Abstract
An integrin found on platelets, αIIbβ3 mediates platelet aggregation, and αIIbβ3 antagonists are effective antithrombotic agents in the clinic. Ligands bind to integrins in part by coordinating a magnesium ion (Mg2+) located in the β subunit metal ion–dependent adhesion site (MIDAS). Drugs patterned on the integrin ligand sequence Arg-Gly-Asp have a basic moiety that binds the αIIb subunit and a carboxyl group that coordinates the MIDAS Mg2+ in the β3 subunits. They induce conformational changes in the β3 subunit that may have negative consequences such as exposing previously hidden epitopes and inducing the active conformation of the receptor. We recently reported an inhibitor of αIIbβ3 (RUC-1) that binds exclusively to the αIIb subunit; here, we report the structure-based design and synthesis of RUC-2, a RUC-1 derivative with a ~100-fold higher affinity. RUC-2 does not induce major conformational changes in β3 as judged by monoclonal antibody binding, light scattering, gel chromatography, electron microscopy, and a receptor priming assay. X-ray crystallography of the RUC-2–IIbβ3 headpiece complex in 1 mM calcium ion (Ca2+)/5 mM Mg2+ at 2.6 Å revealed that RUC-2 binds to αIIb the way RUC-1 does, but in addition, it binds to the β3 MIDAS residue glutamic acid 220, thus displacing Mg2+ from the MIDAS. When the Mg2+ concentration was increased to 20 mM, however, Mg2+ was identified in the MIDAS and RUC-2 was absent. RUC-2′s ability to inhibit ligand binding and platelet aggregation was diminished by increasing the Mg2+ concentration. Thus, RUC-2 inhibits ligand binding by a mechanism different from that of all other αIIbβ3 antagonists and may offer advantages as a therapeutic agent.
INTRODUCTION
Integrin receptors are heterodimeric complexes composed of α and β subunits that bind ligand and transduce signals bidirectionally (1, 2). They contribute to many different biologic and pathologic processes, including hemostasis, thrombosis, angiogenesis, immunity, development, bone resorption, and metastases (3–7). The platelet αIIbβ3 receptor is a validated therapeutic target, with three separate agents that inhibit ligand binding to the receptor (abciximab, eptifibatide, and tirofiban) approved for human use. These have shown clinical benefit in controlled trials in selected high-risk patients when used as adjunctive therapy to prevent ischemic complications of percutaneous coronary interventions and in other clinical conditions (8). The current agents have several limitations, however, including the need for intravenous administration and the induction of thrombocytopenia in some patients (9, 10). A number of oral αIIbβ3 antagonists patterned after the Arg-Gly-Asp (RGD) integrin binding sequence have been developed, but none have achieved regulatory approval because they were not efficacious when used as chronic therapy (11). Treatment with several of the agents was associated with an increased risk of death (11, 12), as well as with thrombocytopenia and an increased risk of bleeding in a small percentage of patients (13). Both the increased risk of death associated with the oral agents and the thrombocytopenia associated with both the intravenous and the oral agents have been hypothesized to result, at least in part, from conformational changes in the receptor induced by the binding of the agents (9, 10, 13–20).
On the basis of electron microscopy (EM) and x-ray crystallography studies, the two best documented conformational changes in the receptor are headpiece extension and headpiece opening, in which the β3 hybrid domain swings away from the αIIb β-propeller domain at its junction with the β3 βI domain (21–24). This latter movement is linked with remodeling of the β3 βI domain at the ligand-binding pocket formed at its interface with the αIIb subunit β-propeller domain. Crystal structures of the αIIbβ3 binding pocket in complex with eptifibatide, tirofiban, and other RGD-based antagonists, as well as the binding of the fibrinogen γ-chain C-terminal peptide, have identified a common binding mechanism involving binding to Asp224 in αIIb via the compound’s Arg (or its equivalent basic or Lys moiety) and coordinating the metal ion–dependent adhesion site (MIDAS) Mg2+ ion in the β3 subunit via one of the oxygen atoms in the compound’s Asp carboxyl or an equivalent carboxyl (22, 23). The binding of these agents was associated with the receptor adopting the β3 swing-out conformation as judged by x-ray crystallography (23). Because very early treatment of myocardial infarction with αIIbβ3 antagonists can prevent cardiac damage (25–27), it would be desirable to have an orally active agent that inhibits the receptor but does not induce the global conformational changes in the receptor.
We recently reported on a small-molecule inhibitor of αIIbβ3 termed RUC-1 (Fig. 1) that was identified by high-throughput screening with an assay based on the adhesion of platelets to immobilized fibrinogen (28, 29). RUC-1 is specific for αIIbβ3 relative to αVβ3, α2β1, and glycoprotein Ib (GPIb) and has antithrombotic effects in murine models in both large and small blood vessels when administered at 25.6 mg/kg. RUC-1 differs from the RGD-based αIIbβ3 antagonists eptifibatide and tirofiban in producing less extensive exposure of β3 ligand–induced binding sites as detected by monoclonal antibodies (mAbs), suggesting that it also produces less extensive conformational changes in the β3 subunit (28). Computer-assisted molecular docking studies corroborated by molecular dynamic (MD) simulations suggested that RUC-1 binds exclusively to the αIIb subunit, providing a potential explanation for its reduced ability to expose β3 ligand–induced binding sites (28–30). Subsequent studies in which RUC-1 was soaked into crystals of the αIIbβ3 headpiece confirmed that it binds exclusively to αIIb and, thus, unlike the RGD-based compounds, does not coordinate the β3 MIDAS metal ion (30). RUC-1 also does not initiate the reorganization of the β3 metal ions that is associated with the binding of RGD-based antagonists and the adoption of the β3 swing-out orientation. Additional gel permeation and dynamic light scattering (DLS) studies demonstrated that, unlike eptifibatide and tirofiban, RUC-1 does not induce conformational changes detectable by these techniques (30).
Fig. 1.
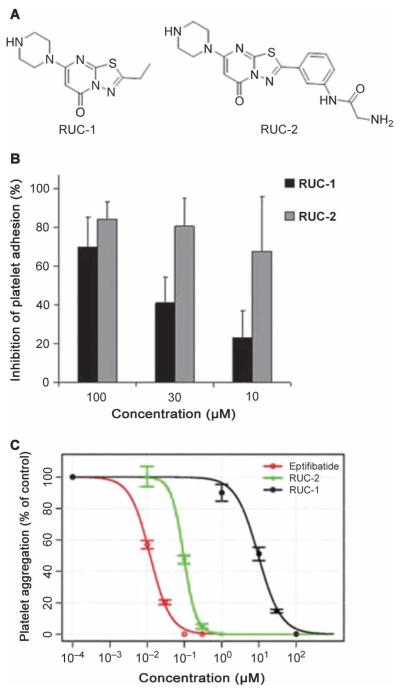
Chemical structure of RUC-2 and effects on platelet adhesion and aggregation. (A) Chemical structures of RUC-1 (left) and RUC-2 (right). (B) Effect of RUC-1 and RUC-2 on platelet adhesion to immobilized fibrinogen. RUC-1 and RUC-2 were tested at the indicated concentrations. Data are means ± SD and n =5.(C) Effect of RUC-1, RUC-2, and eptifibatide on platelet aggregation induced by ADP. Citrated PRP was incubated with either RUC-1, RUC-2, or eptifibatide at the indicated concentrations for 15 min, and then aggregation was induced by adding ADP (5 μM). The initial slope of aggregation was measured, and the inhibition was assessed as a percentage of the aggregation in the absence of an αIIbβ3 antagonist (designated as a concentration of 10−∞). The IC50 for RUC-2 (96 ± 5 nM) was more than two logs lower than the IC50 for RUC-1 (9.7 ± 1 μM) and nearly a log higher than the IC50 for eptifibatide (12 ± 1 nM).
To further explore the αIIbβ3 binding pocket and obtain additional information on the correlation between binding mechanism and induction of conformational changes in the receptor, we synthesized a series of derivatives of RUC-1 guided by structural and energetic considerations. One of these, termed RUC-2 (Fig. 1A and fig. S1), was more than 100 times more potent in inhibiting platelet aggregation than RUC-1, and so it was selected for further evaluation.
RESULTS
RUC-2′s chemical and biologic properties are summarized in Table 1.
Table 1.
Properties of RUC-2, 2-amino-N-(3-(5-oxo-7-(piperazin-1-yl)-5H-[1,3,4]thiadiazolo[3,2-a]pyrimidin-2-yl)phenyl)acetamide (NCGC00183896-01) (ML165).
| Category | Parameter | Description |
|---|---|---|
| Compound | Citation | |
| Name | (RUC-2) (NCGC00183896-01) (ML165) | |
| Chemical descriptors | ||
| Molecular weight | 385.443 g/mol | |
| Entries in chemical databases |
PubChem: CID 44820665, SID 8944968 | |
| Additional comments |
pKa values by titration in water: 6.41 and 8.08 |
|
| In vitro profiling |
Target | αIIbβ3 receptor |
| Potency | 96 ± 5 nM in ADP-induced platelet aggregation assay |
|
| Selectivity | 6 ± 15% inhibition of ligand binding to the αVβ3 receptor. |
|
| SAR | A manuscript detailing the SAR is in preparation. |
|
| Mechanism of inhibition |
Orthosteric inhibition at the RGD binding domain as determined by x-ray crystallography |
|
| Structure of target-probe complex |
PDB code 3T3M | |
| Cellular profiling |
Validation of cellular target |
RUC-2 inhibited αIIbβ3-dependent platelet adhesion to fibrinogen in a screening assay and αIIbβ3-dependent platelet aggregation. It was found to bind to the target via x-ray crystallography (PDB code 3T3M). |
| Validation of cellular specificity |
RUC-2 had minimal effect on αVβ3-mediated cell adhesion to vitronectin. |
Platelet adhesion screening assay
In five separate experiments, RUC-2 produced 83 ± 7% (mean ± SD) inhibition of platelet adhesion to fibrinogen at a concentration of 100 μM, 80 ± 13% inhibition at 30 μM, and 66 ± 10% inhibition at 10 μM (Fig. 1B); the comparable values for RUC-1 at the same concentrations were 70 ± 15%, 38 ± 10%, and 22 ± 8%. For further comparison, tirofiban produced 87 ± 9% inhibition at 10 μM.
Platelet aggregation Human platelets
RUC-2 inhibited adenosine diphosphate (ADP)–induced platelet aggregation of citrated platelet-rich plasma (PRP) with an IC50 (the concentration of a substance required to inhibit the activity of another substance by 50%) of 96 ± 5 nM (n = 4) (Fig. 1C). By comparison, RUC-1′s IC50 was more than two logs higher, at 9.7 ± 1.0 μM. For comparison, the IC50 for eptifibatide was 12 ± 1 nM when tested on the same PRP samples. When PPACK (Phe-Pro-Arg chloromethyl ketone) was used as the anticoagulant instead of citrate, the IC50 for RUC-2 was more than twofold higher (220 nM; n =4).
Platelet aggregation: Mouse and rat platelets
At doses that nearly completely inhibited human platelet aggregation, RUC-2 (1 μM), like RUC-1 (100 μM), did not inhibit either mouse or rat platelet aggregation induced by ADP (fig. S2, A and B). In contrast, RUC-2 essentially completely inhibited the aggregation of platelets from a mouse expressing human αIIb and mouse β3 (hαIIb/mβ3) (fig. S2C).
Platelet adhesion/aggregation to collagen
Consistent with our previous reports (28, 31), the anti-α2β1 mAb 6F1 produced 95% inhibition of platelet adhesion/aggregation to collagen, whereas the anti-αIIbβ3 mAb 10E5 and the anti-αIIbβ3 + αVβ3 mAb 7E3 produced ~30% inhibition (fig. S3). RUC-2 at 1 to 100 μM also inhibited adhesion/aggregation by ~30%, and combining RUC-2 with the anti-αIIbβ3 antibody 10E5 did not further inhibit adhesion/aggregation. Microscopic analysis indicated that, as reported with RUC-1 and the anti-mAb 10E5, RUC-2 did not decrease platelet adhesion to collagen, but decreased the recruitment of additional platelets to the adherent platelets.
αVβ3-mediated cell adhesion to vitronectin and αIIbβ3-mediated cell adhesion to fibrinogen
The αVβ3-specific mAb LM609 inhibited adhesion of human embryonic kidney (HEK) 293 cells expressing αVβ3 to vitronectin by 74 ± 27% (n =4) at 20 μg/ml, and the anti-αVβ3 + αIIbβ3 mAb 7E3 inhibited adhesion by 80 ± 12% (n = 4) at 40 μg/ml (fig. S4A). In contrast, RUC-1 at 100 μM produced only 5 ± 7% (n = 4) inhibition and RUC-2 at 10 μM produced only 6 ± 15% (n = 4). The RUC-1 and RUC-2 data are both similar to the 2 ± 9% (range, –9.4 to +7.6%) (n = 3) inhibition produced by the αIIbβ3-specific mAb 10E5.
LM609 did not inhibit the adhesion of HEK293 cells expressing αIIbβ3 to fibrinogen (fig. S4B), whereas 10E5 produced 79 ± 10% inhibition, 7E3 produced 87 ± 9% inhibition, RUC-1 produced 55 ± 5% inhibition, and RUC-2 produced 65 ± 5% inhibition (all n =4) at the same concentrations indicated for the αVβ3 experiments.
Induction of ligand-induced binding site epitopes
Two different β3 ligand–induced binding site antibodies were tested, AP5 and LIBS1, which bind to the PSI (plexin-semaphorin-integrin) domain and the distal leg region, respectively (32, 33). The net normalized fluorescence intensity in the presence of eptifibatide (10 μM, 30-min incubation at 22°C) was assigned the value of 100%. Untreated platelets bound 7 ± 3% (n = 5) of the amount of AP5 bound in the presence of eptifibatide (fig. S5). In the presence of RUC-1, platelets bound 10 ± 4% of the amount of AP5 bound in the presence of eptifibatide, and in the presence of RUC-2, they bound 18 ± 5%. The comparable data for LIBS1 binding after 30 min were 22 ± 3% (n =5) for untreated platelets, 18 ± 2% for platelets in the presence of RUC-1, and 21 ± 3% for platelets in the presence of RUC-2. The αIIb LIBS antibody PMI-1 was also tested (34). Untreated platelets bound 46 ± 5% (n =4) of the amount of PMI-1 that platelets treated with eptifibatide bound; both RUC-1 and RUC-2 increased PMI-1 binding to similar extents (73 ± 9 and 82 ± 13%, respectively).
Effect of RUC-1 and RUC-2 on recruitment of αIIbβ3-dependent antibodies to platelets
Sera from 20 patients who developed thrombocytopenia after treatment with the RGD-mimetic platelet inhibitors tirofiban (5 cases) or eptifibatide (15 cases) were studied for reactivity with normal human platelets pretreated with tirofiban (4.0 μM), eptifibatide (2.4 μM), RUC-1 (100 μM), RUC-2 (3.9 μM), or, as a control, a structurally related derivative of RUC-1 that does not inhibit ligand binding. In accord with our previous studies (9), pretreatment of platelets with either tirofiban or eptifibatide enhanced recruitment of immunoglobulin G (IgG) to the platelet surface from the sera of all of the patients. The strength of these reactions, as judged by median fluorescence intensity (MFI), ranged from 2.2 to 54 (median, 8.0) times the strength of the reactions obtained with control platelets treated with the inactive derivative of RUC-1. Neither RUC-1 nor RUC-2 induced recruitment of patient IgG to platelets when tested with all 5 tirofiban samples and 13 of 15 eptifibatide samples. Two sera from patients who experienced eptifibatide-induced thrombocytopenia recognized platelets treated with RUC-1 or RUC-2. One of these produced median MFI values with RUC-1– and RUC-2–treated platelets that were 6.8 and 4.2 times greater than the signals obtained with control platelets, but these increases were only 13 and 8%, respectively, of the values obtained with eptifibatide-treated platelets. The second recognized platelets pretreated with RUC-2, but not RUC-1, producing a signal that was 14 times the signal obtained with control platelets and 1.4 times the signal obtained with the eptifibatide-treated platelets. These two eptifibatide-treated patient samples were also unusual in that both tirofiban and the peptide RGDW were able to recruit patient IgG.
Effect of RUC-2 on extension of purified αIIbβ3 as judged by EM
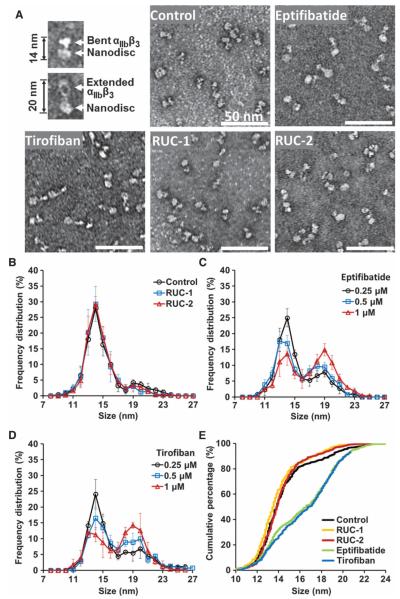
In accord with the data of Ye et al. (35), in the absence of compounds, αIIbβ3 primarily adopted a compact conformation adjacent to the nanodisc (Fig. 2), giving nanodisc-integrin length (NIL) values primarily between 11 and 17 nm (Fig. 2, A, B, and E). Occasional nanodiscs, however, contained αIIbβ3 molecules that were extended, giving NIL values between 18 and 23. As a result, the NIL frequency distribution showed a bimodal pattern, with a marked predominance of αIIbβ3 nanodiscs in the range of 11 to 17 nm, and a small subpopulation in the range 18 to 23 nm (Fig. 2, B and E). Both eptifibatide and tirofiban shifted the distribution in a dose-dependent manner such that, at the highest doses, most αIIbβ3 nanodiscs had NIL values in the range of 18 to 23 nm (Fig. 2, A and C to E) (P <0.001 and P < 0.001, respectively). In contrast, neither RUC-1 at concentrations up to 100 μM nor RUC-2 at concentrations up to 10 μM produced a significant shift in NIL values (P = 0.23 and 0.37, respectively)(Fig. 2, A, B, and E).
Fig. 2.
Negative stain EM of αIIbβ3 nanodiscs in the absence and presence of αIIbβ3 antagonists. (A) Representative images of bent and extended αIIbβ3 nanodiscs and images of αIIbβ3 nanodiscs in the presence of buffer, eptifibatide (1 μM), tirofiban (1 μM), RUC-1 (100 μM), or RUC-2 (10 μM). (B to D) Quantitative measurements of αIIbβ3 NIL values in the absence and presence of αIIbβ3 antagonists. (B) NIL value distributions in the presence of buffer, 100 μM RUC-1, or 10 μM RUC-2. (C and D) Dosedependent NIL value distributions in the presence of eptifibatide (C) or tirofiban (D). The mean ± SD of five separate experiments is depicted for each condition; a total of 600 to 700 particles contained in five separate electron microscopic images were measured at × 33,000 magnification in each experiment. (E) Cumulative percentage of NIL values in the presence of buffer, 100 μMRUC-1, 10 μM RUC-2, 1 μM eptifibatide, or 1 μM tirofiban.
Effect of RUC-2 on the Stokes radius of the soluble αIIbβ3 headpiece
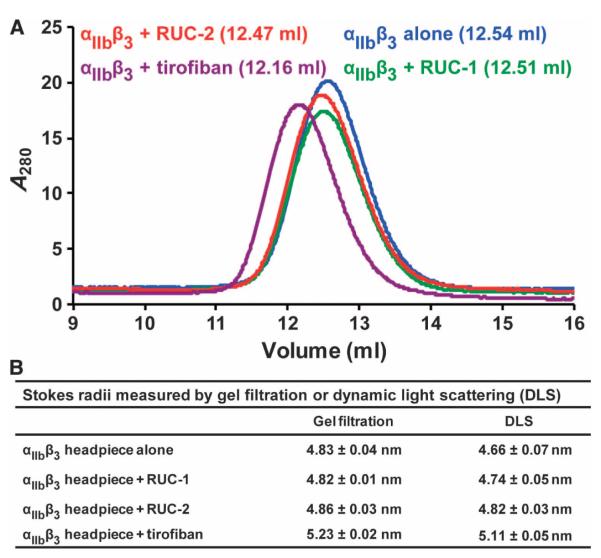
We examined the effect of RUC-2 on the conformation of the αIIbβ3 headpiece in solution by gel filtration or DLS with or without near-saturating concentrations of RUC-1, RUC-2, or tirofiban. Consistent with our previous results (30), gel filtration showed that tirofiban induced a substantial reduction in the αIIbβ3 headpiece elution volume (0.38 ml) (Fig. 3A). In contrast, RUC-2 and RUC-1 had little effect on elution volume of the αIIbβ3 headpiece (0.07 and 0.03 ml, respectively) (Fig.3A).These changes in elution volumes corresponded to a 0.4-nm increase in Stokes radius with tirofiban, and little or no increases with RUC-1 or RUC-2 (Fig. 3B). When measured by DLS, a similar increase in Stokes radius was found with tirofiban (0.45 nm). RUC-1 and RUC-2 produced considerably smaller but measurable increases in Stokes radius (0.08 and 0.16 nm, respectively) (Fig. 3B). These results suggest that RUC-2 induces less opening of the αIIbβ3 headpiece in solution than tirofiban.
Fig. 3.
Stokes radius determinations by gel filtration and DLS. (A) Gel filtration profile of αIIbβ3 headpiece alone or bound with antagonists. The untagged αIIbβ3 headpiece was mixed with near-saturating concentrations of RUC-1, RUC-2, or tirofiban and incubated at room temperature for 1 hour before chromatography on Superdex 200 in tris-buffered saline plus 1 mM Ca2+/Mg2+. The elution volumes are shown in parentheses. (B) Stokes radii calculated from gel filtration or DLS. Data are means ± SD (n = 2 for gel filtration; n =3forDLS).
Effect of RUC-2 on αIIbβ3 high-affinity ligand-binding conformation
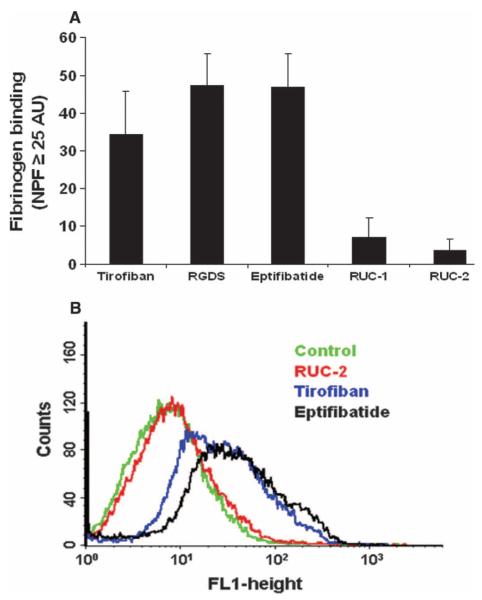
Because small-molecule αIIbβ3 antagonists based on the RGD motif induce a high-affinity ligand-binding conformation in αIIbβ3 (16, 20, 36–38), and because this activity has been proposed to explain the paradoxical increase in thrombotic death associated with the small-molecule oral αIIbβ3 antagonists (11, 12, 14, 18, 37), we tested the “priming” effect of RUC-2, that is, its ability to induce fibrinogen binding to platelet αIIbβ3. Incubation of washed platelets with eptifibatide (1 μM), tirofiban (0.5 μM), or an RGDS peptide (100 μM), followed by fixation in paraformaldehyde and washing, increased the binding of fluorescent fibrinogen to platelets as judged by the percentage of platelets with fluorescence values above those in the absence of the agents. The values were 47 ± 9, 34 ± 12, and 48 ± 8% (n = 4), respectively (Fig. 4). In contrast, the value for RUC-1 (100 μM) was only 8 ± 3% (n = 4), and for RUC-2 (1 μM), it was only 4 ± 3% (n = 4). Increasing the RUC-2 concentration to 5 μM, more than 20-fold its IC50, did not increase the fibrinogen binding. The specificity of the fibrinogen binding induced by the agents was established by the ability of eptifibatide to block the binding when present during the fibrinogen binding step [for example, in one experiment, the incremental fibrinogen binding values for eptifibatide (34%), tirofiban (17%), the RGDS peptide (36%), and RUC-1 (2%) were all reduced by eptifibatide to 0%; RUC-2 did not produce any incremental binding in this experiment].
Fig. 4.
Effect of RUC-2 on priming platelets to bind fibrinogen. Washed platelets were incubated with eptifibatide (1 μM), tirofiban (0.5 μM), RGDS (100 μM), RUC-1 (100 μM), or RUC-2 (1 μM) for 20 min and fixed with 1% paraformaldehyde for 40 min. After the paraformaldehyde was quenched with glycine (5 mM), platelets were washed and incubated with fluorescent fibrinogen (200 μg/ml) in the presence of 2 mM Ca2+ and 1 mM Mg2+. After washing, the platelets were analyzed by flow cytometry. (A) Mean ± SD (n = 4) net platelet fluorescence (NPF), defined as the percentage of platelets with fluorescence intensity values above 25 arbitrary units (AU) in the presence of one of the antagonists minus the percentage in the absence of the antagonist. Eptifibatide blocked the binding of fibrinogen induced by the antagonists, yielding values equal to or below the control value. (B) Fluorescence data from one of the four similar experiments.
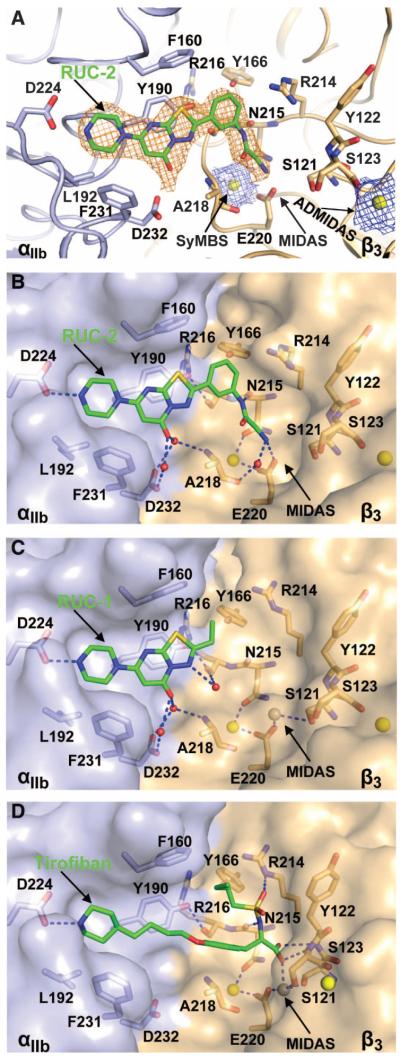
Mechanism of RUC-2 binding to αIIbβ3 as revealed by crystallography
An αIIbβ3 headpiece–Fab complex was crystallized in the closed headpiece conformation (30). We obtained a diffraction data set to 2.6 Å from a crystal soaked with RUC-2 in 1 mM Ca2+ and 5 mM Mg2+ (Table 2). Clear electron densities for RUC-2 and Ca2+ ions at the synergy metal binding site (SyMBS) and the adjacent to MIDAS metal binding site (ADMIDAS) were found at the RGD-binding pocket in each of the two crystallographically independent molecules in the asymmetric unit (Fig. 5A). However, we saw no density for a metal ion at the MIDAS (Fig. 4A). Compared to the native structure (21, 30) or the structure of the RUC-1 complex (30), soaking RUC-2 into the crystal did not induce any significant change in the αIIb or β3 subunits, except for the absence of the Mg2+ ion at the MIDAS (Fig. 5A). To test for competition between RUC-2 and Mg2+, we also obtained 2.2 and 2.4 Å diffraction data sets from crystals soaked with RUC-2 in the presence of 1 mM Ca2+ and 20 mM Mg2+. Clear electron densities of Ca2+ ions at the SyMBS and ADMIDAS, as well as a Mg2+ ion at the MIDAS, were found in both crystals, but no densities of RUC-2 were visible in either of the two independent molecules in the asymmetric unit in the crystals (fig. S6). Instead, the ligand-binding pocket was occupied by water molecules.
Table 2.
Statistics of x-ray diffraction data and structure refinement. RMSD, root mean square deviation.
| Ligand | RUC-2 (1 mM Ca/5 mM Mg) | RUC-2 (1 mM Ca/20 mM Mg) |
|---|---|---|
| Space group | P21212 | P21212 |
| Unit cell | ||
| (a, b, c) (Å) | 261.2, 145.3, 104.7 | 259.5, 145.3, 104.8 |
| (α, β, γ (°) | 90, 90, 90 | 90, 90, 90 |
| Wavelength (Å) | 1.0331 | 1.0332 |
| Resolution (Å) | 50–2.6/2.74–2.60* | 50–2.2/2.32–2.20* |
| Number of reflections (total/unique) | 743,660/118,868 | 1,236,472/199,292* |
| Completeness (%) | 96.7/92.3* | 99.3/96.2* |
| I/σ(I) | 6.2/1.8* | 13.4/1.6* |
| Rmerge(%)† | 17.6/89.7* | 8.1/102.5* |
| Rwork‡/Rfree§ | 0.179/0.222 | 0.189/0.220 |
| RMSD | ||
| Bond (Å) | 0.006 | 0.009 |
| Angle (°) | 0.696 | 0.850 |
| Ramachandran plot∥ | 95.0%/4.7%/0.3% | 95.8%/4.0%/0.2% |
| Molecules per asymmetric unit | 2 | 2 |
| Residues, αIIb/β3 | 1–454 (453)/1–466 (471)¶ | 1–454 (453)/1–466 (471)¶ |
| Non-H atoms, protein/carbohydrate/water | 20,770/195/855 | 20,866/195/1,291 |
| PDB code | 3T3M | 3T3P |
Numbers correspond to the last resolution shell.
Rmerge = ∑h∑i|Ii(h) − ⟨I(h)⟩|/∑h∑i|Ii(h), where Ii(h) and ⟨I(h)⟩ are the ith and mean measurement of the intensity of reflection h.
Rwork = ∑h||Fobs (h)| − |Fcalc (h)||/∑h|Fobs (h)|, where Fobs (h) and Fcalc (h) are the observed and calculated structure factors, respectively. No I/s cutoff was applied.
Rfree is the R value obtained for a test set of reflections consisting of a randomly selected 0.86 or 0.5% subset of data excluded from refinement.
Residues in favorable, allowed, and outlier of the Ramachandran plot as reported by MolProbity.
Numbers in parentheses correspond to chains C and D.
Fig. 5.
The binding pocket of RUC-2 in the closed αIIbβ3 headpiece crystal structure. (A) Electron density maps of RUC-2 and metal ions. αIIb β-propeller (light blue) and β3 βI(wheat) domainsare shownascartoon.Ca2+ ions of the SyMBS and ADMIDAS are shown as yellow spheres. RUC-2 and selected αIIbβ3 side-chain and backbone atoms are shown as sticks with green (RUC-2), light blue (αIIb), or wheat carbon (β3), red oxygen, blue nitrogen, and yellow sulfur atoms. 2Fobs – Fcalc maps at 1.5 σ for RUC-2 and Ca2+ ions are shown in orange and blue, respectively. (B to D) Comparison of RUC-2, RUC-1 (PDB code 3NIF), and tirofiban (PDB code 2VDM) binding modes. Color code is the same as in (A). αIIb and β3 are shown as solvent-accessible surfaces. Ca2+ ions of SyMBS or ADMIDAS (yellow) and the Mg2+ ion of MIDAS (silver) are shown as spheres. Water molecules are small red spheres. Hydrogen and metal coordination bonds are shown as blue dashed lines. The RUC-1 and tirofiban structures are after superposition on the RUC-2 complex using super command in PyMOL with the αIIb β-propeller and β3 βIdomains.
RUC-2 fits into the same binding pocket in the αIIb β-propeller domain as RUC-1, which is lined with residues Phe160, Tyr190, Leu192, Asp224, Phe231, and Asp232 (Fig. 5, B and C). RUC-2 maintains the same interactions as seen with RUC-1, including the hydrogen bonding with αIIb Asp224, the π-π stacking interaction with αIIb Tyr190, and the water-mediated hydrogen bonding with αIIb Asp232 (Fig. 5, B and C). In addition to the interactions with αIIb, RUC-2 makes direct contacts with the β3 βI domain. Its primary amine group forms hydrogen bonds with one of the oxygens of the β3 Glu220 carboxyl side chain and with the carbonyl oxygen of β3 ALA218 through a water molecule (Fig. 5B). In addition, RUC-2′s phenylacetamide nitrogen forms a hydrogen bond with the carbonyl oxygen of β3 Asn215. The phenyl group in RUC-2 that replaces the ethyl group in RUC-1 also increases hydrophobic interactions with the binding pocket. These additional interactions with the β3 subunit can account for the higher affinity of RUC-2 compared with RUC-1.
Like the RGD-mimetic drug tirofiban, RUC-2′s Arg-mimetic piperazinyl group interacts with αIIb Asp224. However, RUC-2 lacks an Asp-mimetic terminal carboxyl group with which to interact with the MIDAS Mg2+ ion and the backbone nitrogen atoms of the MIDAS residues Tyr122 and Ser123 (Fig.5, B and D). Instead, RUC-2 has a terminal primary amine group that interacts directly with the side-chain oxygen of Glu220 that ordinarily contributes to coordinating the MIDAS Mg2+ ion. The absence of the MIDAS Mg2+ metal ion in the presence of RUC-2 thus likely reflects the loss of metal ion coordination by the Glu220 carboxyl oxygen, steric hindrance, and/or electrostatic repulsion (Fig. 5B). When a higher concentration of Mg2+ was used (20 mM) during crystal soaking, we identified the Mg2+ but not RUC-2 (fig. S6), suggesting that RUC-2 competes with Mg2+ for interaction with Glu220.
Molecular docking and MD simulations
A single RUC-1–like docking pose of RUC-2 was identified in the absence of the MIDAS Mg2+ ion, regardless of whether the primary amine was uncharged or positively charged, and this pose remained stable throughout 10 ns of MD simulation (fig. S7). As inferred from the crystal structure, the primary amine group of RUC-2 interacted directly with the carboxyl oxygen of the β3 Glu220 residue regardless of whether the RUC-2 primary amine was uncharged (fig. S7, A and C) or positively charged (fig. S7, B and D). The interaction remained stable in both simulations with slightly different relative average distances (~3.5 Å versus ~2.8 Å, respectively).
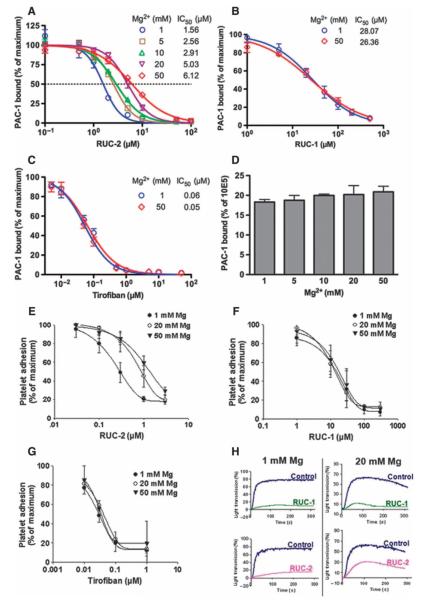
Mg2+ effects on the IC50 of RUC-2, RUC-1, and tirofiban
Both crystallographic and computational data supported a model in which RUC-2 and Mg2+ compete for binding to the same Glu220 carboxyl oxygen. To test this hypothesis, we assessed the binding of the ligand-mimetic mAb PAC-1 to Chinese hamster ovary (CHO) cells expressing recombinant human αIIbβ3 after activation with the mAb PT25-2 at different Mg2+ concentrations(Fig.6, A to D). In going from 1 to 50 mM Mg2+, the RUC-2 IC50 increased by ~3.9-fold, reflecting nearly a 75% reduction in affinity. In contrast, the IC50 of RUC-1 was increased by only ~6% and that of tirofiban was completely unchanged. Thus, as predicted from the structural studies, higher Mg2+ concentrations can decrease RUC-2′s inhibition of ligand binding.
Fig. 6.
Effect of Mg2+ concentration on RUC-2′s ability to inhibit ligand binding to αIIbβ3. (A to C) Inhibition of PAC-1 binding to αIIbβ3 in CHO-K cells in the presence of the activating mAb PT25-2 by RUC-2 (A), RUC-1 (B), or tirofiban (C) at indicated concentrations of Mg2+ plus 1 mM Ca2+. Data are means ± SD of percentage of MFI in the presence versus absence of drug (maximum binding) (n =3).(D) PAC-1 binding to CHO-K cells expressing αIIbβ3 in the presence of PT25-2, normalized for αIIbβ3 expression (mAb 10E5 binding) at indicated concentrations of Mg2+ plus 1 mM Ca2+ in the absence of drug. PAC-1 binding is the mean ± SD of percentage of MFI of PAC-1 binding to the MFI of 10E5 binding (n =3).(E to G) Effect of Mg2+ concentration on platelet adhesion to fibrinogen in the presence of RUC-2 (E), RUC-1 (F), or tirofiban (G). Washed platelets in buffers containing 1 mM Ca2+ plus 1, 20, or 50 mM Mg2+ were added to wells precoated with fibrinogen (50 μg/ml) for 60 min at 22°C. After washing, adherent platelets were detected by acid phosphatase activity. Results are expressed as means ± SD (n = 3 for RUC-2 and RUC-1; n = 2 for tirofiban). (H) Effect of Mg2+ on platelet aggregation induced by ADP in the presence of RUC-2 (1 μM) or RUC-1 (100 μM). Washed platelets were resuspended in buffer containing fibrinogen (200 μg/ml) and 1 mM Ca2+ in combination with either 1 or 20 mM Mg2+. Aggregation was induced by adding a thrombin receptor–activating peptide (SFLLRN) at 10 μM. Results from one of two similar experiments are shown.
To further test the effects of varying the Mg2+ concentration on RUC-2′s ability to inhibit αIIbβ3 ligand binding, we performed the platelet adhesion to fibrinogen assay at different Mg2+ concentrations, keeping the Ca2+ concentration at 1 mM. In three separate experiments, the IC50 for RUC-2 increased from 0.29 ± 0.1 μM (mean ±SD) at 1 mM Mg2+ to 0.91 ± 0.21 μM at 20 mM Mg2+ (P <0.01) and to 1.3 ± 0.35 μM at 50 mM Mg2+ (P < 0.01) (Fig.6, E to G). Thus, in going from 1 to 50 mM Mg2+, there was an ~4.5-fold increase in IC50, corresponding to ~80% decrease in affinity. Neither RUC-1 nor tirofiban showed a comparable increase in IC50 at higher Mg2+ concentrations. Finally, we tested the effect of RUC-2 (1 μM) and RUC-1 (100 μM) on platelet aggregation induced by a thrombin receptor–activating peptide (SFLLRN) at different Mg2+ concentrations and observed that the inhibitory effect of RUC-2 was clearly attenuated at 20 mM Mg2+, whereas the effect on RUC-1 was much less evident (Fig. 6H).
DISCUSSION
We previously demonstrated that a small, αIIb-specific compound (RUC-1) could effectively inhibit ligand binding, platelet aggregation, and in vivo thrombus formation mediated by human αIIbβ3 (28, 29). Docking studies, MD simulations, and crystallographic structural data defined its mode of binding, which was further supported by cross-species and mutagenesis studies (28, 29). Here, we have built on these data by rationally designing, synthesizing, and then analyzing the binding of RUC-2, a RUC-1 derivative that is greater than 100 times more potent in inhibiting platelet aggregation.
RUC-2 is somewhat larger than RUC-1 (molecular weight, 385 versus 265) but still well below the 500 molecular weight cutoff commonly used to assess a compound’s suitability to function as an oral therapeutic. It retains many of RUC-1′s properties, including selectivity for αIIbβ3 compared to αVβ3, and for human αIIbβ3 compared to mouse or rat αIIbβ3. It also shares RUC-1′s decreased ability to induce the β3 LIBS epitopes compared to eptifibatide, suggesting that it induces less extensive conformational changes in the receptor. In accord with this hypothesis and in contrast with the results with both eptifibatide and tirofiban, RUC-2 did not induce αIIbβ3 receptor extension as judged by electron micrographs of αIIbβ3 inserted into nanodiscs, nor did it produce the major shift in elution volume in gel filtration or the major change in DLS produced by these drugs. Studies by several investigators on different RGD-based αIIbβ3 antagonists showed that the antagonists that induce exposure of β3 LIBS epitopes all share a structure that includes a carboxyl capable of coordinating the MIDAS metal ion (14, 16, 37–39). Moreover, unlike eptifibatide and tirofiban, RUC-2 did not “prime” the αIIbβ3 receptor, that is, induce it to adopt a high-affinity fibrinogen binding conformation (16, 20, 36, 37, 40). This is of particular note because the capacity to prime the receptor may explain the lack of efficacy and variable increase in mortality associated with the first generation of oral αIIbβ3 antagonists, which were based on the RGD sequence (11, 12, 14). We used a modification of the original priming assay developed by Du et al. (36), which uses fixation before washing away the agent. We and others have developed variations of this assay, some of which do not involve fixation (16, 28, 37, 40, 41), and we obtained similar results with RUC-1 using one of these assays (28). After evaluating a number of different assays, we found that the assay we used in this study provided the most consistent results. However, none of these assays has been validated with in vivo data.
After ligand binding, integrin receptors are capable of initiating outside-in signaling (7). We did not directly study the effect of RUC-2 on such signaling, but because ligand binding is associated with conformational changes in the β3 subunit (23), RUC-2′s inability to induce such changes may make it less likely to induce outside-in signaling.
To further explore the conformational changes induced in αIIbβ3 by RUC-1 and RUC-2, we analyzed the effect of the compounds on inducing platelet recruitment of IgG from the serum of patients who developed thrombocytopenia when treated with either tirofiban or eptifibatide. In these cases, the drug used to treat the patient induced recruitment of IgG to the platelet. In contrast, neither RUC-1 nor RUC-2 induced recruitment of IgG compared to control in all five patients studied with tirofiban-dependent thrombocytopenia. Similarly, in 13 of 15 patients with eptifibatide-dependent thrombocytopenia, neither RUC-1 nor RUC-2 induced IgG recruitment. In one patient with eptifibatide dependent thrombocytopenia, however, both RUC-1 and RUC-2 enhanced platelet IgG recruitment to a minor degree, and in another, RUC-2, but not RUC-1, induced substantial IgG recruitment. Thus, in most cases, RUC-2 does not expose and/or contribute to the neoepitopes induced and/or created by tirofiban or eptifibatide that are recognized by patient IgG and that are associated with the development of clinical thrombocytopenia. It is possible, however, that RUC-2 can induce platelet recruitment of other individuals’ IgG and that this could lead to thrombocytopenia.
To assess the structural basis for RUC-2′s greater potency than RUC-1, we used both computational and x-ray crystallographic techniques. By soaking RUC-2 into the closed αIIbβ3 headpiece crystal in the presence of 1 mM Ca2+ and 5 mM Mg2+, we obtained good electron density for RUC-2 at the RGD binding pocket of αIIbβ3. The RUC-2 crystal structure showed the same αIIb subunit binding features as did RUC-1. In addition, the primary amine of RUC-2 interacted with the β3 subunit by forming a hydrogen bond to the carboxyl oxygen of Glu220 that ordinarily coordinates the MIDAS Mg2+, and RUC-2′s phenylacetamide nitrogen formed a hydrogen bond to the backbone oxygen of β3 Asn215. These additional interactions and the hydrophobic interactions of the phenyl ring of RUC-2 with the β3 subunit most likely account for its more than 100-fold higher affinity for αIIbβ3 than RUC-1. Remarkably, no electron density for the Mg2+ ion at the MIDAS was visible in the αIIbβ3 headpiece–RUC-2 complex structure.This is in contrast to our previously reported native crystal structures in the presence of 5 mM Mg2+, which showed clear electron density for the Mg2+ ion in the MIDAS (30). To assess whether RUC-2 was competing for binding to the β3 Glu220 MIDAS carboxyl oxygen, we also soaked RUC-2 into the crystal in the presence of 20 mM Mg2+. Both of the two higher-resolution data sets obtained under these conditions showed clear densities for a Mg2+ at the MIDAS, but no densities for RUC-2. These results indicate that RUC-2 and the Mg2+ ion compete with each other for binding to β3. In the absence of ligand, the MIDAS Mg2+ ion is held in place by direct coordination by one of the side-chain oxygens of Glu220 and the side-chain oxygen of Ser121, as well as indirect coordination by Asp119 and Ser123 through water molecules. The other Glu220 carboxyl oxygen directly coordinates the Ca2+ in the SyMBS, and thus, it plays a major role in the structures of both the SyMBS and the MIDAS. The importance of the MIDAS metal ion in ligand binding is well established from mutational studies of cells expressing recombinant αIIbβ3, studies of patients with the hemorrhagic disorder Glanzmann thrombasthenia who harbor naturally occurring mutations (42, 43), and the crystallographic evidence that ligands bind to αIIbβ3 through direct coordination of the MIDAS Mg2+ by a carboxyl oxygen (22, 23). The crystal structure also provides important information on RUC-2′s propensity to induce conformational changes in αIIbβ3. Thus, RUC-2, like RUC-1, does not induce conformational changes in either the αIIb or the β3 subunits in the αIIbβ3 headpiece.
The results of molecular docking and MD simulation studies of RUC-2 performed in the absence of the MIDAS Mg2+ support the stability of the hydrogen bond between RUC-2′s primary amine group and the carboxyl oxygen of Glu220 that ordinarily coordinates the MIDAS Mg2+ ion. This interaction was observed whether the primary amine was uncharged or positively charged, but the interaction was tighter when the primary amine group of RUC-2 was positively charged. The pKa (where Ka is the acid dissociation constant) 1 and pKa 2 values of RUC-2 (corresponding to the primary amine and piperazine nitrogen, respectively) determined directly by titration in water were 6.41 and 8.08, respectively. The value of pKa 1 is significantly lower than that of primary amines in amino acids (~8.8 to 10.8); however, the functional pKa value of the primary amine is likely influenced by the binding pocket microenvironment of αIIbβ3.
A review of the Mg2+ concentration data in the crystal structure studies of αIIbβ3 and αVβ3 demonstrates that the MIDAS was not clearly filled with Mg2+ in the absence of exogenous Mg2+ or in the presence of 1 mM Mg2+, but was filled at concentrations of 5 mM or above (21, 23, 30, 44). We could not identify any studies in which the affinity of Mg2+ for the αIIbβ3 MIDAS was directly determined, but Pesho et al. (45) found that it required millimolar concentrations of Mg2+ to displace Ca2+ from the MIDAS of an isolated β3 βI domain containing a mutation in the ADMIDAS (D126A). Our crystal structure data obtained at 5 and 20 mM Mg2+ suggest that RUC-2 can compete successfully with Mg2+ for binding to Glu220 at 5 mM, but not 20 mM. In support of a competition between Mg2+ and RUC-2 for binding to β3, we found that high concentrations of Mg2+ increased RUC-2′s IC50 for inhibiting both ligand binding to recombinant αIIbβ3 and platelet αIIbβ3-mediated adhesion to fibrinogen, as well as platelet aggregation induced by a thrombin receptor–activating peptide. In contrast, increasing the Mg2+ concentration had little effect on inhibition by tirofiban and/or RUC-1 in the same systems.
RUC-2 may offer advantages over existing intravenous αIIbβ3 antagonists if its reduced capacity to induce conformational changes in the receptor translates into a reduced incidence of thrombocytopenia and a decreased capacity to paradoxically induce ligand binding. If it is rapidly orally bioavailable or can be modified to become such, it may be especially valuable in the prehospital treatment of myocardial infarction because, currently, more than half of the deaths from myocardial infarction occur during the prehospital phase (~300,000 deaths per year in the United States alone) (46), and early treatment with αIIbβ3 antagonists can increase blood flow, decrease mortality, and even abort the progression of myocardial infarction in more than 25% of cases if administered in the first hour (25, 26, 47–49). Definitive testing of the value of RUC-2 or a related derivative of RUC-2 in the prehospital setting could be performed in a randomized study of ambulance administration of RUC-2 versus placebo; indeed, in a study that enrolled just 179 patients with this trial design, abciximab demonstrated statistically significant benefits in infarct size, left ventricle ejection fraction, major adverse coronary events, ST segment elevation resolution, and heart failure (50).
Although RUC-2 is highly specific for human αIIb, preclinical testing can be performed in mice that have targeted deletion of murine αIIb and insertion of human αIIb, as we demonstrated with RUC-1 (29). In addition, preclinical testing of RUC-2′s effect on the ability of human platelets to form thrombi in vivo can be performed in mice that carry an engineered von Willebrand factor that interacts with human GPIb (51). The clinical relevance of this model is supported by the strong correlation between the antithrombotic effects of the currently approved antiplatelet agents in this model and their observed clinical efficacy (52).
In conclusion, we report that RUC-2, a high-affinity derivative of RUC-1, specifically inhibits ligand binding by a novel mechanism, leading to loss of the MIDAS metal ion while producing only minor changes in the conformation of β3. Although there are theoretical reasons to hope that RUC-2 will have therapeutic advantages over existing αIIbβ3 antagonists, this remains to be tested directly.
MATERIALS AND METHODS
Synthesis of RUC-2
The synthesis of RUC-2 (NCGC00183896-01) is shown in fig. S1 and described in detail in the Supplementary Materials.
Purification of integrin αIIβ3
αIIbβ3 was purified from outdated single-donor platelet concentrates obtained from the New York Blood Center as described in detail in the Supplementary Methods. In brief, washed platelets were lysed in n-octyl-β-d-glucoside and αIIbβ3 was purified by sequential concanavalin A, heparin, Q-Sepharose, and Sephacryl S300 HR chromatography.
Preparation of αIIbβ3-containing nanodiscs
αIIbβ3-containing nanodiscs were prepared by a modification of previously described techniques (35, 53, 54). In brief, a His-tagged membrane scaffold protein was prepared as a recombinant protein in Escherichia coli, and final assembly consisted of solubilizing an equimolar mixture of 1, 2-dimyristoyl-sn-glycero-3-phosphocholine and 1, 2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) in octylglucoside and cholate and then adding the purified αIIbβ3.The detergents were removed with macroporous polymeric beads (Bio-Bead SM-2), and then the αIIbβ3 nanodiscs were separated from the empty nanodiscs by gel filtration.
Negative staining EM and evaluation of αIIbβ3 nanodisc particle size
αIIbβ3 nanodiscs were treated with eptifibatide, tirofiban, RUC-1, or RUC-2 for 1 hour at room temperature at the concentrations indicated in the text. Samples were loaded onto carbon-coated copper grids that were glow-discharged and then stained with 2% uranyl acetate, followed by drying. Imaging of αIIbβ3 nanodiscs was performed with a JEOL JEM 100CX transmission electron microscope at 80 kV and magnifications of ×33,000 and ×50,000. The images of αIIbβ3 nanodiscs were taken from randomly chosen fields on each EM grid. Between 100 and 150 αIIbβ3 nanodisc particles were present in each image at a magnification of ×33,000. The lengths of all of the particles in each image were measured with Image J [National Institutes of Health (NIH)].
Platelet function assays
The following assays were all carried out as previously described (28, 30): platelet and HEK293 cell adhesion to fibrinogen, platelet adhesion/aggregation on collagen, platelet aggregation to ADP (5 μM), binding of fluorescent fibrinogen to platelets in the presence of the activating mAb PT25-2, binding of the αIIb-specific (PMI-1) and β3-specific (AP5 and LIBS1) LIBS mAbs to platelets, and HEK293 cell αVβ3-mediated adhesion to vitronectin.
Priming assay
To assess the ability of eptifibatide, tirofiban, RUC-1, and RUC-2 to induce the high-affinity, ligand-binding state of the αIIbβ3 receptor, we used a modified version of the assay developed by Du et al. (36). Platelets washed in Hepes-modified Tyrode’s buffer were incubated with the compounds for 20 min at room temperature, fixed with 1% paraformaldehyde for 40 min at room temperature, incubated with 5 mM glycine for 5 min at room temperature, washed four times, resuspended in buffer containing 2 mM Ca2+ and 1 mM Mg2+, incubated with Alexa Fluor 488–conjugated fibrinogen (200 μg/ml; Invitrogen) (with or without 10 μM eptifibatide) for 30 min at 37°C, washed, diluted 10-fold, and analyzed by flow cytometry. The net fluorescence was calculated by determining the percentage of platelets with fluorescence values greater than 25 arbitrary units and subtracting the percentage in the untreated samples. In the four experiments, the mean ± SD values in the untreated samples were 9 ± 3%.
Protein expression, purification, and crystallography
The expression, purification, and crystallization of the αIIbβ3 headpiece (αIIb β-propeller, thigh, and calf-1 domains and β3 βI, hybrid, PSI, and IEGF-1 domains) in complex with 10E5 Fab were performed as previously described (30). RUC-2 was soaked into the αIIbβ3/Fab crystals at 37.5 μM in the crystallization well solution containing 1 mM Ca2+ and 5 mM (or 20 mM) Mg2+ for 3 to 5 days. Crystals were harvested in 15% PEG 8000 (polyethylene glycol, molecular weight 8000), 0.2 M ammonium sulfate, 0.1 M tris-HCl (pH 8.9) plus 1 mM Ca2+ and 5 mM (or 20 mM) Mg2+; cryoprotected with additional glycerol in 5% increments up to a 20% final concentration; and then flash-frozen in liquid nitrogen. Diffraction data collected at ID-23 of APS were solved by molecular replacement. Final refinement with Phenix used translation-libration-screw and noncrystallographic symmetry analyses.
Gel filtration and DLS
The purified αIIbβ3 headpiece at 2.0 μM was incubated with RUC-1, RUC-2, or tirofiban at 500, 100, and 56 μM, respectively, at 25°C for 1 hour and subjected to Superdex 200 chromatography in tris-buffered saline plus 1 mM Ca2+/Mg2+. DLS of the purified αIIbβ3 headpiece alone at 20 μM or after mixing with RUC-1, RUC-2, or tirofiban at 500, 100, and 56 μM, respectively, was measured at 25°C with a Viscotek 802 DLS (Viscotek Corp.) in tris-buffered saline plus 1 mM Ca2+/Mg2+.
Molecular docking
The crystal structure of the αIIbβ3 headpiece cocrystallized with the inhibitor RUC-1 (28–30) was used for the docking of RUC-2 [Protein Data Bank (PDB) code 3NIF]. After RUC-1 was removed from the structure, RUC-2 docking was performed in the absence of the MIDAS Mg2+ ion. The SyMBS and ADMIDAS Ca2+ ions were retained, as were the crystallographic water molecules around the ions and the two water molecules close to Asp232. In the latter case, only the MIDAS Mg2+ was removed. Further details are provided in the Supplementary Materials.
MD simulations
The MD simulations of the αIIbβ3 complex with RUC-2 bound in a similar fashion to RUC-1 were carried out on truncated forms of the protein system (that is, αIIb residues 1 to 452 and β3 residues 108 to 352) with the Amber10.0 suite of programs. Further details are provided in the Supplementary Materials.
Detection of antibodies from patients with tirofiban- or eptifibatide-induced thrombocytopenia
To assess whether RUC-1 or RUC-2 induces conformational changes in αIIbβ3 similar to those produced by eptifibatide and tirofiban that result in recruitment of IgG to the platelet surface in patients who develop thrombocytopenia after treatment with one or the other drug, we used the assay described previously (9). Further details are provided in the Supplementary Materials.
Effect of Mg2+ concentration on RUC-2′s ability to inhibit ligand binding to αIIbβ3
CHO-K cells stably expressing human αIIbβ3 were incubated with or without drugs at the indicated Mg2+ concentrations plus 1 mM Ca2+ for 30 min, and then incubated with mAb PAC-1 (5 μg/ml) (ligand-mimetic IgM, selective for the activated conformation of αIIbβ3) plus activating mAb PT25-2 (5 μg/ml) for another 30 min at 25°C. Cells were washed and incubated with phycoerythrin-labeled goat antimouse IgM on ice for 30 min and analyzed by flow cytometry after washing. The expression of αIIbβ3 was detected with mAb 10E5 and Alexa Fluor 488–labeled goat anti-mouse IgG. PAC-1 binding is presented as mean ± SD of percentage of MFI of PAC-1 binding to the MFI of 10E5 binding.
Titration analysis of RUC-2 in water was performed by Analiza Inc. by retention time analysis using parallel capillary electrophoresis separation.
Supplementary Material
Acknowledgments
We thank S. Rivera for administrative assistance and T. Song and M. Suarez-Farinas for biostatistical support.
Funding: Supported, in part, by grants HL19278, HL13629, and HL48675 from the National Heart, Lung, and Blood Institute; Clinical and Translational Science Award grant ULRR024143 from the National Center for Research Resources, NIH; the Molecular Libraries Initiative of the NIH Roadmap for Medical Research and the Intramural Research Program of the National Human Genome Research Institute; funds from Stony Brook University; and the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-352-E00042). The computations were supported in part by the NSF through TeraGrid advanced computing resources provided by Texas Advanced Computing Center under grant TG-MCB080109N (principal investigator: M.F.).
Footnotes
SUPPLEMENTARY MATERIALS www.sciencetranslationalmedicine.org/cgi/content/full/4/125/125ra32/DC1 Materials and Methods
Author contributions: Jieqing Zhu designed, performed, and interpreted the crystallography, Stokes radius, and RUC-2–Mg2+ competition studies; W.-S.C. designed, performed, and interpreted the nanodisc studies; J.G.M. designed and performed the syntheses of RUC-1 and RUC-2 along with M.S. and W.H.; A.N. designed, performed, and analyzed the MD simulations and docking studies; Jianghai Zhu collected the x-ray diffraction data and refined and interpreted the crystal structures; S.N. and J.L. performed and interpreted the platelet function studies; D.B. designed, conducted, and interpreted the platelet antibody recruitment studies along with M.R.; R.A. designed, oversaw, and interpreted the platelet antibody recruitment studies; C.J.T. designed, oversaw, and interpreted the synthesis studies; M.F. designed, oversaw, and interpreted the MD simulations and docking studies; T.A.S. designed, oversaw, and interpreted the crystallography and Stokes radius studies; and B.S.C. designed, oversaw, and interpreted the platelet function studies. B.S.C. had primary responsibility for writing the manuscript with contributions from W.-S.C., J.G.M., A.N., J.Z., D.B., R.A., C.J.T., M.F., and T.A.S.
Competing interests: B.S.C. is an inventor of abciximab (Centocor) and, in accord with Federal law and the policies of the Research Foundation of the State University of New York, receives royalties based on the sales of abciximab. He is also an inventor of the Verify Now assays (Accumetrics) and, in accord with Federal law and the policies of the Mount Sinai School of Medicine, receives royalties based on the sales of the VerifyNow assays. Rockefeller University has applied for patents on RUC-1 and RUC-2.
Data and materials availability: The structural data have been deposited at the Protein Database (PDB code 3T3M). RUC-2 is available from the authors (B.S.C. and C.J.T.).
REFERENCES AND NOTES
- 1.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Zou W, Teitelbaum SL. Integrins, growth factors, and the osteoclast cytoskeleton. Ann. N. Y. Acad. Sci. 2010;1192:27–31. doi: 10.1111/j.1749-6632.2009.05245.x. [DOI] [PubMed] [Google Scholar]
- 5.Caswell PT, Vadrevu S, Norman JC. Integrins: Masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Lu D, Scully M, Kakkar V. The role of integrins in cancer and the development of anti-integrin therapeutic agents for cancer therapy. Perspect. Medicin. Chem. 2008;2:57–73. [PMC free article] [PubMed] [Google Scholar]
- 7.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin αIIbβ3) odyssey: A technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch X, Marrugat J, Sanchis J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. Cochrane Database Syst. Rev. 2010;9:CD002130. doi: 10.1002/14651858.CD002130.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Bougie DW, Wilker PR, Wuitschick ED, Curtis BR, Malik M, Levine S, Lind RN, Pereira J, Aster RH. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100:2071–2076. [PubMed] [Google Scholar]
- 10.Gao C, Boylan B, Bougie D, Gill JC, Birenbaum J, Newman DK, Aster RH, Newman PJ. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcγRIIa and the integrin β3 cytoplasmic domain. J. Clin. Invest. 2009;119:504–511. doi: 10.1172/JCI36745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chew DP, Bhatt DL, Topol EJ. Oral glycoprotein IIb/IIIa inhibitors: Why don’t they work? Am. J. Cardiovasc. Drugs. 2001;1:421–428. doi: 10.2165/00129784-200101060-00002. [DOI] [PubMed] [Google Scholar]
- 12.Cox D. Oral GPIIb/IIIa antagonists: What went wrong? Curr. Pharm. Des. 2004;10:1587–1596. doi: 10.2174/1381612043384673. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Cannon CP, Cooper R, Aster RH, Brassard J, McCabe CH, Charlesworth A, Skene AM, Braunwald E. Drug-induced thrombocytopenia and thrombosis: Evidence from patients receiving an oral glycoprotein IIb/IIIa inhibitor in the Orbofiban in Patients with Unstable coronary Syndromes- (OPUS-TIMI 16) trial. J. Thromb. Thrombolysis. 2006;22:95–102. doi: 10.1007/s11239-006-8669-4. [DOI] [PubMed] [Google Scholar]
- 14.Jennings LK, Haga JH, Slack SM. Differential expression of a ligand induced binding site (LIBS) by GPIIb-IIIa ligand recognition peptides and parenteral antagonists. Thromb. Haemost. 2000;84:1095–1102. [PubMed] [Google Scholar]
- 15.Kouns WC, Kirchhofer D, Hadváry P, Edenhofer A, Weller T, Pfenninger G, Baumgartner HR, Jennings LK, Steiner B. Reversible conformational changes induced in glycoprotein IIb-IIIa by a potent and selective peptidomimetic inhibitor. Blood. 1992;80:2539–2547. [PubMed] [Google Scholar]
- 16.Peter K, Schwarz M, Ylänne J, Kohler B, Moser M, Nordt T, Salbach P, Kübler W, Bode C. Induction of fibrinogen binding and platelet aggregation as a potential intrinsic property of various glycoprotein IIb/IIIa (αIIbβ3) inhibitors. Blood. 1998;92:3240–3249. [PubMed] [Google Scholar]
- 17.Epelman S, Nair D, Downey R, Militello M, Askari AT. Eptifibatide-induced thrombocytopenia and thrombosis. J. Thromb. Thrombolysis. 2006;22:151–154. doi: 10.1007/s11239-006-8785-1. [DOI] [PubMed] [Google Scholar]
- 18.Chew DP, Bhatt DL, Sapp S, Topol EJ. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: A meta-analysis of phase III multicenter randomized trials. Circulation. 2001;103:201–206. doi: 10.1161/01.cir.103.2.201. [DOI] [PubMed] [Google Scholar]
- 19.Seiffert D, Stern AM, Ebling W, Rossi RJ, Barrett YC, Wynn R, Hollis GF, He B, Kieras CJ, Pedicord DL, Cromley DA, Hua TA, Stein RB, Daly RN, Sferruzza A, Pieniaszek HJ, Billheimer JT. Prospective testing for drug-dependent antibodies reduces the incidence of thrombocytopenia observed with the small molecule glycoprotein IIb/IIIa antagonist roxifiban: Implications for the etiology of thrombocytopenia. Blood. 2003;101:58–63. doi: 10.1182/blood-2002-02-0471. [DOI] [PubMed] [Google Scholar]
- 20.Bassler N, Loeffler C, Mangin P, Yuan Y, Schwarz M, Hagemeyer CE, Eisenhardt SU, Ahrens I, Bode C, Jackson SP, Peter K. A mechanistic model for paradoxical platelet activation by ligand-mimetic αIIbβ3 (GPIIb/IIIa) antagonists. Arterioscler. Thromb. Vasc. Biol. 2007;27:e9–e15. doi: 10.1161/01.ATV.0000255307.65939.59. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer TA, Zhu J, Xiao T. Structural basis for distinctive recognition of fibrinogen γC peptide by the platelet integrin αIIbβ3. J. Cell Biol. 2008;182:791–800. doi: 10.1083/jcb.200801146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi J, Strokovich K, Springer TA, Walz T. Structure of integrin α5β1 in complex with fibronectin. EMBO J. 2003;22:4607–4615. doi: 10.1093/emboj/cdg445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan AK, Jukema JW, van der Laarse A, Hasan-Ali H, Wolterbeek R, van der Kley F, Spano F, Atsma DE, Schalij MJ. Incidence, patient characteristics and predictors of aborted myocardial infarction in patients undergoing primary PCI: Prospective study comparing pre- and in-hospital abciximab pretreatment. EuroIntervention. 2009;4:662–668. doi: 10.4244/eijv4i5a110. [DOI] [PubMed] [Google Scholar]
- 26.De Luca G, Gibson CM, Bellandi F, Murphy S, Maioli M, Noc M, Zeymer U, Dudek D, Arntz HR, Zorman S, Gabriel HM, Emre A, Cutlip D, Biondi-Zoccai G, Rakowski T, Gyongyosi M, Marino P, Huber K, van’t Hof AW. Early glycoprotein IIb–IIIa inhibitors in primary angioplasty (EGYPT) cooperation: An individual patient data meta-analysis. Heart. 2008;94:1548–1558. doi: 10.1136/hrt.2008.141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, Boulenc JM, Morice MC, Maillard L, Pansiéri M, Choussat R, Pinton P, ADMIRAL Investigators Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-up, Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N. Engl. J. Med. 2001;344:1895–1903. doi: 10.1056/NEJM200106213442503. [DOI] [PubMed] [Google Scholar]
- 28.Blue R, Murcia M, Karan C, Jirousková M, Coller BS. Application of high-throughput screening to identify a novel αIIb-specific small-molecule inhibitor of αIIbβ3-mediated platelet interaction with fibrinogen. Blood. 2008;111:1248–1256. doi: 10.1182/blood-2007-08-105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blue R, Kowalska MA, Hirsch J, Murcia M, Janczak CA, Harrington A, Jirouskova M, Li J, Fuentes R, Thornton MA, Filizola M, Poncz M, Coller BS. Structural and therapeutic insights from the species specificity and in vivo antithrombotic activity of a novel αIIb-specific αIIbβ3antagonist. Blood. 2009;114:195–201. doi: 10.1182/blood-2008-08-169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Zhu J, Negri A, Provasi D, Filizola M, Coller BS, Springer TA. Closed headpiece of integrin αIIbβ3 and its complex with an αIIbβ3-specific antagonist that does not induce opening. Blood. 2010;116:5050–5059. doi: 10.1182/blood-2010-04-281154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coller BS, Beer JH, Scudder LE, Steinberg MH. Collagen-platelet interactions: Evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989;74:182–192. [PubMed] [Google Scholar]
- 32.Honda S, Tomiyama Y, Pelletier AJ, Annis D, Honda Y, Orchekowski R, Ruggeri Z, Kunicki TJ. Topography of ligand-induced binding sites, including a novel cation-sensitive epitope (AP5) at the amino terminus, of the human integrin β3 subunit. J. Biol. Chem. 1995;270:11947–11954. doi: 10.1074/jbc.270.20.11947. [DOI] [PubMed] [Google Scholar]
- 33.Frelinger AL, III, Cohen I, Plow EF, Smith MA, Roberts J, Lam SC, Ginsberg MH. Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J. Biol. Chem. 1990;265:6346–6352. [PubMed] [Google Scholar]
- 34.Shadle PJ, Ginsberg MH, Plow EF, Barondes SH. Platelet-collagen adhesion: Inhibition by a monoclonal antibody that binds glycoprotein IIb. J. Cell Biol. 1984;99:2056–2060. doi: 10.1083/jcb.99.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, McLean MA, Sligar SG, Taylor KA, Ginsberg MH. Recreation of the terminal events in physiological integrin activation. J. Cell Biol. 2010;188:157–173. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du XP, Plow EF, Frelinger AL, III, O’Toole TE, Loftus JC, Ginsberg MH. Ligands “activate” integrin αIIbβ3 (platelet GPIIb-IIIa) Cell. 1991;65:409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- 37.Hantgan RR, Stahle MC. Integrin priming dynamics: Mechanisms of integrin antagonist promoted αIIbβ3:PAC-1 molecular recognition. Biochemistry. 2009;48:8355–8365. doi: 10.1021/bi900475k. [DOI] [PubMed] [Google Scholar]
- 38.Hantgan RR, Stahle MC, Connor JH, Connor RF, Mousa SA. αIIbβ3 priming and clustering by orally active and intravenous integrin antagonists. J. Thromb. Haemost. 2007;5:542–550. doi: 10.1111/j.1538-7836.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 39.Honda S, Tomiyama Y, Aoki T, Shiraga M, Kurata Y, Seki J, Matsuzawa Y. Association between ligand-induced conformational changes of integrin αIIbβ3 and αIIbβ3-mediated intracellular Ca2+ signaling. Blood. 1998;92:3675–3683. [PubMed] [Google Scholar]
- 40.Hantgan RR, Stahle MC, Lord ST. Dynamic regulation of fibrinogen: Integrin αIIbβ3 binding. Biochemistry. 2010;49:9217–9225. doi: 10.1021/bi1009858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frelinger AL, III, Furman MI, Krueger LA, Barnard MR, Michelson AD. Dissociation of glycoprotein IIb/IIIa antagonists from platelets does not result in fibrinogen binding or platelet aggregation. Circulation. 2001;104:1374–1379. doi: 10.1161/hc3701.095950. [DOI] [PubMed] [Google Scholar]
- 42.Baker EK, Tozer EC, Pfaff M, Shattil SJ, Loftus JC, Ginsberg MH. A genetic analysis of integrin function: Glanzmann thrombasthenia in vitro. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1973–1978. doi: 10.1073/pnas.94.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loftus JC, O’Toole TE, Plow EF, Glass A, Frelinger AL, III, Ginsberg MH. A β3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990;249:915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- 44.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin αVβ3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pesho MM, Bledzka K, Michalec L, Cierniewski CS, Plow EF. The specificity and function of the metal-binding sites in the integrin β3 A-domain. J. Biol. Chem. 2006;281:23034–23041. doi: 10.1074/jbc.M602856200. [DOI] [PubMed] [Google Scholar]
- 46.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold HK, Garabedian HD, Dinsmore RE, Guerrero LJ, Cigarroa JE, Palacios IF, Leinbach RC. Restoration of coronary flow in myocardial infarction by intravenous chimeric 7E3 antibody without exogenous plasminogen activators. Observations in animals and humans. Circulation. 1997;95:1755–1759. doi: 10.1161/01.cir.95.7.1755. [DOI] [PubMed] [Google Scholar]
- 48.Siudak Z, Rakowski T, Dziewierz A, Janzon M, Birkemeyer R, Stefaniak J, Partyka Ł, Zmudka K, Dudek D. Early abciximab use in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention improves long-term outcome. Data from EUROTRANSFER Registry. Kardiol. Pol. 2010;68:539–543. [PubMed] [Google Scholar]
- 49.De Luca G. Glycoprotein IIb-IIIa inhibitors. Cardiovasc. Ther. 2011 doi: 10.1111/j.1755-5922.2011.00293.x. 10.1111/j.1755-5922.2011.00293.x. [DOI] [PubMed] [Google Scholar]
- 50.Hassan AK, Liem SS, van der Kley F, Bergheanu SC, Wolterbeek R, Bosch J, Bootsma M, Zeppenfeld K, van der Laarse A, Atsma DE, Jukema JW, Schalij MJ. In-ambulance abciximab administration in STEMI patients prior to primary PCI is associated with smaller infarct size, improved LV function and lower incidence of heart failure: Results from the Leiden MISSION! acute myocardial infarction treatment optimization program. Catheter Cardiovasc. Interv. 2009;74:335–343. doi: 10.1002/ccd.21980. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Tan K, Zhou H, Lo HF, Roux DT, Liddington RC, Diacovo TG. Modifying murine von Willebrand factor A1 domain for in vivo assessment of human platelet therapies. Nat. Biotechnol. 2008;26:114–119. doi: 10.1038/nbt1373. [DOI] [PubMed] [Google Scholar]
- 52.Magallon J, Chen J, Rabbani L, Dangas G, Yang J, Bussel J, Diacovo T. Humanized mouse model of thrombosis is predictive of the clinical efficacy of antiplatelet agents. Circulation. 2011;123:319–326. doi: 10.1161/CIRCULATIONAHA.110.951970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. Chapter 11—Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009;464:211–231. doi: 10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayburt TH, Sligar SG. Membrane protein assembly into nanodiscs. FEBS Lett. 2010;584:1721–1727. doi: 10.1016/j.febslet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.