Abstract
Intense pulsed light technologies have evolved significantly since their introduction to the medical community 20 years ago. Now such devices can be used safely and effectively for the cosmetic treatment of many vascular lesions, unwanted hair, and pigmented lesions. Newer technologies often give results equal to those of laser treatments.
Introduction
Intense pulsed light (IPL) devices are non-laser high intensity light sources that make use of a high-output flashlamp to produce a broad wavelength output of noncoherent light, usually in the 500 to 1200nm range. Light pulses generated by most modern devices are produced by bursts of electrical current passing through a xenon gas-filled chamber.1 The lamp output is then directed toward the distal end of the handpiece, which, in turn, releases the energy pulse onto the surface of the skin via a sapphire or quartz block. Individual systems use different cooling systems, such as a cryogen spray, contact cooling, or forced refrigerated air, to protect the epidermis in contact with the conduction crystal of the handpiece.2
Individual light pulses have a specific duration, intensity, and spectral distribution allowing for a controlled and confined energy delivery into tissue. IPL use in dermatology relies on the basis that certain targets for energy absorption (chromophores) are capable of absorbing energy from this broad spectrum of light wavelength (absorptive band) without exclusively being targeted by their highest absorption peak. The working basis of the IPL rests on the principle of selective photothermolysis, in which thermally mediated radiation damage is confined to chosen epidermal and/or dermal pigmented targets at the cellular or tissue structural levels.3 Tissues surrounding these targeted structures, including overlying or immediately neighboring cells, are spared, potentially reducing nonspecific, widespread thermal injury. The three main chromophores (hemoglobin, water, and melanin) in human skin all have broad absorption peaks of light energy, allowing them to be targeted by a range as well as a specific wavelength of light. Therefore, monochromaticity of the light beam is not a prerequisite for selective heating of target structures in human skin. The broad wavelength range discharged from an IPL device leads to the simultaneous emission of green, yellow, red, and infrared wavelengths allowing the various chromophores to be targeted concurrently.
Most of the currently available IPL emission devices however, can be limited at the lower end of the emission spectrum by using dichroic or “cut off” filters to more selectively target desired cellular or structural elements. Although most IPL devices have one or two cutoff filters, available cutoff filters include 515, 550, 560, 570, 590, 615, 645, 690, and 755nm and function by blocking emission of shorter wavelength light. Apart from wavelength, a wide range of other treatment parameters including pulse duration, pulse sequences, and pulse delay time may be customized on most devices, affording users greater versatility and precision. Therefore, one attractive feature of IPL devices is their ability to treat various targets with the same device by applying different filters.
Another well-recognized advantage of IPL devices is the relatively large footprint of their spot size and their resulting treatment speed, allowing one to limit the total number of pulses per treatment to a minimum and affording a swift treatment of large anatomical areas. Nonetheless, the larger handpieces and spot sizes can pose a potential maneuverability disadvantage when treating uneven skin surfaces.
The first report of the use of an IPL device in dermatology dates back to 1996, when it was successfully utilized to treat a cohort of 80 patients with treatment-resistant facial port wine stains in Germany.4,5 The device, which employed a light source emitting noncoherent light with a wavelength spectrum of 515 to 1200nm, had been originally developed for the treatment of a wide range of benign vascular lesions, including telangiectasias and reticular varicose leg veins.6
Soon after, the same authors published two cases of effective permanent removal of terminal hair in two patients who underwent multiple treatments with IPL to their beard area.7 This initial report was followed by several more standardized studies that demonstrated the safety and efficacy of IPL for long-term hair removal.8,9
The first IPL device obtained United States Food and Drug Administration (FDA) clearance in 1995 for treatment of lower extremity telangiectasias. Since then, its favorable cost and versatility in contrast to many single-spectrum lasers, has lead to its rapid proliferation and use in a number of different clinical settings. Despite early claims of having too many side effects and too little efficacy, innovations in technology have resulted in the development of more powerful, predictable, and reliable devices enhancing their usefulness in skin rejuvenation
Intense Pulsed Light Clinical Uses
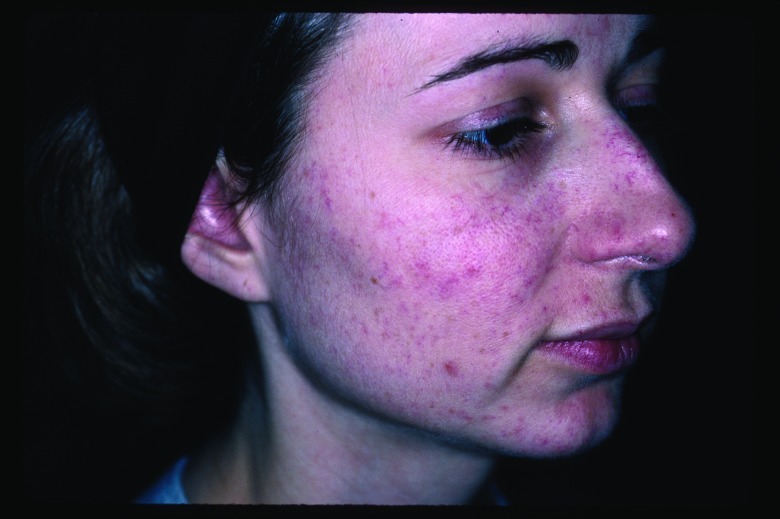
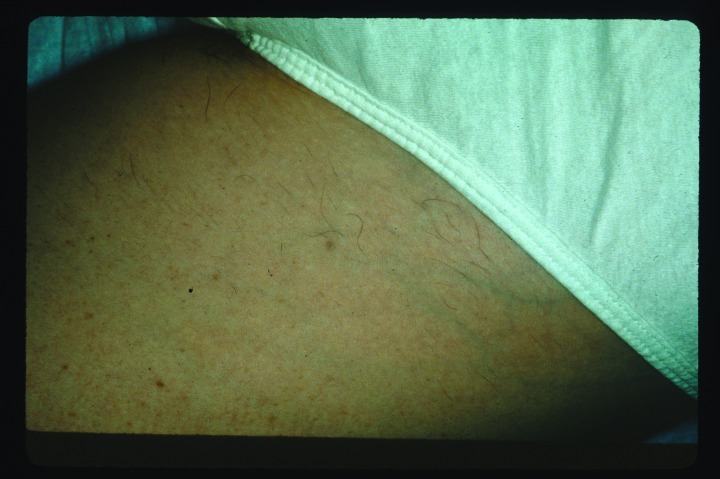
Vascular lesions (Figure 1 and Figure 2). There are multiple well-established and effective laser treatments for targeting blood vessels in the skin, with the pulsed dye laser being the workhorse in many practices nationwide. However, one limitation of the latter is the need to achieve purpura in several clinical scenarios to achieve acceptable results. In contrast, one of the main advantages of IPL technology is the absence of postoperative purpura, which minimizes postprocedure downtime substantially. Rather than inducing immediate purpura, the goal of treating vascular lesions with IPL is to raise the blood vessel temperature high enough to cause its coagulation, leading to its destruction and replacement by fibrous granulation tissue. Because of its polychromaticity, IPL can target oxyhemoglobin (predominantly found in clinically red lesions), deoxygenated hemoglobin (predominantly in blue lesions), and methemoglobin, with absorption peak wavelengths of 418, 542, and 577.3
Figure 1.
Angiomas before intense pulsed light treatment
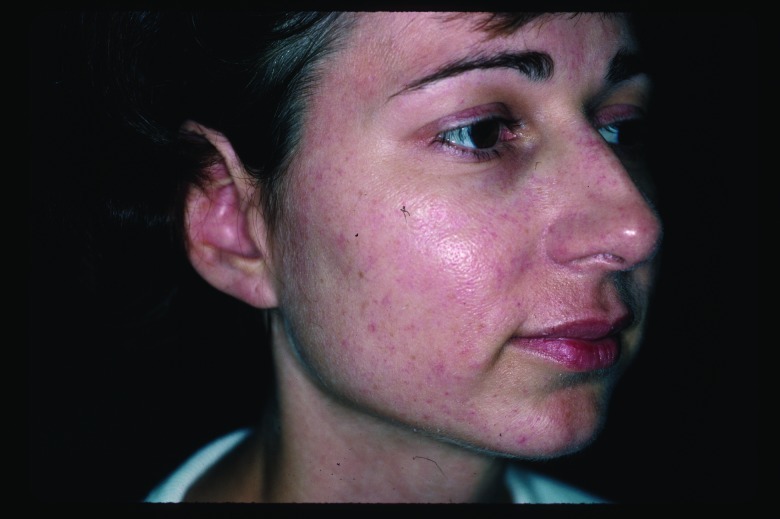
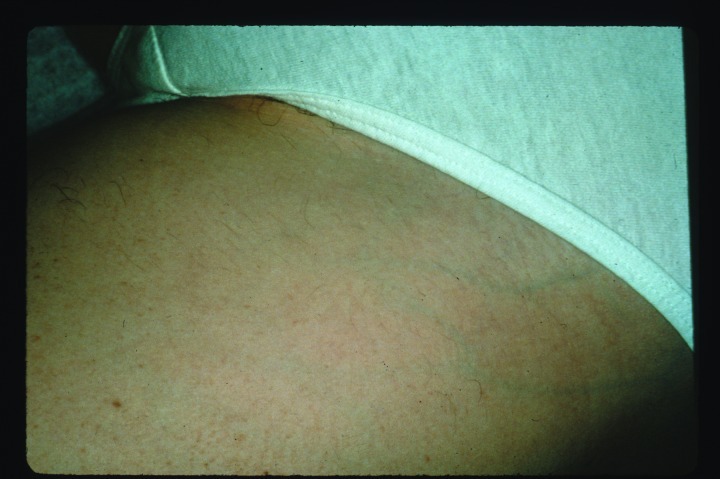
Figure 2.
Improvement in angiomas three months after three intense pulsed light treatments
The successful treatment of vascular lesions with IPL depends on the type and size of vessels targeted, with cherry angiomas and superficial telangiectatic veins typically demonstrating the best response. In contrast to the pulsed dye laser, which delivers a 585nm wavelength at a relatively short-pulse duration (450 microseconds), thereby limiting its depth of penetration to a maximum depth of 1.5mm, the broader wavelength range emitted by IPL devices and delivered through variable pulse durations and multiple-pulse sequences enables deeper-seated vessels and cavernous vascular lesions to be targeted.10 Ideally, the pulse duration should be compatible with the vessel diameter and be about equal or below the thermal relaxation time for that dimension cutaneous blood vessel so that the surrounding tissue is barely harmed.11 Modern IPL devices provide pulse durations up to 100 milliseconds, which enables delivery of light energy to vessels over longer periods of time, resulting in gentle, uniform heating or even coagulation across the entire vessel while reducing vessel rupture and its associated purpura and hyperpigmentation.4,12 The diameter of the targeted vessels is an important consideration when choosing the treatment settings of the IPL device to adequately account for epidermal relaxation times. For standard-sized small vessels in the papillary dermis with a diameter of 100 microns (0.1mm), the thermal relaxation time is approximately 10 milliseconds; for larger vessels of 300 microns (0.3mm) in diameter, the thermal relaxation time is approximately 100 milliseconds. Assuming that the thermal relaxation time of the human epidermis (average thickness of 100 microns or 0.1mm) is 10 milliseconds, the treatment of smaller vessels should ideally be done with multiple pulses with at least 10 millisecond delays between pulses to allow for adequate epidermal cooling.
IPL systems have been used effectively in the treatment of cavernous hemangiomas, venous and capillary malformations, facial and leg telangiectasias, and poikiloderma of Civatte.4,5,13,14 When treating port wine stains, especially those with a nodular component with external light sources, an old challenge has been targeting deeper-seated vessels located at the base of such lesions. It has been shown that using a 585nm wavelength pulsed dye laser source, most energy is deposited in the superficial vessels and that this decreases the amount of light available to deeper vessels (shadowing effect).15 Thus, clinical response of lightening in port wine stains with pulsed dye laser is dependent on vessel depth, diameter, and wall thickness. With IPL in contrast, the variable pulse durations and the multiple split-light pulses cause additive heating, leading to coagulation of vessels of different diameters and, theoretically, better concurrent heating of superficial and deeper vessels. A 5 to 100 millisecond delay between pulses allows the epidermis to cool down, preventing damage. In most clinical studies of port wine stains, IPL treatments were well tolerated. Side effects were infrequently observed and included temporary erythema, superficial blistering, hypopigmentation, and hyperpigmentation.4,16
Other vascular lesions commonly targeted by IPL are telangiectasias and cherry angiomas of various anatomical locations.17,18 The most frequently used intervals between treatments reported in the literature vary between 3 and 8 weeks. In a study of 1,000 consecutive patients with facial telangiectasias or vascular marks treated from 1998 to 2005 with the Photoderm VL (Lumenis Aesthetic, Santa Clara, California), IPL was shown to be a fast, safe, and effective modality. In this study, large facial veins were treated in triple-pulse mode using the 590 cutoff filter with pulse times of 2.4, 3.0, and 3.5 milliseconds; a delay of 30 and 25 milliseconds; and an energy flow of 50 to 56 J/cm2. Red fine telangectasias usually were treated in double-pulse mode using the 570nm cutoff filter with pulse times of 2.8 and 4.5 milliseconds, a delay of 30 milliseconds, and energy levels of 38 to 42J/cm2. Spider lesions were treated with the same settings as for large veins using a white screen with a varying size hole (1–4mm) to hit the arteriolar part of the lesion. Perilesional erythema, blanching, and vessel clearance were considered optimal treatment endpoints.
IPL has also been shown to be an effective treatment for the telangiectasias and background erythema/flushing seen in patients with erythematotelangiectatic rosacea. Recently, it has been shown that IPL is at least equally effective as nonpurpuragenic pulsed dye laser in reducing both signs and symptoms of rosacea.19
Hair removal (Figure 3 and Figure 4). The application of light based technology for hair removal is also based on the principle of selective photothermolysis, using melanin as the target chromophore. Since melanin is found in the hair follicle epithelium as well as throughout the epidermis, it is important to selectively target the pigment deposits inside the hair shafts while leaving the epidermal melanin aggregates untouched. However, melanin’s absorption peak is best at lower wavelengths, making deeper penetration of light to the base of the hair follicle more challenging. The use of longer wavelengths to deliver more energy to deeper layers of the skin can be used to target larger, more dense melanin collections in hair follicles.20 Once melanin absorbs the light energy, it is transformed into thermal energy causing necrosis of the hair follicle.21 The hair follicle in the anagen phase is the most responsive to this treatment, given the fact that the hair shaft contains the largest amount of melanin.
Figure 3.
Lentigines before intense pulsed light treatment
Figure 4.
Improvement in lentigines three months after four intense pulsed light treatment
As a result, hair color and size are important determinants to predicting the efficacy of hair removal using IPL. Coarser, darker hair tends to absorb more energy and respond better than lighter fine hair. In fact, blond hair will typically not respond to IPL or laser treatment and is better addressed with alternate hair removal modalities that target hair follicles independent of their pigment content.22 To protect the epidermal melanin from thermal injury, IPL pulses can be divided in synchronized millisecond pulses separated by short thermal relaxation times.
IPL can be used to treat unwanted hair in a variety of anatomic locations. The large spot size makes it easy to treat larger surface areas, such as the back, chest, or legs, which would otherwise be cumbersome to treat with smaller spot-sized devices.
Pretreatment patient counseling and managing individual patient’s expectations is an integral part of successful light-assisted or laser hair removal. It is important for patients to understand that multiple treatment sessions will be needed and that their hair will gradually become finer and sparser.
Treatment with the IPL device is generally well tolerated by most people when using standard energy settings. Priming the skin with topical anesthetic creams for an hour prior to treatment will further help alleviate some of the discomfort, but is not always necessary. Although rarely observed in the hands of an experienced user, potential complications from IPL hair removal with excessive energy or improper technique include hyperpigmentation, hypopigmentation, folliculitis, and paradoxical hypertrichosis.23
To avoid such complications it is essential to use the appropriate energy settings and to ensure complete contact between the crystal square of the handpiece and the skin. For some commercially available systems, it is wise to apply a water-based conduction gel to enhance this contact. Furthermore, the risk of residual dyschromia is greater in darker skin types and should be taken into account when selecting the fluence and pulse duration prior to treatment. In these cases, it is recommended that the highest available filter (755nm) be used with interpulse delays of 50 to 100 milliseconds to allow sufficient time for the epidermis to cool to minimize thermal damage.24
Recently, a handheld, low-fluence, home-use intense pulsed light device has gained FDA approval for treatment of unwanted facial hair. Although this technology appears to be safe, results from clinical studies suggest that its effectiveness is lower than in-office treatments performed with lasers or IPL devices at higher energies.25
Pigmented lesions and dyschromia (Figure 5 and Figure 6). Among the variety of methods used to treat photoaged skin, IPL has gained significant interest because of the patient’s rapid recovery after the procedure. Dyschromia, especially of the face, is a common complaint of patients seeking photorejuvenation with laser or light technology. Localized epidermal melanocyte, melanin, or ecstatic vessel aggregates enhance the color contrast with the surrounding skin giving it an uneven, heterogeneous tone. Breaks in the homogeneous color palette of the skin are perceived by the human eye as “imperfections” and has been shown to be one of the principal defining features of “old”-appearing skin.26 Solar lentigines are hyperchromatic macules typically resultant of chronic sun exposure. UV light induces and aggravates them, and they have been associated with an increased risk of skin cancer. Various modalities, including cryotherapy, lasers, and topical and chemical peeling agents have been reported to treat solar lentigines with varying success.
Figure 5.
Unwanted hair before intense pulsed light treatment
Figure 6.
Improvement in unwanted hair six months after five intense pulsed light treatments
When utilizing a light source for treatment of pigmented skin lesions, the localization of the pigment target is a key determinant for the selection of the appropriate therapeutic wavelength. Wavelengths in the range of 630 to 1100nm account for both a preferential absorption of melanin over hemoglobin as well as an effective dermal penetration depth.
The mechanism of action of IPL for treating pigmentary lesions is thought to be the result of rapid differentiation of keratinocytes induced by thermal heating. This process results in an upward transfer of melanosomes along with necrotic keratinocytes, resulting in their elimination as the microcrusts are removed from the skin surface. These effects have been demonstrated in vivo using both reflectance-mode confocal microscopy and optical coherence tomography, which allow precise visualization of melanosomes in the skin in horizontal and vertical dimensions, respectively.27
Ephelides, senile, and solar lentigines are among the many examples of pigmented lesions that have been successfully treated with IPL.28,29 As discussed earlier, telangiectasias are also easily treated by this modality, making IPL a good treatment for overall dyschromia regardless of its origin. With the use of different filters, the IPL device is capable of emitting wavelength spectra in the ranges of 500 to 670 and 870 to 1,400nm, allowing it to target vascular and pigmented lesions, respectively. Filtering wavelengths between 670 and 870nm aids in preservation of the melanin-rich epidermis and improves the ratio of vascular-to-pigment destruction.30 As such, lower cutoff filters can be used to treat superficial pigmentation, such as freckles, and higher cutoff filters for deeper lesions, such as nevus spilus.31 IPL has also been used with varied success in Asian patients with refractory melasma.32
The effect of IPL treatment for dyschromia is cumulative and repeat treatments (typically 3–6) every 3 to 4 weeks are generally necessary for complete clearance. The author has noted that lesser treatments with associated less discomfort have been seen when IPL is combined with associated photopneumatic treatment. As expected, darker lesions and those where the pigment is localized to deeper layers of the dermis typically respond slower and require a higher total number of treatments. In these cases, despite adequate treatment regimens, lesions are often persistent.
It is important to carefully assess each patient’s skin type preoperatively and adjust the IPL settings appropriately to avoid complications. In darker skin types, there is a risk of inducing hyperpigmentation. The immediate endpoint from IPL treatment of dyschromia should be visible darkening of the treated brown spots. These typically crust over 24 to 48 hours and peel off within seven days. This process can be accelerated by having patients apply a moisturizer twice a day or by performing microdermabrasion of the treated area 1 to 2 days following treatment.
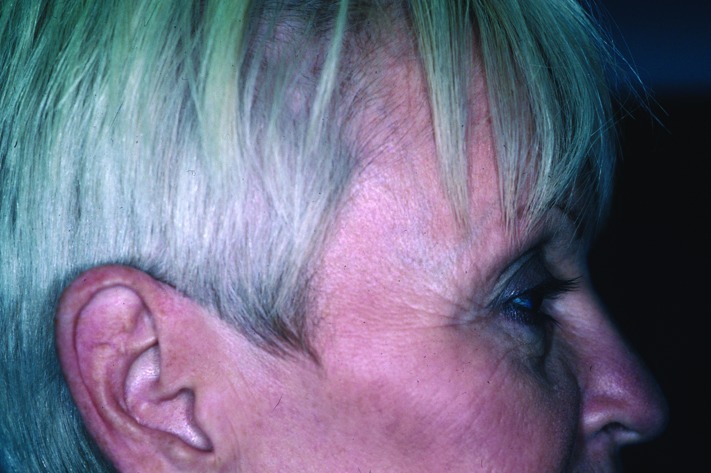
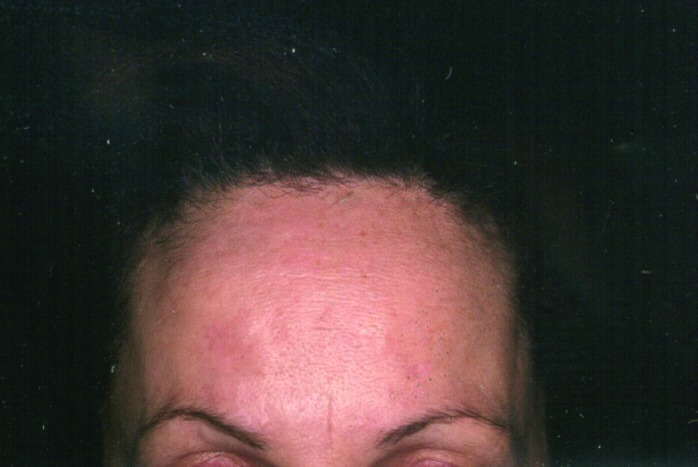
Rhytids and skin tightening (Figure 7 and Figure 8). Fine superficial wrinkles in the skin are the result of a combination of intrinsic and extrinsic aging processes that affect the skin with the passage of time. Intrinsic aging results in the gradual reduction of extracellular matrix proteins, such as collagen and hyaluronic acids, in the dermis due to a chronological senescence of dermal fibroblasts. Extrinsic aging of the skin is mainly the result of long-term exposure to UV radiation from the sun, which leads to further breakdown of collagen fibers by oxygen radicals. The overall result is a decrease in dermal structural support and volume, leading to a reduction in skin torsion extensibility, thereby causing the overlying redundant epidermis to wrinkle.
Figure 7.
Rhytids before intense pulsed light treatment
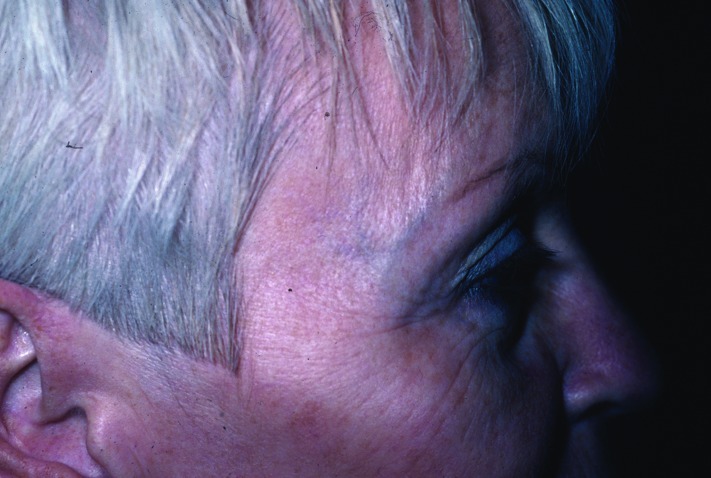
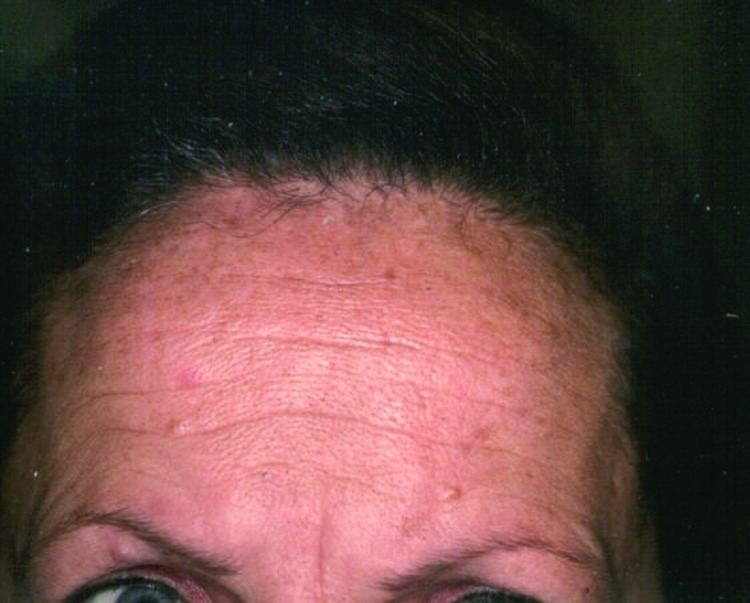
Figure 8.
Improvement in rhytids one month after three intense pulsed light treatments
In recent years, there has been a surge of noninvasive rejuvenation modalities aimed at the treatment of fine wrinkles and tightening of lax skin, using a variety of external laser and light sources. A series of 4 to 6 IPL treatments termed “photorejuvenation” has recently been popularized and is available in many dermatology practices nationwide.33 The principle behind tightening of the skin using IPL rests on the theory that heating collagen fibers with high intensity light energy leads to their contracture. This may account for the textural change described in skin treated with IPL, which has been reported as a secondary observation in several studies.34 Furthermore, the thermal stimulation of dermal fibroblasts by the higher wavelengths within the IPL spectrum has been shown to result in increased synthesis of extracellular matrix proteins, leading to at least partial replacement of the lost dermal volume. Specifically, wavelenghts in the 1200nm spectrum are absorbed by water in the dermis, triggering a cytokine reaction, which, in turn, stimulates the formation of new collagen I, III, and elastin.35 Histological evaluation of the effects of five subsequent IPL treatments with 570 to 645nm wavelengths showed epidermal thickening of 100 to 300µm, a decrease in horny plugs, new rete ridge formation, a decrease in proportion of degenerated elastic fibers, and new dermal collagen formation.36,37
The most commonly targeted wrinkles are those in the perioral and periorbital regions, with finer, more superficial lines typically responding better than deeper furrows. Results are often subtle and require multiple treatment sessions. Recent studies suggest that combining localized or full-face IPL treatments with concomitant botulinum toxin injections for dynamic rhytids produce better results than IPL treatment alone.38 The first report of the benefits of such a combination was published by Carruthers and Carruthers in 2004, showing that combined IPL-BTX treatment was associated with improvements not only in rhytids but also in telangiectasias, lentigines, apparent pore size, and skin texture.39 The basis for this synergy is thought to be the result of the immobilization of dermal matrix proteins achieved by paralyzing the underlying musculature, leading to smoother collagen lay down.
Conclusions
Intense pulsed technology is a highly versatile, safe, and effective modality for the treatment of vascular and pigmented lesions, hypertrichosis, and epidermal and dermal atrophy associated with photoaging, as well as acne, rosacea, actinic keratoses, and nonmelanoma skin cancers. As our understanding of the biological efficacy of various wavelength distributions evolves, so to will the range of IPL technology, particularly with regard to different wavelength filters, pulse durations, pulse frequencies, and cooling modalities to protect from side effects. The end result will be a widening domain of IPL’s clinical applications and indications. It will be incumbent on clinicians who use these devices with regularity for such new and emerging indications to report their clinical experiences in order to sustain our continued understanding of the technology’s long-term safety and efficacy profile.
Footnotes
DISCLOSURE:Dr. Goldberg reports no relevant conflicts of interest.
References
- 1.Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78–87. doi: 10.1002/lsm.10145. [DOI] [PubMed] [Google Scholar]
- 2.Weiss RA, Sadick NS. Epidermal cooling crystal collar device for improved results and reduced side effects on leg telangiectasias using intense pulsed light. Dermatol Surg. 2000;26(11):1015–1018. doi: 10.1046/j.1524-4725.2000.0260111015.x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RR, Parrish RR. Selective photothermolysis: precise microsurgery by selective absorption of pulse radiation. Science. 1983;220:524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 4.Raulin C, et al. Treatment of a nonresponding port wine stain with a new pulsed light source (PhotoDerm VL) Lasers Surg Med. 1997;21(2):203–208. doi: 10.1002/(sici)1096-9101(1997)21:2<203::aid-lsm13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Raulin C, et al. Treatment of essential telangiectasias with an intense pulsed light source (PhotoDerm VL) Dermatol Surg. 1997;23(10):941–945; discussion 945-946. doi: 10.1111/j.1524-4725.1997.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 6.Hellwig S, Schroter C, Raulin C. [Behandlung essesntieller Teleangiektasien durch das Photoderm VL. Z Hautkr. 1996;71:44–47. [Google Scholar]
- 7.Raulin C, Werner S, et al. Effective treatment of hypertrichosis with pulsed light: a report of two cases. Ann Plast Surg. 1997;39(2):169–173. doi: 10.1097/00000637-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gold MH, Bell MW, et al. Long-term epilation using the EpiLight broad band, intense pulsed light hair removal system. Dermatol Surg. 1997;23(10):909–913. doi: 10.1111/j.1524-4725.1997.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 9.Weiss RA, Weiss MA, et al. Hair removal with a non-coherent filtered flashlamp intense pulsed light source. Lasers Surg Med. 1999;24(2):128–132. doi: 10.1002/(sici)1096-9101(1999)24:2<128::aid-lsm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Haedersdal M, Efsen J, et al. Changes in skin redness, pigmentation, echostructure, thickness, and surface contour after 1 pulsed dye laser treatment of port-wine stains in children. Arch Dermatol. 1998;134:175–181. doi: 10.1001/archderm.134.2.175. [DOI] [PubMed] [Google Scholar]
- 11.Kimel S, Svaasand LO, et al. Differential vascular response to laser photothermolysis. J Invest Dermatol. 1994;103:693–700. doi: 10.1111/1523-1747.ep12398548. [DOI] [PubMed] [Google Scholar]
- 12.Schroeter CA, Neumann HAM. An intense light source: the Photoderm VL flashlamp as a new treatment possibility for vascular skin lesions. Dermatol Surg. 1998;24:743–748. [PubMed] [Google Scholar]
- 13.Raulin C, Raulin SJ, et al. Treatment of benign venous malformations with an intense pulsed light source (PhotoDermTVL) Eur J Dermatol. 1997;7:279–282. [Google Scholar]
- 14.Raulin C, Careen A, et al. Treatment of port-wine stains with a noncoherent pulsed light source: a retrospective study. Arch Dermatol. 1999;135:679–683. doi: 10.1001/archderm.135.6.679. [DOI] [PubMed] [Google Scholar]
- 15.Lucassen GW, Verkruysse W, et al. Light distributions in a port wine stain model containing multiple cylindrical and curved blood vessels. Lasers Surg Med. 1996;18:345–357. doi: 10.1002/(SICI)1096-9101(1996)18:4<345::AID-LSM3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Li YH, Chen JZ, et al. Efficacy and safety of intense pulsed light in treatment of melasma in Chinese patients. Dermatol Surg. 2008;34(5):693–700; discussion 700-701. doi: 10.1111/j.1524-4725.2008.34130.x. [DOI] [PubMed] [Google Scholar]
- 17.Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95–100. doi: 10.1080/14628839950516922. [DOI] [PubMed] [Google Scholar]
- 18.Clementoni MT, Gilardino P, et al. Intense pulsed light treatment of 1,000 consecutive patients with facial vascular marks. Aesthetic Plast Surg. 2006;30(2):226–232. doi: 10.1007/s00266-005-0086-0. [DOI] [PubMed] [Google Scholar]
- 19.Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009 Apr 6. doi: 10.1111/j.1524-4725.2009.01156.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RR, Laser tissue interactions. In: Goldman MP, Fitzpatrick RE. eds. Cutaneous Laser Surgery. St. Louis: Mosby Year Book; 1994:9-10. [Google Scholar]
- 21.Sadick NS, Christopher R, et al. High-intensity flashlamp photoepilation: a clinical, histological, and mechanistic study in human skin. Arch Dermatol. 1999;135:668–676. doi: 10.1001/archderm.135.6.668. [DOI] [PubMed] [Google Scholar]
- 22.Gold MH, et al. Long-term epilation using the EpiLight broad band, intense pulsed light hair removal system. Dermatol Surg. 1997;23(10):909–913. doi: 10.1111/j.1524-4725.1997.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 23.Radmanesh M, Azar-Beig M, et al. Burning, paradoxical hypertrichosis, leukotrichia and folliculitis are four major complications of intense pulsed light hair removal therapy. J Dermatolog Treat. 2008;19(6):360–363. doi: 10.1080/09546630802132627. [DOI] [PubMed] [Google Scholar]
- 24.Goldman MP, Weiss RA, Weiss MA. Intense pulsed light as a nonablative approach to photoaging. Dermatol Surg. 2005;31(9 Pt 2):1179–1187. doi: 10.1111/j.1524-4725.2005.31924. [DOI] [PubMed] [Google Scholar]
- 25.Alster TS, Tanzi EL. Effect of a novel low-energy pulsed-light device for home-use hair removal. Dermatol Surg. 2009;35(3):483–489. doi: 10.1111/j.1524-4725.2009.01089.x. [DOI] [PubMed] [Google Scholar]
- 26.Matts PJ, et al. Color homogeneity and visual perception of age, health, and attractiveness of female facial skin. J Am Acad Dermatol. 2007;57(6):977–984. doi: 10.1016/j.jaad.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita T, Negishi K, et al. Intense pulsed light therapy for superficial pigmented lesions evaluated by reflectance-mode confocal microscopy and optical coherence tomography. J Invest Dermatol. 2006;126(10):2281–2286. doi: 10.1038/sj.jid.5700414. [DOI] [PubMed] [Google Scholar]
- 28.Kawada A, Asai M, Kameyama H, et al. Videomicroscopic and histopathological investigation of intense light therapy for solar lentigines. J Dermatol Sci. doi: 10.1016/s0923-1811(02)00014-2. 29:91-96. [DOI] [PubMed] [Google Scholar]
- 29.Kawada A, Shiraishi H, et al. Clinical improvement of solar lentigines and ephelides with an intense pulsed light source. Dermatol Surg. 2002;28(6):504–508. doi: 10.1046/j.1524-4725.2002.01175.x. [DOI] [PubMed] [Google Scholar]
- 30.Ross EV, Smirnov M, et al. Intense pulsed light and laser treatment of facial telangiectasias and dyspigmentation: some theoretical and practical comparisons. Dermatol Surg. 2005;31(9 Pt 2):1188–1198. doi: 10.1111/j.1524-4725.2005.31925. [DOI] [PubMed] [Google Scholar]
- 31.Moreno Arias GA, Ferrando J. Intense pulsed light for melanocytic lesions. Dermatol Surg. 2001;27:397–400. doi: 10.1046/j.1524-4725.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang CC, Hui CY, et al. Intense pulsed light for the treatment of refractory melasma in Asian persons. Dermatol Surg. 2004;30(9):1196–1200. doi: 10.1111/j.1524-4725.2004.30371.x. [DOI] [PubMed] [Google Scholar]
- 33.Bitter PH. Noninvasive rejuvenation of photodamaged skin using serial, full-face intense pulsed light treatments. Dermatol Surg. 2000;26:835–842. doi: 10.1046/j.1524-4725.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 34.Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28(12):1115–1119. doi: 10.1046/j.1524-4725.2002.02113.x. [DOI] [PubMed] [Google Scholar]
- 35.Wong WR, et al. Intense pulsed light effects on the expression of extracellular matrix proteins and transforming growth factor beta-1 in skin dermal fibroblasts cultured within contracted collagen lattices. Dermatol Surg. 2009;35(5):816–825. doi: 10.1111/j.1524-4725.2009.01138.x. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Pérez E, Ibiett EV. Gross and microscopic findings in patients submitted to nonablative full-face resurfacing using intense pulsed light: a preliminary study. Dermatol Surg. 2002;28(8):651–655. doi: 10.1046/j.1524-4725.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- 37.Zelickson B, Kist D. Pulsed dye laser and photoderm treatment stimulates production of type-1 collagen and collagenase transcripts in papillary dermis fibroblasts. Lasers Surg Med. 2001;13(Suppl):132. [Google Scholar]
- 38.Khoury JG, Saluja R, Goldman MP. The effect of botulinum toxin type A on full-face intense pulsed light treatment: a randomized, double-blind, split-face study. Dermatol Surg. 2008;34(8):1062–1069. Epub 2008 May 6. doi: 10.1111/j.1524-4725.2008.34207.x. [DOI] [PubMed] [Google Scholar]
- 39.Carruthers J, Carruthers A. The effect of full-face broadband light treatments alone and in combination with bilateral crow’s feet Botulinum toxin type A chemodenervation. Dermatol Surg. 2004;30(3):355–366. doi: 10.1111/j.1524-4725.2004.30101.x. [DOI] [PubMed] [Google Scholar]