
| Triglycerides (mg/dL) | 98.77 ± 45.7 | 81.58 ± 29.7 | 0.03* |
| HDL cholesterol (mg/dL) | 51.84 ± 9.7 | 53.09 ± 8.1 | 0.46* |
| LDL cholesterol (mg/dL) | 129.05 ± 29.8 | 126.41 ± 27.5 | 0.62* |
| Plasma glucose (mg/dL) | 97.16 ± 14.3 | 97.31 ± 17.5 | 0.95* |
| Systolic pressure (mmHg) | 126.99 ± 14.9 | 119.37 ± 12.4 | 0.004* |
| Diastolic pressure (mmHg) | 75.94 ± 9.3 | 72.93 ± 9.8 | 0.07* |
| Ocular perfusion pressure (mmHg) | 41.83 ± 6.8 | 38.57 ± 8.0 | 0.009* |
| Sex (M/F) | 106/120 | 16/20 | 0.86† |
| n | 226 | 36 | |
SD, Standard deviation; C/D, = vertical cup-to-disc ratio in stereophotographs; MD, mean deviation; PSD = pattern standard deviation; (M/F), (male/female); n, number of cases.
< 0.002 was considered statistically significant (in bold print).
Student's t-test.
Chi-square test.
Forty-seven eyes (17.9%) showed a significant negative trend based on the mean deviation of SAP. Twenty MRA converters also showed visual field worsening (55.5%). The agreement for detecting conversion to glaucoma evaluated by structural and functional tests was fair, with a kappa statistic of 0.38 (standard error, 0.07) between MRA conversion and visual field deterioration.
Table 2 shows the color-coded parameters of the MRA classification of the population at baseline. Most sectors in most individuals were within normal limits, and the most frequent abnormal sector was the nasal inferior (11.1%). At the beginning of the study, very few MRA sectors were outside normal limits, but around 90% of participants had at least a borderline sector.
Table 2. .
Moorfields Regression Analysis Classification of the Study Sample at Baseline
|
HRT3 Parameters |
Number of Cases |
Percentage |
|
| Temporal superior | WNL | 229 | 87.4 |
| Borderline | 30 | 11.5 | |
| ONL | 3 | 1.1 | |
| Nasal superior | WNL | 210 | 80.2 |
| Borderline | 32 | 12.2 | |
| ONL | 20 | 7.6 | |
| Temporal inferior | WNL | 213 | 81.3 |
| Borderline | 37 | 14.1 | |
| ONL | 12 | 4.6 | |
| Nasal inferior | WNL | 185 | 70.6 |
| Borderline | 48 | 18.3 | |
| ONL | 29 | 11.1 | |
HRT3, Heidelberg retina tomograph; ONL, outside normal limits; WNL, within normal limits.
EDV, RI, PI, and S/D of the OA were significantly different at baseline (Student t-test, P < 0.05) between MRA converters and nonconverters (Table 3). The OA was the vessel with the largest difference between groups, and all hemodynamic parameters of the OA, except PSV and MV, were significantly different. The MRA converters had reduced EDV of the OA and increased RI and PI of the OA compared with the nonconverters.
Table 3. .
Comparison of Hemodynamic Parameters at Baseline (Student's t-test) between the MRA-Based Converter and Nonconverter Groups.
|
Parameter |
Group of Nonconverters |
Group of MRA Converters |
P |
|
| OA | PSV (cm/s) | 32.24 ± 11.0 | 29.36 ± 11.1 | 0.15 |
| EDV (cm/s) | 7.87 ± 3.5 | 5.76 ± 2.7 | 0.001 | |
| MV (cm/s) | 15.17 ± 5.9 | 12.48 ± 5.0 | 0.01 | |
| RI | 0.75 ± 0.1 | 0.80 ± 0.1 | <0.001 | |
| PI | 1.67 ± 0.4 | 1.95 ± 0.5 | <0.001 | |
| S/D | 4.43 ± 1.3 | 5.44 ± 1.6 | <0.001 | |
| CRA | PSV (cm/s) | 8.53 ± 2.2 | 8.00 ± 2.7 | 0.20 |
| EDV (cm/s) | 2.13 ± 0.7 | 1.98 ± 0.8 | 0.29 | |
| MV (cm/s) | 4.11 ± 1.2 | 3.81 ± 1.6 | 0.19 | |
| RI | 0.74 ± 0.1 | 0.75 ± 0.1 | 0.84 | |
| PI | 1.59 ± 0.4 | 1.65 ± 0.4 | 0.42 | |
| S/D | 4.28 ± 1.4 | 4.30 ± 1.3 | 0.92 | |
| TPCA | PSV (cm/s) | 15.70 ± 6.1 | 15.88 ± 6.4 | 0.87 |
| EDV (cm/s) | 4.63 ± 2.1 | 4.17 ± 2.0 | 0.22 | |
| MV (cm/s) | 8.47 ± 3.4 | 8.34 ± 3.7 | 0.83 | |
| RI | 0.70 ± 0.1 | 0.73 ± 0.1 | 0.01 | |
| PI | 1.33 ± 0.3 | 1.46 ± 0.4 | 0.05 | |
| S/D | 3.60 ± 1.1 | 4.16 ± 1.6 | 0.009 | |
| NPCA | PSV (cm/s) | 13.01 ± 5.7 | 13.41 ± 5.1 | 0.75 |
| EDV (cm/s) | 3.78 ± 2.0 | 3.57 ± 1.9 | 0.65 | |
| MV (cm/s) | 7.01 ± 3.3 | 6.78 ± 3.2 | 0.76 | |
| RI | 0.71 ± 0.1 | 0.73 ± 0.1 | 0.29 | |
| PI | 1.37 ± 0.3 | 1.52 ± 0.5 | 0.04 | |
| S/D | 3.72 ± 1.0 | 4.11 ± 1.5 | 0.12 | |
| CRV | Vmax (cm/s) | 4.39 ± 1.7 | 3.82 ± 1.1 | 0.06 |
| Vmin (cm/s) | 2.81 ± 0.7 | 2.48 ± 0.7 | 0.008 | |
| MV (cm/s) | 3.45 ± 0.8 | 3.12 ± 0.9 | 0.03 | |
| PI | 0.42 ± 0.1 | 0.43 ± 0.1 | 0.70 |
Values are expressed as mean ± standard deviation.
< 0.002 was considered statistically significant (in bold print).
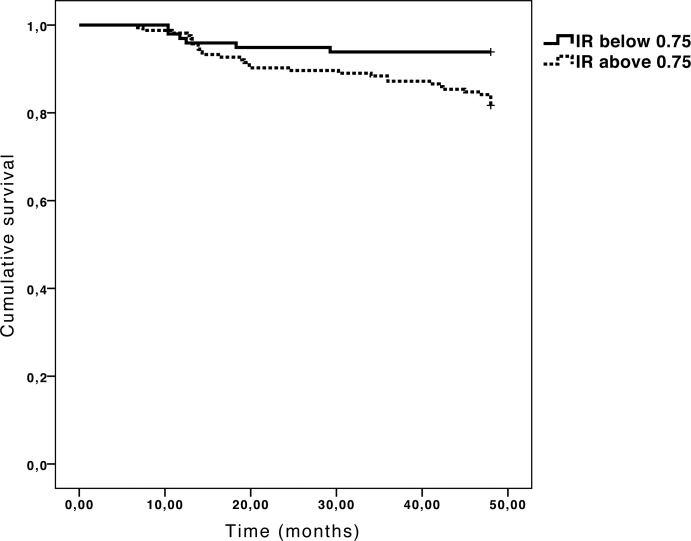
Because RI is one of the most reliable hemodynamic parameters34–38 (in this study, intraclass correlation coefficient for three consecutive measurements of RI of the OA was 0.968) and the greatest number of significant differences between MRA converters and nonconverters was in the OA, study authors calculated the survival and HR of glaucoma suspects with an RI of the OA, choosing 0.75 as the RI cut-off point for conversion to glaucoma (which was the mean RI of the OA obtained in the group of nonconverters and the mean RI of the OA described by Galassi et al. in a healthy population).33 Thus, the whole sample was classified into two subgroups, according to the RI of the OA: 98 individuals had an RI lower than 0.75 and the remaining 164 had an RI greater than 0.75. At the end of the 48-month follow-up period, 30 patients in the group with an RI greater than 0.75 and 6 patients in the group with an RI lower than 0.75 met the criteria for conversion to glaucoma. The group with RI values lower than 0.75 in the OA had a survival rate of 93.9% at the 48-month follow-up (Fig. 2), while the probability of survival in the group with RI values greater than 0.75 decreased to 81.7% (P = 0.007; log-rank test). RI values over 0.75 in the OA positively correlated with conversion to glaucoma (HR: 3.306; 95% CI = 1.448–7.547; P = 0.002). Based on the results of Galassi et al.,34 study authors also selected an additional cut-off point of 0.78 for the RI of the OA (150 participants had an RI of the OA < 0.78 and 112 had an RI of the OA ≥ 0.78). With this condition, the HR increased slightly up to 3.757 (95% CI = 1.812–7.794; P < 0.001). Table 4 shows the RBF velocities for both the 0.75 and 0.78 RI-defined subgroups. RI, PI, and S/D of the OA, CRA, and TPCA differed between each RI-defined subgroup. EDV and MV only differed in the OA, and RI of NPCA was only different for the 0.78 RI-defined subgroups.
Figure 2. .
Kaplan-Meier survival plots of the resistivity index of the ophthalmic artery (P = 0.007; log-rank test).
Table 4. .
Comparison of Hemodynamic Parameters at Baseline between the 0.75 and 0.78 RI-Defined Subgroups (Student's t-test).
|
Parameter |
RI ≤ 0.75 |
RI > 0.75 |
p |
RI ≤ 0.78 |
RI > 0.78 |
P |
|
| OA | PSV (cm/s) | 33.01 ± 11.2 | 31.09 ± 10.9 | 0.17 | 32.08 ± 11.5 | 31.34 ± 10.2 | 0.60 |
| EDV (cm/s) | 10.02 ± 3.6 | 6.11 ± 2.5 | <0.001 | 8.88 ± 3.6 | 5.35 ± 2.0 | <0.001 | |
| MV (cm/s) | 17.35 ± 6.3 | 13.23 ± 4.5 | <0.001 | 16.11 ± 6.2 | 12.50 ± 4.3 | <0.001 | |
| RI | 0.70 ± 0.0 | 0.80 ± 0.0 | <0.001 | 0.73 ± 0.0 | 0.83 ± 0.0 | <0.001 | |
| PI | 1.35 ± 0.2 | 1.93 ± 0.4 | <0.001 | 1.48 ± 0.2 | 2.12 ± 0.4 | <0.001 | |
| S/D | 3.34 ± 0.4 | 5.30 ± 1.3 | <0.001 | 3.71 ± 0.5 | 6.03 ± 1.1 | <0.001 | |
| CRA | PSV (cm/s) | 8.11 ± 2.2 | 8.66 ± 2.4 | 0.06 | 8.26 ± 2.1 | 8.79 ± 2.5 | 0.07 |
| EDV (cm/s) | 2.22 ± 0.8 | 2.05 ± 0.8 | 0.09 | 2.21 ± 0.8 | 1.95 ± 0.7 | 0.01 | |
| MV (cm/s) | 4.07 ± 1.3 | 4.06 ± 1.3 | 0.95 | 4.10 ± 1.2 | 4.01 ± 1.3 | 0.60 | |
| RI | 0.73 ± 0.1 | 0.76 ± 0.1 | <0.001 | 0.73 ± 0.1 | 0.77 ± 0.1 | <0.001 | |
| PI | 1.49 ± 0.3 | 1.67 ± 0.4 | <0.001 | 1.52 ± 0.3 | 1.75 ± 0.4 | <0.001 | |
| S/D | 3.87 ± 0.9 | 4.53 ± 1.5 | <0.001 | 3.94 ± 1.0 | 4.86 ± 1.7 | <0.001 | |
| TPCA | PSV (cm/s) | 15.62 ± 6.1 | 15.76 ± 6.1 | 0.86 | 15.40 ± 6.0 | 16.22 ± 6.2 | 0.30 |
| EDV (cm/s) | 4.86 ± 2.1 | 4.40 ± 2.1 | 0.09 | 4.71 ± 2.0 | 4.36 ± 2.1 | 0.19 | |
| MV (cm/s) | 8.58 ± 3.3 | 8.36 ± 3.5 | 0.62 | 8.42 ± 3.3 | 8.49 ± 3.6 | 0.87 | |
| RI | 0.68 ± 0.1 | 0.72 ± 0.1 | <0.001 | 0.69 ± 0.1 | 0.73 ± 0.1 | <0.001 | |
| PI | 1.26 ± 0.3 | 1.41 ± 0.4 | 0.001 | 1.28 ± 0.3 | 1.48 ± 0.4 | <0.001 | |
| S/D | 3.32 ± 0.8 | 3.89 ± 1.3 | <0.001 | 3.39 ± 0.8 | 4.15 ± 1.5 | <0.001 | |
| NPCA | PSV (cm/s) | 13.32 ± 5.9 | 13.08 ± 5.5 | 0.81 | 13.33 ± 6.2 | 12.94 ± 4.9 | 0.67 |
| EDV (cm/s) | 4.13 ± 2.2 | 3.62 ± 1.9 | 0.15 | 4.04 ± 2.2 | 3.45 ± 1.7 | 0.07 | |
| MV (cm/s) | 7.25 ± 3.5 | 6.90 ± 3.2 | 0.54 | 7.20 ± 3.5 | 6.77 ± 3.0 | 0.43 | |
| RI | 0.69 ± 0.1 | 0.73 ± 0.1 | 0.005 | 0.70 ± 0.1 | 0.74 ± 0.1 | 0.001 | |
| PI | 1.32 ± 0.3 | 1.43 ± 0.3 | 0.05 | 1.33 ± 0.3 | 1.47 ± 0.4 | 0.01 | |
| S/D | 3.53 ± 1.2 | 3.89 ± 1.0 | 0.06 | 3.56 ± 1.1 | 4.05 ± 1.1 | 0.005 | |
| CRV | Vmax (cm/s) | 4.12 ± 1.1 | 4.42 ± 1.9 | 0.17 | 4.30 ± 1.9 | 4.32 ± 1.1 | 0.92 |
| Vmin (cm/s) | 2.76 ± 0.8 | 2.77 ± 0.6 | 0.90 | 2.75 ± 0.7 | 2.80 ± 0.7 | 0.56 | |
| MV (cm/s) | 3.38 ± 1.0 | 3.43 ± 0.8 | 0.66 | 3.38 ± 0.9 | 3.46 ± 0.8 | 0.45 | |
| PI | 0.41 ± 0.2 | 0.43 ± 0.1 | 0.28 | 0.42 ± 0.2 | 0.44 ± 0.1 | 0.45 | |
| n | 98 | 164 | 165 | 97 |
Values are expressed as mean ± standard deviation.
< 0.002 was considered statistically significant (in bold print).
Neither the clinical variables (age, triglycerides, HDL cholesterol, LDL cholesterol, glycemia, systolic blood pressure, diastolic blood pressure, and OPP) nor the ophthalmic parameters (IOP, central corneal thickness, mean deviation, and pattern deviation of SAP) were significant in the Cox regression model; only the vertical cup-to-disc ratio evaluated by stereophotographs was significant (HR 1.973 per 0.1 increase; 95% CI = 1.106–3.518; P = 0.021).
With respect to the RBF velocities, each unit increase in the PSV of the OA resulted in a 6.2% increase in the risk of developing glaucoma (HR: 1.062; 95% CI = 1.008–1.120; P = 0.024). By contrast, each unit increase in the EDV of the OA decreased the risk of conversion to glaucoma by 30% (HR: 0.699; 95% CI = 0.552–0.884; P = 0.003). With respect to the CRV, each centimeter per second decrease in Vmin was associated with a 66% greater increase in the risk of developing glaucoma (HR: 0.342; 95% CI = 0.152–0.767; P = 0.008).
Because the vertical cup-to-disc ratio in the stereophotographs differed at baseline between the MRA converter and nonconverter groups, further analysis was performed. The whole sample was stratified into two subgroups according to this parameter. A 0.5 cut-off point was selected because it was the mean cup-to-disc ratio of the nonconverter group and the median value of the range for this variable. The clinical characteristics of both subgroups are detailed in Table 5. The vertical cup-to-disc ratio did not differ between MRA converters and nonconverters of each subgroup. The RI of the OA was higher in the MRA converters with a cup-to-disc ratio higher than 0.5 (Table 6). In the subgroup with a cup-to-disc ratio ≤0.5, the Mann-Whitney U test was used because the MRA converters group included only six cases. RBF velocities in the OA, CRA, TPCA, NPCA, and CRV were not significantly different in this subgroup.
Table 5. .
Clinical Characteristics of Both Subgroups at Baseline, Stratified by the Vertical Cup-to-Disc Ratio.
|
C/D ≤0.5 |
C/D > 0.5 |
|||||
|
Nonconverter Group (Mean ± SD) |
MRA-Converter Group (Mean ± SD) |
P |
Nonconverter Group (Mean ± SD) |
MRA-Converter Group (Mean ± SD) |
P |
|
| Age (years) | 51.76 ± 10.6 | 45.50 ± 12.1 | 0.19* | 52.30 ± 11.3 | 49.63 ± 11.9 | 0.28† |
| BCVA (Snellen) | 0.86 ± 0.1 | 0.91 ± 0.1 | 0.24* | 0.89 ± 0.1 | 0.86 ± 0.2 | 0.30† |
| IOP (mmHg) | 23.40 ± 2.3 | 23.62 ± 2.6 | 0.82* | 23.32 ± 2.0 | 23.86 ± 2.3 | 0.23† |
| Pachymetry (μm) | 567.00 ± 38.1 | 539.50 ± 35.0 | 0.09* | 554.09 ± 38.4 | 544.83 ± 41.7 | 0.27† |
| C/D | 0.41 ± 0.2 | 0.44 ± 0.3 | 0.73* | 0.57 ± 0.5 | 0.64 ± 0.6 | 0.54† |
| MD of SAP (dB) | −0.53 ± 1.4 | −0.32 ± 1.0 | 0.72* | −0.38 ± 1.4 | −0.66 ± 1.3 | 0.34† |
| PSD of SAP | 1.52 ± 0.5 | 1.38 ± 0.4 | 0.51* | 1.62 ± 0.6 | 1.41 ± 0.5 | 0.09† |
| Triglycerides (mg/dL) | 101.11 ± 47.9 | 85.10 ± 17.1 | 0.42* | 95.00 ± 42.2 | 80.09 ± 28.3 | 0.08† |
| HDL cholesterol (mg/dL) | 54.41 ± 9.5 | 55.12 ± 8.1 | 0.86* | 48.54 ± 9.2 | 52.85 ± 7.7 | 0.02† |
| LDL cholesterol (mg/dL) | 121.76 ± 28.6 | 130.25 ± 37.7 | 0.58* | 137.61 ± 29.2 | 125.23 ± 25.5 | 0.04† |
| Plasma glucose (mg/dL) | 96.57 ± 14.4 | 102.37 ± 15.3 | 0.34* | 98.07 ± 14.4 | 92.75 ± 6.4 | 0.05† |
| Systolic pressure (mmHg) | 128.13 ± 15.8 | 122.80 ± 14.5 | 0.42* | 126.81 ± 13.6 | 120.68 ± 12.2 | 0.03† |
| Diastolic pressure (mmHg) | 76.24 ± 9.4 | 71.40 ± 7.7 | 0.22* | 75.46 ± 9.0 | 73.24 ± 10.2 | 0.26† |
| Ocular perfusion pressure (mmHg) | 41.92 ± 6.7 | 37.62 ± 8.1 | 0.13* | 41.70 ± 7.0 | 38.76 ± 8.1 | 0.06† |
| Sex (M/F) | 65/76 | 4/2 | 0.42‡ | 41/44 | 12/18 | 0.53‡ |
| n | 141 | 6 | 85 | 30 | ||
< 0.002 was considered statistically significant.
Mann-Whitney's U test.
Student's t-test.
Chi-square test.
Table 6. .
Comparison of Hemodynamic Parameters at Baseline between MRA-Converter and Nonconverter Groups of Subgroups Stratified by the Vertical Cup-to-Disc Ratio.
|
Parameter |
C/D ≤0.5 (Mann-Whitney's U test) |
C/D > 0.5 (Student's t-test) |
|||||
|
Group of Nonconverters |
Group of MRA Converters |
P |
Group of Nonconverters |
Group of MRA Converters |
P |
||
| OA | PSV (cm/s) | 32.16 ± 10.6 | 25.70 ± 4.9 | 0.08* | 32.26 ± 11.7 | 30.10 ± 11.9 | 0.40† |
| EDV (cm/s) | 7.85 ± 3.6 | 4.32 ± 1.4 | 0.006* | 7.88 ± 3.5 | 6.06 ± 2.8 | 0.006† | |
| MV (cm/s) | 15.15 ± 5.8 | 9.87 ± 2.2 | 0.01* | 15.11 ± 6.0 | 13.00 ± 5.3 | 0.07† | |
| RI | 0.76 ± 0.1 | 0.83 ± 0.1 | 0.009* | 0.75 ± 0.1 | 0.80 ± 0.1 | 0.001† | |
| PI | 1.68 ± 0.4 | 2.19 ± 0.4 | 0.005* | 1.68 ± 0.4 | 1.90 ± 0.5 | 0.04† | |
| S/D | 4.44 ± 1.2 | 6.28 ± 1.7 | 0.009* | 4.42 ± 1.4 | 5.27 ± 1.5 | 0.01† | |
| CRA | PSV (cm/s) | 8.59 ± 2.3 | 8.52 ± 4.5 | 0.35* | 8.43 ± 2.0 | 7.90 ± 2.4 | 0.28† |
| EDV (cm/s) | 2.14 ± 0.8 | 1.97 ± 1.2 | 0.22* | 2.12 ± 0.7 | 1.99 ± 0.8 | 0.44† | |
| MV (cm/s) | 4.13 ± 1.2 | 3.97 ± 2.5 | 0.13* | 4.07 ± 1.2 | 3.78 ± 1.4 | 0.33† | |
| RI | 0.75 ± 0.1 | 0.76 ± 0.1 | 0.39* | 0.75 ± 0.1 | 0.75 ± 0.1 | 0.99† | |
| PI | 1.60 ± 0.4 | 1.74 ± 0.5 | 0.45* | 1.59 ± 0.4 | 1.64 ± 0.4 | 0.63† | |
| S/D | 4.31 ± 1.5 | 4.58 ± 1.3 | 0.39* | 4.23 ± 1.2 | 4.25 ± 1.3 | 0.93† | |
| TPCA | PSV (cm/s) | 15.38 ± 6.0 | 18.23 ± 5.8 | 0.14* | 16.18 ± 6.2 | 15.40 ± 6.5 | 0.58† |
| EDV (cm/s) | 4.57 ± 2.2 | 4.32 ± 0.8 | 0.71* | 4.76 ± 1.9 | 4.15 ± 2.2 | 0.19† | |
| MV (cm/s) | 8.28 ± 3.4 | 9.35 ± 2.2 | 0.19* | 8.77 ± 3.3 | 8.14 ± 3.9 | 0.45† | |
| RI | 0.70 ± 0.1 | 0.75 ± 0.1 | 0.21* | 0.70 ± 0.1 | 0.73 ± 0.1 | 0.07† | |
| PI | 1.35 ± 0.4 | 1.46 ± 0.3 | 0.30* | 1.32 ± 0.4 | 1.47 ± 0.4 | 0.10† | |
| S/D | 3.59 ± 1.0 | 4.34 ± 1.5 | 0.22* | 3.62 ± 1.2 | 4.12 ± 1.6 | 0.13† | |
| NPCA | PSV (cm/s) | 12.76 ± 5.8 | 14.30 ± 0.3 | 0.38* | 13.74 ± 5.7 | 13.33 ± 5.3 | 0.78† |
| EDV (cm/s) | 3.67 ± 2.0 | 3.15 ± 0.2 | 0.84* | 4.07 ± 2.1 | 3.62 ± 2.0 | 0.41† | |
| MV (cm/s) | 6.78 ± 3.3 | 7.85 ± 0.5 | 0.37* | 7.53 ± 3.4 | 6.69 ± 3.4 | 0.35† | |
| RI | 0.72 ± 0.1 | 0.78 ± 0.0 | 0.14* | 0.71 ± 0.1 | 0.73 ± 0.1 | 0.38† | |
| PI | 1.39 ± 0.3 | 1.42 ± 0.0 | 0.77* | 1.34 ± 0.3 | 1.54 ± 0.5 | 0.09† | |
| S/D | 3.74 ± 1.0 | 4.55 ± 0.4 | 0.15* | 3.69 ± 1.1 | 4.07 ± 1.5 | 0.32† | |
| CRV | Vmax (cm/s) | 4.45 ± 1.96 | 3.77 ± 0.8 | 0.16* | 4.28 ± 1.2 | 3.83 ± 1.2 | 0.09† |
| Vmin (cm/s) | 2.84 ± 0.7 | 2.77 ± 0.6 | 0.77* | 2.78 ± 0.6 | 2.43 ± 0.8 | 0.03† | |
| MV (cm/s) | 3.48 ± 0.8 | 3.22 ± 0.7 | 0.39* | 3.42 ± 0.9 | 3.10 ± 1.0 | 0.14† | |
| PI | 0.43 ± 0.2 | 0.32 ± 0.1 | 0.05* | 0.43 ± 0.1 | 0.46 ± 0.1 | 0.26† | |
| n | 141 | 6 | 85 | 30 | |||
Values are expressed as mean ± standard deviation.
< 0.002 was considered statistically significant (in bold print).
Mann-Whitney's U test.
Student's t-test.
All 36 MRA converters were treated with ocular hypotensive drugs and neither HRT3 nor SAP showed any significant worsening through the end of the follow-up period.
Discussion
Based on currently available data, this is the first study prospectively evaluating the predictive value of RBF velocities for conversion to glaucoma in glaucoma suspects. In contrast to a retrospective design in which the follow-up duration, frequency, and number of investigations may vary considerably among patients, all participants were followed up for 48 months with examinations performed using a standardized protocol. Moreover, conversion to glaucoma was defined by objective changes measured by HRT, which is a validated technology for diagnosing and monitoring ONH changes in patients with glaucoma or at risk for developing the disease.19,24–26,28,29 Our long follow-up period allowed us to obtain enough MRA converters to provide reliable results, especially because the glaucomatous ONH changes usually occur slowly and progressively in this kind of patient.
The findings of the present study confirm the relationship between abnormal RBF velocities and conversion to glaucoma. Glaucoma suspects who converted to glaucoma during the 48-month follow-up period based on MRA exhibited significantly lower EDV and significantly higher RI and PI values in the OA at baseline, compared with the group of glaucoma suspects who did not convert to glaucoma. Similar outcomes were reported by Galassi et al.34 comparing patients with stable glaucoma and glaucoma progression, and Butt et al.39 comparing healthy and glaucoma patients.
Differences in the RBF velocities between MRA converters and nonconverters were observed in the OA. Although most of the blood coming from the OA does not go into the eye, this is the retrobulbar artery with the largest lumen. Thus, the OA is the most easily located by the investigator and their measurements are more reproducible than those of other small diameter vessels.35 Because the IOP was not different between both groups, these RBF velocities in individuals at risk for glaucoma may play a role in the development of the disease. In the multivariate analysis, hemodynamic variables were the only factors related to conversion to glaucoma aside from the vertical cup-to-disc ratio evaluated by stereophotographs (HR: 1.973). Nevertheless, this result must be analyzed carefully. Because one condition included in this study was glaucomatous ONH appearance, and study criteria for glaucoma conversion were based on changes in the MRA of HRT3—which is a linear regression that takes into account the relationship between optic disc size (optic disc area) and rim area or cup-disc area ratio17—study definitions for glaucoma suspect and glaucoma conversion may have produced a bias toward this ONH parameter.
The OA is the main source of blood supply to the optic nerve and the short posterior ciliary arteries are the main source for ONH perfusion.40 Some previous studies reported that glaucoma patients had reduced circulation in these vessels measured by scanning laser Doppler flowmetry,41 or by CDI,11,42–46 while other authors34 reported no differences in the short posterior ciliary arteries. The results of the current study confirmed those of Galassi,34 who reported no differences in the CRA between patients with stable and deteriorating visual fields. In the same way, the authors of this present study did not demonstrate that RBF velocities of the CRA differed between glaucoma suspects and patients with structural changes of the ONH.
Different study designs, techniques for evaluating the RBF variables, and criteria for classifying the groups make it difficult to compare results among diverse studies. Moreover, CDI has some limitations. On the one hand, it is very difficult to determine if the vessel being studied is actually a short ciliary artery or a long ciliary artery. On the other hand, given the small size of these vessels, the proximity between them, and the variability in number and position, it is possible that more than one ciliary artery was analyzed at the same time. Furthermore, adjustments of the insonation angle are required, although in many cases it is difficult to obtain an adequate record. Thus, measurements of the short posterior ciliary arteries have the lowest reproducibility.34–38 Because these vessels are branches of the OA, however, it can be assumed that factors that modify blood flow in the OA also affect blood flow in the short posterior ciliary arteries, and hemodynamic differences are likely to be found for CRA, TPCA, and NPCA similar to those observed for the OA between MRA converters and nonconverters. In the present study, the higher variability of RBF velocities for the CRA, TPCA, and NPCA compared with the OA, may explain why study authors were unable to observe those differences in vessels smaller than the OA.
The ability of the HRT to detect glaucomatous changes of the ONH has been widely validated. Based on several studies,19,24–26,28,29 the temporal and nasal sectors are generally less sensitive for detecting glaucomatous changes, which is why these sectors were excluded from the statistical analysis. The retinal nerve fiber layer bundles are thicker in the superior and inferior regions and thinner in the temporal and nasal areas; consequently, the HRT can more easily measure changes in the vertical axis. Moreover, the superior and inferior poles of the ONH are affected first in the early stages of glaucoma (inferior–superior–nasal–temporal rule).19,23–29
We evaluated the survival of glaucoma suspects based on a 0.75 cut-off point for the RI of the OA because this parameter is independent of the insonation angle, includes systolic and diastolic velocity values, and is the most reproducible parameter in Doppler ultrasound (coefficient of variation around 6%).40–44 We found statistical differences in the RI of the OA between the MRA converters and nonconverters (P = 0.007, log-rank test). This analysis revealed a significantly higher conversion rate to glaucoma in subjects with baseline RI values greater than 0.75. Study data are consistent with those of Galassi et al.34 reporting an odds ratio of visual field deterioration of 6.61 (95% CI: 1.67–26.1; P = 0.007) in glaucoma patients with an OA RI of 0.78 or higher. We found that an OA RI ≥ 0.78 increased the risk of MRA conversion (HR: 3.757; 95% CI = 1.812–7.794; P < 0.001) by 3.75 times, and that individuals with an OA RI ≥ 0.75 had a 330% greater probability of worsening in three MRA sectors (HR: 3.306; 95% CI = 1.448–7.547; P = 0.002). Study authors also observed that although PSV, EDV, and MV did not differ for the CRA and TPCA, the indices derived from these velocities (RI, PI, and S/D) were different in these vessels for both IR-defined subgroups. Thus, study authors expected to find glaucomatous changes in smaller retrobulbar vessels than OA if the accuracy of the CDI measurements could be increased and the operator variability of RBF velocities for the CRA, TPCA, and NPCA could be reduced.
When the sample was divided into subgroups according to the vertical cup-to-disc ratio in stereophotographs, this variable did not differ between MRA converters and nonconverters in either subgroup; and the RI of the OA was higher in the MRA-converter group with a cup-to-disc ratio higher than 0.5. In the subgroup with a cup-to-disc ratio ≤0.5, study authors did not observe any significant difference in the RBF velocities in the OA (or other smaller vessels) because the MRA-converter group included only six cases and differences were only considered significant when P < 0.002. Although RBF velocities for the OA in this subgroup were not significantly different, they should be taken into account clinically because they would likely be significant in a larger sample.
The usefulness of ultrasonographic measurement of RI in ocular blood vessels, however, is not fully understood. Polska et al.47 reported that RI is not a valid indicator of vascular resistance in the CRA, and suggested that velocity changes as assessed in retrobulbar vessels cannot be extrapolated to changes in blood flow. Because CDI does not measure volumetric blood flow, but rather blood velocities, study results must be interpreted accordingly. Study authors found that RI in the OA was reduced in the MRA-converter group. This is a significant finding in clinical practice, but it is likely that IR in OA does not providing a good measure of the true distal vascular resistance in this vessel.
The aim of this study was not to evaluate the effect of RBF velocities in visual field progression, but in predicting damage to the ONH. Thus, the study was designed such that only the required test was performed to avoid an excess number of visits and exams that may contribute to increased losses though the follow-up period. Currently, there is a lack of general agreement for a definition of clinically significant progression; nevertheless, it has been suggested that event analyses (such as that used to define MRA conversion) and trend-based methods (regression analysis to measure rates of change, used in this study to define SAP deterioration) are the best approaches.48 Study authors compared the performance of SAP and HRT to detect conversion to glaucoma, and their results were similar to those of previous studies reporting a mild agreement between functional and structural tests.29,49,50 With a longer follow-up, it is possible that the changes detected by only MRA or SAP would be detected by both techniques, leading to better agreement between methods.
Among the available methods to evaluate the vascular component in glaucoma, CDI seems to be the most advisable because of its noninvasivity and acceptable reproducibility. The ability of any technology to detect change is related to the magnitude of the change relative to the measurement variability of the instrument. Furthermore, the variability and accuracy to measure RBF velocities depends on the experience of the Doppler ultrasound operator. In the current study, the same trained radiologist (PS) performed all of the CDI tests, to reduce the effect of operator skill. COVs for this radiologist for PSV, EDV, MV, and RI in the OA were 7.4%, 8.5%, 8.2%, and 1.3%, respectively. The variability of CDI measurements increased as the lumen of the examined vessel decreased. These reproducibility measurements are similar to those reported by other authors.34–38
A limitation is that only one vascular bed was evaluated in this study, and no information on retinal or choroid blood flow was analyzed. Another limitation is that while both the MRA-converters and nonconverters had comparable clinical characteristics at baseline, other unevaluated factors may have contributed to the MRA conversion to glaucoma, such as genetics and life habits. Nevertheless, the multivariate analysis revealed no other significant factors among the studied variables.
Previous population-based studies demonstrated that IOP has an important role in the development and progression of glaucomatous optic disc neuropathy.51–55 These previous studies provide strong evidence that the prevalence of primary open-angle glaucoma is higher as the IOP level increases. Thus, we selected individuals with an elevated IOP to obtain a sample population at high risk for conversion to glaucoma. Because the study sample did not include patients with normal-tension glaucoma, the results may not apply to these patients.
The results of this study are consistent with the wide evidence supporting the effect of vascular factors in the pathogenesis of glaucoma.5–7 Significant abnormalities of blood flow in the OA may increase the risk of developing a glaucomatous optic neuropathy.
Footnotes
Supported in part by Instituto de Salud Carlos III Grants PI080976 and PI1101239
Disclosure: P. Calvo, None; A. Ferreras, None; V. Polo, None; N. Güerri, None; P. Seral, None; I. Fuertes-Lazaro, None; L.E. Pablo, None
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Academy of Ophthalmology Glaucoma Panel Preferred Practice Pattern. Primary Open-Angle Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2005:3 [Google Scholar]
- 3. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279 [DOI] [PubMed] [Google Scholar]
- 4. Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972 [DOI] [PubMed] [Google Scholar]
- 5. Graham SL, Drance SM, Wijsman K. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102:61–69 [DOI] [PubMed] [Google Scholar]
- 6. Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393 [DOI] [PubMed] [Google Scholar]
- 7. Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol. 1994;39:23–42 [DOI] [PubMed] [Google Scholar]
- 8. Harris A, Spaeth G, Wilson R, et al. Nocturnal ophthalmic arterial hemodynamics in primary open-angle glaucoma. J Glaucoma. 1997;6:170–174 [PubMed] [Google Scholar]
- 9. Hayreh SS, Revie IH, Edwards J. Vasogenic origin of visual field defects and optic nerve changes in glaucoma. Br J Ophthalmol. 1970;54:461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaoeda K, Shirakashi M, Fukushima A, et al. Relationship between optic nerve head microcirculation and visual field loss in glaucoma. Acta Ophthalmologica Scandinavica. 2003;81:253–259 [DOI] [PubMed] [Google Scholar]
- 11. Zeitz O, Galambos P, Wagenfeld L, et al. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br J Ophthalmol. 2006;90:1245–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plange N, Kaup M, Arend O, et al. Asymmetric visual field loss and retrobulbar haemodynamics in primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244:978–983 [DOI] [PubMed] [Google Scholar]
- 13. Chylack LT, Jr, Wolfe JK, Singer DM, et al. Longitudinal study of cataract study group. The Lens Opacities Classification System III. Arch Ophthalmol. 1993;111:831–836 [DOI] [PubMed] [Google Scholar]
- 14. Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–353 [DOI] [PubMed] [Google Scholar]
- 15. Tuulonen A, Airaksinen PJ. Initial glaucomatous optic disk and retinal nerve fiber layer abnormalities and their progression. Am J Ophthalmol. 1991;111:485–490 [DOI] [PubMed] [Google Scholar]
- 16. Girkin CA. Principles of confocal scanning laser ophthalmoscopy for the clinician. : Fingeret M, Flanagan JG, Liebmann JM, The Essential HRT Primer. Heidelberg, Germany: Heidelberg Engineering; 2005:1–9 [Google Scholar]
- 17. Wollstein G, Garway-Heath DF, Hitchings RA. Identification of early glaucoma cases with the scanning laser ophthalmoscope. Ophthalmology. 1998;105:1557–1563 [DOI] [PubMed] [Google Scholar]
- 18. Swindale NV, Stjepanovic G, Chin A, Mikelberg FS. Automated analysis of normal and glaucomatous optic nerve head topography images. Invest Ophthalmol Vis Sci. 2000;41:1730–1742 [PubMed] [Google Scholar]
- 19. Ferreras A, Pajarín AB, Polo V, Larrosa JM, Pablo LE, Honrubia FM. Diagnostic ability of Heidelberg Retina Tomograph 3 classifications: glaucoma probability score versus Moorfields regression analysis. Ophthalmology. 2007;114:1981–1987 [DOI] [PubMed] [Google Scholar]
- 20. Stalmans I, Vandewalle E, Anderson DR, et al. Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 2011;89:e609–e630 [DOI] [PubMed] [Google Scholar]
- 21. Pourcelot L. Indications of Doppler's ultrasonography in the study of peripheral vessels. Rev Prat. 1975;25:4671–4680 [PubMed] [Google Scholar]
- 22. Williamson TH, Harris A. Ocular blood flow measurement. Br J Ophthalmol. 1994;78:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jonas JB, Gusek GC, Naumann GO. Optic disc morphometry in chronic primary open-angle glaucoma. I Morphometric intrapapillary characteristics. Graefes Arch Clin Exp Ophthalmol. 1988;226:522–530 [DOI] [PubMed] [Google Scholar]
- 24. Uchida H, Brigatti L, Caprioli J. Detection of structural damage from glaucoma with confocal laser image analysis. Invest Ophthalmol Vis Sci. 1996;37:2393–2401 [PubMed] [Google Scholar]
- 25. Miglior S, Guareschi M, Albe' E, et al. Detection of glaucomatous visual field changes using the Moorfields regression analysis of the Heidelberg retina tomograph. Am J Ophthalmol. 2003;136:26–33 [DOI] [PubMed] [Google Scholar]
- 26. Medeiros FA, Zangwill LM, Bowd C, et al. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and StratusOCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol. 2004;122:827–837 [DOI] [PubMed] [Google Scholar]
- 27. Nouri-Mahdavi K, Hoffman D, Tannenbaum DP, et al. Identifying early glaucoma with optical coherence tomography. Am J Ophthalmol. 2004;137:228–235 [DOI] [PubMed] [Google Scholar]
- 28. Ferreras A, Pablo LE, Pajarín AB, Larrosa JM, Polo V, Pueyo V. Diagnostic ability of the Heidelberg retina tomograph 3 for glaucoma. Am J Ophthalmol. 2008;145:354–359 [DOI] [PubMed] [Google Scholar]
- 29. Ferreras A, Pablo LE, Garway-Heath DF, Fogagnolo P, García-Feijoó J. Mapping standard automated perimetry to the peripapillary retinal nerve fiber layer in glaucoma. Invest Ophthalmol Vis Sci. 2008;49:3018–3025 [DOI] [PubMed] [Google Scholar]
- 30. Kamal DS, Garway-Heath DF, Hitchings RA, Fitzke FW. Use of sequential Heidelberg retina tomograph images to identify changes at the optic disc in ocular hypertensive patients at risk of developing glaucoma. Br J Ophthalmol. 2000;84:993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamal DS, Viswanathan AC, Garway-Heath DF, Hitchings RA, Poinoosawmy D, Bunce C. Detection of optic disc change with the Heidelberg retina tomograph before confirmed visual field change in ocular hypertensives converting to early glaucoma. Br J Ophthalmol. 1999;83:290–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zangwill LM, Weinreb RN, Beiser JA, et al. Baseline topographic optic disc measurements are associated with the development of primary open-angle glaucoma: the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123:1188–1197 [DOI] [PubMed] [Google Scholar]
- 33. Galassi F, Sodi A, Rossi MG, Ucci F. Results of color doppler imaging in various types of glaucoma. : Pillunat LE Harris A Anderson DR Greve EL, Current Concepts in Ocular Blood Flow in Glaucoma. The Hague: Kugler Publications; 1999:119–127 [Google Scholar]
- 34. Galassi F, Soi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol. 2003;121:711–715 [DOI] [PubMed] [Google Scholar]
- 35. Kouvidis GK, Benos A, Kyriakopoulou G, Anastopoulos G, Triantafyllou D. Colour Doppler ultrasonography of the ophthalmic artery: flow parameters in normal subjects. The significance of the resistance index. Int Angiol. 2000;19:319–325 [PubMed] [Google Scholar]
- 36. Harris A, Williamson TH, Martin B, et al. Test/retest reproducibility of color Doppler imaging assessment of blood flow velocity in orbital vessels. J Glaucoma. 1995;4:281–286 [PubMed] [Google Scholar]
- 37. Quaranta L, Harris A, Donato F, et al. Color Doppler imaging of ophthalmic artery blood flow velocity. Ophthalmology. 1997;104:653–658 [DOI] [PubMed] [Google Scholar]
- 38. Neméth J, Kovács R, Harkányi Z, et al. Observer experience improves reproducibility of color Doppler sonography of orbital blood vessels. J Clin Ultrasound. 2002;30:332–335 [DOI] [PubMed] [Google Scholar]
- 39. Butt Z, McKillop G, O'Brien C, Allan P, Aspinall P. Measurement of ocular blood flow velocity using colour Doppler imaging in low tension glaucoma. Eye. 1995;9:29–33 [DOI] [PubMed] [Google Scholar]
- 40. Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kerr J, Nelson P, O'Brien C. A comparison of ocular blood flow in untreated primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 1998;126:42–51 [DOI] [PubMed] [Google Scholar]
- 42. Rankin SJ, Walman BE, Buckley AR, Drance SM. Color Doppler imaging and spectral analysis of the optic nerve vasculature in glaucoma. Am J Ophthalmol. 1995;119:685–693 [DOI] [PubMed] [Google Scholar]
- 43. Nicolela MT, Drance SM, Rankin SJ, Buckley AR, Walman BE. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol. 1996;121:502–510 [DOI] [PubMed] [Google Scholar]
- 44. Kaiser HJ, Schoetzau A, Stümpfig D, Flammer J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am J Ophthamol. 1997;123:320–327 [DOI] [PubMed] [Google Scholar]
- 45. Birinci H, Danaci M, Oge I, Erkan ND. Ocular blood flow in healthy and primary open-angle glaucomatous eyes. Ophthalmologica. 2002;216:434–437 [DOI] [PubMed] [Google Scholar]
- 46. Akarsu C, Bilgili MY. Color Doppler imaging in ocular hypertension and open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2004;242:125–129 [DOI] [PubMed] [Google Scholar]
- 47. Polska E, Kircher K, Ehrlich P, Vecsei PV, Schmetterer L. RI in central retinal artery as assessed by CDI does not correspond to retinal vascular resistance. Am J Physiol Heart Circ Physiol. 2001;280:H1442–H1447 [DOI] [PubMed] [Google Scholar]
- 48. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vizzeri G, Bowd C, Weinreb RN, et al. Determinants of agreement between the confocal scanning laser tomograph and standardized assessment of glaucomatous progression. Ophthalmology. 2010;117:1953–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leung CK, Liu S, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology. 2011;118:1551–1557 [DOI] [PubMed] [Google Scholar]
- 51. Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095 [DOI] [PubMed] [Google Scholar]
- 52. Dielemans I, Vingerling JR, Wolfs RC, et al. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology. 1994;101:1851–1855 [DOI] [PubMed] [Google Scholar]
- 53. Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–829 [DOI] [PubMed] [Google Scholar]
- 54. Quigley HA, West SK, Rodriguez J, et al. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–1826 [DOI] [PubMed] [Google Scholar]
- 55. Weih LM, Nanjan M, McCarty CA, Taylor HR. Prevalence and predictors of open-angle glaucoma: results from the visual impairment project. Ophthalmology. 2001;108:1966–1972 [DOI] [PubMed] [Google Scholar]