Abstract
Lipid biosynthesis is essential for the maintenance of cellular homeostasis. The lipids produced by cells (glycerolipids, fatty acids, phospholipids, cholesterol, spingolipids) are used as an energy source/reserve, as building blocks for membrane biosynthesis, as precursor molecules for the synthesis of various cellular products and as signaling molecules. Defects in lipid synthesis or processing contribute to the development of many diseases, including obesity, insulin resistance, type 2 diabetes, non-alcoholic fatty liver disease, and cancer. Studies published over the last few years have shown that the target of rapamycin (TOR), a conserved serine/threonine kinase playing key roles in regulating cell growth, controls lipid biosynthesis through various mechanisms. Here, we review these findings and briefly discuss their potential relevance for human health and disease.
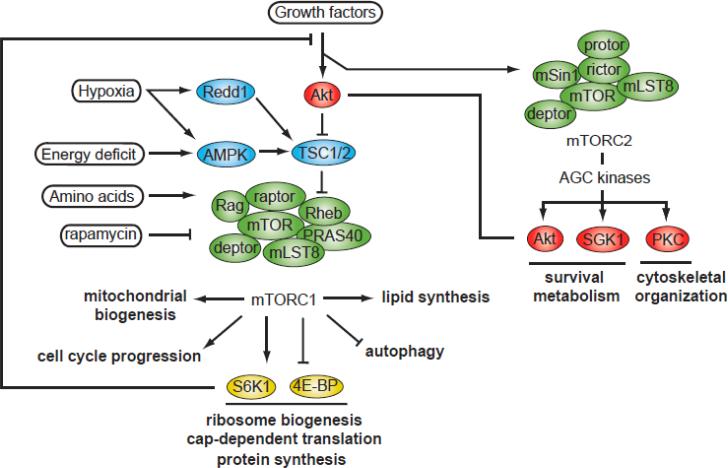
The mammalian TOR (mTOR) protein is a serine-threonine kinase belonging to the phosphoinositide 3-kinase (PI3K)-related kinase family that plays key roles in regulating cell growth and metabolism. As depicted in Figure 1, mTOR nucleates at least two distinct multi-protein complexes, mTOR complex 1 (mTORC1) and complex 2 (mTORC2) (reviewed in [1]). mTORC1 integrates four major signals – growth factors, energy status, oxygen, and amino acids to regulate many processes involved in the promotion of cell growth and metabolism. mTORC2, that is activated by growth factors, regulates cell survival, cell metabolism, and cytoskeletal organization (reviewed in [2]).
Figure 1. Overview of the mTOR signaling pathway.
The mTOR kinase nucleates two characterized protein complexes termed mTORC1 and mTORC2. mTORC1 is composed of 5 subunits : (i) the mTOR catalytic subunit, (ii) Raptor, (iii) mLST8, (iv) PRAS40, and (v) Deptor. The mTORC2 complex contains 6 proteins (i) mTOR, (ii) Rictor, (iii) mSin1, (iv) Protor-1, (v) mLST8, and (vi) Deptor. mTORC1 is sensitive to nutrients and is inhibited by FKBP12-rapamycin. One of the most important sensors involved in the regulation of mTORC1 activity is TSC, which is a heterodimer composed of TSC1 and TSC2. TSC1/2 functions as a GTPase GAP for the small, Ras-related GTPase, Rheb. The active, GTP-bound form of Rheb directly interacts with mTORC1 to stimulate its activity. As a Rheb-specific GAP, TSC1/2 negatively regulates mTORC1 signaling by converting Rheb into its inactive GDP-bound state. Stimulation of cells with growth factors activates Akt, Erk, and Rsk, which inactivates TSC and promotes Rheb activation. Akt activation also promotes mTORC1 activity by phosphorylating PRAS40, a negative regulator of mTORC1. At the opposite, induction of Redd1 and AMPK by hypoxia and energy deficit activates TSC1/2 and turn down mTORC1 signaling. The activation of mTORC1 by amino acids is regulated by the Rag GTPase and is independent of TSC1/2. Activation of mTORC1 by growth factors or amino acids promotes protein synthesis via the phosphorylation of S6K1 and 4EBPs. High activation of S6K1 by mTORC1 induces a feedback inhibition loop in which S6K1 reduces growth factor signaling by promoting the phosphorylation and the degradation of IRS-1. mTORC2 is activated by growth factors and regulates many AGC kinases (Akt, SGK1, PKC-α) playing key roles in controlling cell survival, metabolism and cytoskeletal organization. This complex is insensitive to FKBP12-rapamycin but is inhibited by the newly characterized mTOR inhibitor Torin1. Akt, protein kinase B; AMPK, AMP-activated protein kinase; Deptor, DEP domain containing protein associated with mTOR; Erk, extracellular signal-regulated kinase; FKBP12, intracellular receptor for rapamycin; GAP, GTPase activating protein; IRS-1, insulin receptor substrate-1; mLST8, mammalian lethal with Sec13 protein 8; mSIN1, mammalian stress-activated protein kinase interacting protein PKC-α, protein kinase C-α; PRAS40, proline-rich Akt substrate 40kDa; Protor1, protein observed with rictor-1; Raptor, regulatory-associated protein of mTOR; Redd1, transcriptional regulation of DNA damage response-1; Rheb, Ras homolog enriched in brain; Rictor, rapamycin insensitive companion of mTOR; Rsk, ribosomal S6 kinase; SGK1, serum- and glucocorticoid-induced protein kinase 1; S6K1, p70 ribosomal S6 kinase 1; TSC1/2, tuberous sclerosis complex 1 and 2; 4EBPs, eukaryotic initiation factor 4E-binding proteins.
From yeast to mammals, favorable conditions such as nutrient and oxygen availability induce cell growth by activating the TOR pathway. For many years, regulation of cell growth by mTOR has been essentially studied in relation to protein synthesis, the best-characterized biological output controlled by this pathway. However, it is becoming increasingly clear that mTOR also controls cell growth by promoting the activation of other anabolic processes leading to the synthesis of many classes of lipids (unsaturated and saturated fatty acids, phosphatidylcholine, phosphatidylglycerol, and sphingolipids) required for membrane biosynthesis and energy storage. Here, we review these findings and discuss their potential relevance for the treatment of human diseases including cancer, obesity, and non-alcoholic fatty liver disease (NAFLD).
mTORC1 controls lipid synthesis through various effectors
SREBP-1
When total energy intake exceeds energy expenses, excess carbohydrates are converted to fatty acids and deposited for storage as triglycerides in hepatic and adipose tissues. This process, defined as de novo lipogenesis, is strongly driven by insulin (reviewed in [3]). The binding of insulin to its cell surface receptor activates PI3K, promotes the production of phosphatidylinositol (3,4,5)-triphosphate (PIP3), and increases the recruitment and the activation of Akt at the plasma membrane (reviewed in [4]). When activated, Akt positively regulates de novo lipogenesis by promoting glucose uptake, glycolysis, and the expression of genes playing key roles in lipid biosynthesis (reviewed in [5]). Many lines of evidence suggest that Akt mediates the effect of insulin on de novo lipogenesis by activating a transcription factor named sterol regulatory element-binding protein-1 (SREBP-1) (reviewed in [6]).
SREBPs are basic helix-loop-helix transcription factors that regulate lipid homeostasis by controlling the expression of genes required for cholesterol, fatty acids, triglycerides, and phospholipids synthesis. Three members of the SREBP family have been described in mammals, SREBP-1a and -1c, which are produced by alternative splicing, and SREBP-2 that is encode by another gene. SREBP-1c and -2 are considered the most physiologically relevant because of their high expression in lipogenic tissues (reviewed in [7]). The SREBPs are synthesized in the endoplasmic reticulum (ER) in the form of a precursor protein. In order to reach the nucleus and act as a transcription factor, the NH2-terminal domain of each SREBP must be released from the ER proteolytically. SREBP-1 and -2 are cleaved and activated in response to different signals. SREBP-2 is activated in response to cellular sterol depletion whereas SREBP-1c, that is insensitive to sterol levels, is activated by insulin (reviewed in [7]). Accordingly, studies using genetically engineered mouse models show that SREBP-2 mainly controls cholesterol biosynthesis whereas SREBP-1c is involved in insulin-mediated fatty acid synthesis (reviewed in [6]).
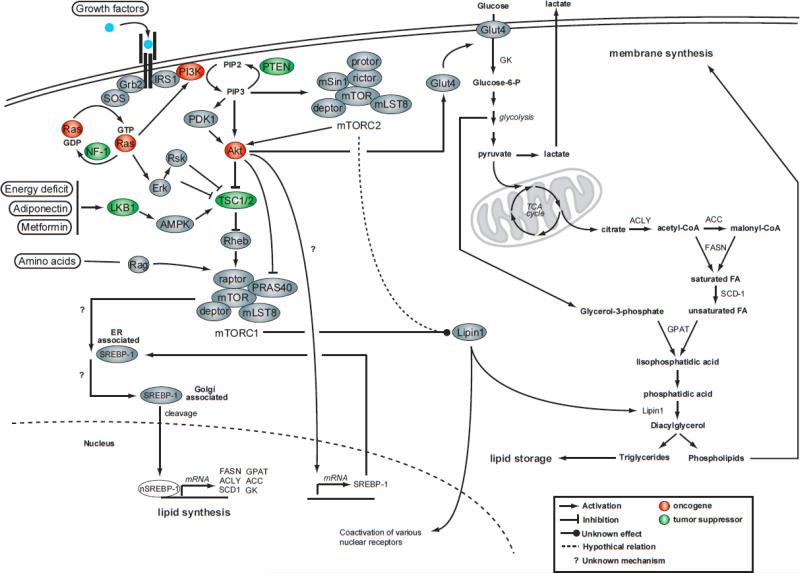
Insulin treatment or constitutive Akt activation rapidly induces nuclear accumulation of SREBP-1 and the expression of lipogenic genes [8, 9]. The precise mechanism by which insulin signaling promotes SREBP-1 cleavage has remained evasive for many years. As shown in Figure 1 and 2, growth factors such as insulin increase Akt activity, which in turns promotes mTORC1 activation by directly phosphorylating the tuberous sclerosis complex 1/2 (TSC1/2) and PRAS40. Porstmann et al. observed that impairing mTORC1 activity with rapamycin blocks Akt-induced SREBP-1 nuclear localization, the expression of lipogenic genes, and the production of various classes of lipids (unsaturated and saturated fatty acids, phosphatidylcholine, and phosphatidylglycerol) [10]. The knock down of raptor, but not rictor, showed similar effects, indicating that SREBP-1 activation by Akt depends on mTORC1 but not mTORC2. This supports previous work that showed that rapamycin reduces the expression of many SREBP-1 target genes including acetyl-CoA carboxylase (ACC) [11], fatty acid synthase (FASN) [12], and stearoyl-CoA desaturase 1 (SCD-1) [13]. In addition to establishing a link between mTORC1 and SREBP-1, Porstmann et al. nicely demonstrated that SREBP-1 silencing restricts mammalian cell growth and fly size, suggesting that the PI3K/Akt/TORC1 pathway regulates cell size by promoting both protein and lipid biosynthesis [10]. The exact mechanism by which mTORC1 promotes SREBP-1 cleavage and nuclear localization remains to be determined.
Figure 2. mTORC1 promotes de novo lipogenesis through the activation of SREBP-1.
The activation of mTORC1 in response to growth factors induces many anabolic processes that favors cell growth and proliferation. In addition of promoting protein synthesis through the phosphorylation of S6K1 and 4EBPs, mTORC1 controls de novo lipid synthesis by regulating the activation state of SREBP-1. In details, growth factors activate Akt, Erk and Rsk, which induce TSC1/2 phosphorylation and lead to mTORC1 activation. Akt also directly promotes mTORC1 action through the phosphorylation of PRAS40. When activated, mTORC1 favors the cleavage of SREBP-1 by a mechanism that remains to be established. The cleaved form of SREBP-1 then translocates to the nucleus where it induces the expression of many lipogenic genes including ACC, ACLY, FASN, GPAT, GK and SCD1. Mutations in tumor suppressors or oncogenes that lead to the overactivation of Ras and insulin signaling pathways are commonly observed in cancer and are thought to promote cell growth and proliferation by inducing many anabolic processes including lipid biosynthesis. The induction of lipogenesis through the activation of SREBP-1 promotes cancer progression by providing the lipids required for membrane synthesis. The fact that mTORC1 promotes SREBP-1 activation indicates that this protein complex may play a central role in cancer cell growth/proliferation by relaying growth factor signals to lipid synthesis. ACC, acetyl-CoA carbolxylase; ACLY, acyl-CoA lyase; FASN, fatty acid synthase; ER, endoplasmic reticulum; SCD-1, stearoyl-CoA desaturase-1; GPAT, glycerol-3-phosphate acyltransferase; GK, glucokinase; GLUT4, glucose transporter 4; Grb2, growth factor receptor bound protein 2; NF-1, neurofibromatosis type 1; PDK1, phosphoinositide-dependent kinase 1; PTEN, phosphatase and tensin homologue deleted on chromosome 10; SOS, son of sevenless; nSREBP-1, nuclear form of sterol regulatory element-binding protein-1; TCA cycle, tricarboxilic acid cycle.
Increased de novo lipid synthesis is a hallmark of proliferating cancer cells (reviewed in [14]). Uncontrolled growth factor signaling caused by the mutation/amplification of genes coding for proteins involved in PI3K and Ras signaling pathways is commonly observed in tumors and serves as a key signaling event leading to the activation of SREBP-1 and de novo lipogenesis (Figure 2). A significant body of evidences suggests that the induction of lipid biosynthesis through the activation of SREBP-1 facilitates cancer progression by providing the lipids required for membrane synthesis (reviewed in [14]). The observation that mTORC1 promotes SREBP-1 activation indicates that this protein complex may play a central role in cancer cell growth/proliferation by relaying growth factor signals to lipid synthesis (Figure 2). These results strongly suggest that mTORC1 inhibition with rapamycin, or with the newly characterized mTOR inhibitors [15-17], may reduce cancer cell growth/proliferation by blocking both protein and lipid biosynthesis. Further investigations are needed to support this hypothesis.
Non-alcoholic fatty liver disease (NAFLD), a condition produced by fat accumulation in the liver, is the most common liver disease and can eventually lead to non-alcoholic steatohepatitis, cirrhosis and hepatocellular carcinomas (reviewd in [18]). NAFLD is commonly observed in obese subjects and is closely link to insulin resistance and the metabolic syndrome. A recent study performed in obese humans pointed out an important role of de novo lipogenesis in the excessive accumulation of hepatic triglycerides [19]. Although highly dependent on insulin for its activation, lipogenesis is paradoxically very active in the liver of obese rodents, which are characterized by severe hepatic insulin resistance. Interestingly, mTORC1 is also highly active in the liver of obese rodents [20]. In addition to reducing insulin signaling by promoting insulin receptor substrate-1 (IRS-1) phosphorylation/degradation (see Figure 1), mTORC1 hyperactivation by overfeeding may promote lipogenesis by inducing SREBP-1c cleavage and activation. The nutrient-dependent activation mTORC1 may explain why hepatic SREBP-1c is highly activated even in the context of profound liver insulin resistance. Additionally, it is interesting to note that metformin, an antidiabetic drug known to activate AMP-activated protein kinase (AMPK), reduces hepatic lipid content by promoting fatty acid oxidation and by impairing SREBP-1c expression and cleavage [21]. Because AMPK negatively regulates mTORC1 (Figures 1 and 2), this suggests that AMPK activators (metformin, adiponectin) may reduce fat deposition in the liver by affecting the expression of lipogenic genes through an mTORC1/SREBP-1c-dependent mechanism. The involvement of the mTORC1/SREBP-1c axis for the control of lipid synthesis in the other important lipogenic tissue, i.e adipose tissue, will be discussed in the next section.
PPAR-γ and Lipin1
Adipogenesis is the biological process leading to the formation of mature adipocytes from adipose cell precursors. This process follows a well-orchestrated program in which the CCAAT/enhancer binding protein-β (C/EBP-β) and C/EBP-δ triggers the expression of C/EBP-α that in turns induces the expression of peroxisome proliferator-activated receptor-γ (PPAR-γ), a member of the nuclear-receptor superfamilly of ligand-activated transcription factors (reviewed in [22]). The activation of PPAR-γ leads to profound changes in gene expression that ultimately lead to the stimulation of fatty acid uptake, synthesis, esterification, and storage in the newly formed adipose cell.
Rapamycin strongly inhibits the adipogenic program in mouse and human pre-adipocytes in vitro [23-29]. It was initially shown that rapamycin blocks adipogenesis by impairing the clonal expansion of pre-adipocytes [29], an early event thought to be required for the establishment of the normal adipogenic program in vitro (reviewed in [22]). Over the following years, it was found that rapamycin severely reduces lipid accumulation in fat cells even when added after clonal expansion, indicating that mTORC1 affects adipogenesis and adipocyte maintenance through additional mechanisms [23, 24, 26, 28]. Most of the studies linking mTOR signaling to adipogenesis were based on long-term rapamycin treatments, an experimental condition that can also lead to mTORC2 inhibition [30]. To determine the specific role of mTORC1 in adipogenesis and adipocyte maintenance, Polak et al. knocked down raptor in 3T3-L1 adipocytes and observed a reduction in adipogenesis [28]. Additionally, it was recently shown that adipose-specific deletion of rictor does not affect adipocyte size and adipose tissue mass in vivo [31]. Although it remains to be determined if acute knock down of rictor leads to similar effects, these observations strongly suggest that mTORC1, but not mTORC2, plays a key role in adipogenesis in mammalian cells.
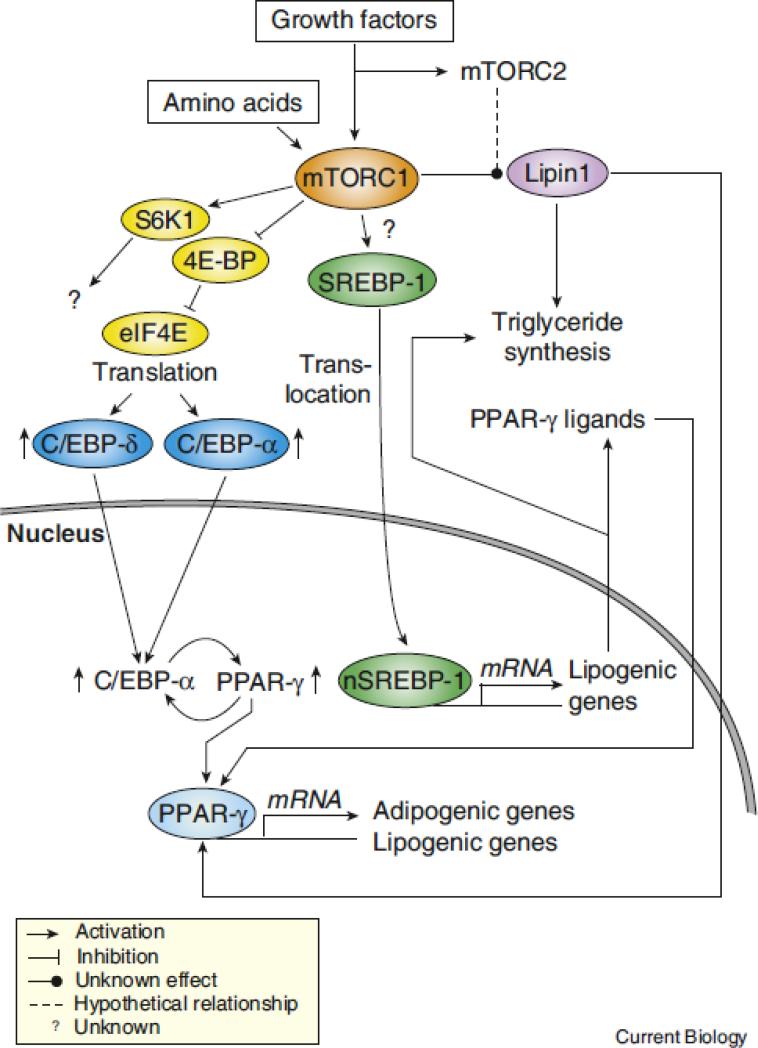
Although the exact mechanism linking mTORC1 signaling to adipogenesis and adipocyte maintenance is unknown, observations suggest that mTORC1 may control these processes by regulating the expression and the activation state of PPAR-γ (Figure 3). Many independent groups have reported that mTOR inhibition with rapamycin reduces mRNA and protein levels of PPAR-γ and C/EBP-α and the expression of numerous lipogenic genes in vitro [23, 24, 26]. Accordingly, constitutive activation of mTORC1 through TSC2 deletion increases PPAR-γ and C/EBP-α expression and promotes adipogenesis [32]. Some evidences indicate that 4E-BP1 and 2, which are negative regulators of translation that are inhibited by mTORC1, are important for the regulation of PPAR-γ and C/EBPs by mTORC1 [27, 33]. Disruption of 4E-BP1/2 promotes the expression of PPAR-γ, C/EBP-α, and C/EBP-δ and increases adipogenesis/lipid synthesis in mouse embryonic fibroblasts [33]. Although the activation of S6K1, and probably other mTORC1 substrates, is induced by the deletion of 4E-BP1/2 [33], these results suggest that mTORC1 controls adipogenesis/lipogenesis by inducing the translation of mRNA coding for key components of the adipogenic program, namely PPAR-γ and C/EBP-α. Additionally, the observation that S6K1 deficient mice have a reduced adipose tissue mass and are protected against diet-induced obesity indicates that mTORC1 may also control adipogenesis/lipogenesis through this effector [34]. The molecular mechanism by which S6K1 may control lipid metabolism is unknown.
Figure 3. mTORC1 promotes adipogenesis and adipocyte maintenance through various mechanisms.
Many evidences indicates that mTORC1 controls adipogenesis by activating PPAR-γ, a nuclear receptor controlling the expression of numerous genes involved in fatty acid uptake, synthesis, esterification, and storage. Many mechanisms are though to be involved in the regulation of PPAR-γ by mTORC1. Activation of mTORC1 induces the phosphorylation of 4E-BPs, which in turn releases eIF4E and increases the translation of C/EBP-α and -δ, which are key components required for the establishment of the adipogenic cascade. C/EBP-δ is known to drive the expression of C/EBP-α and PPAR-γ and to trigger the activation of a feed-forward loop in which these two transcription factors reciprocally induce their expression. When sufficient levels of PPAR-γ proteins are produced, this transcription factor promotes adipogenesis and lipid synthesis by inducing the expression of many lipogenic genes. SREBP-1 cleavage by mTORC1 may also contribute to the induction of adipognesis by directly favoring triglyceride synthesis and by promoting the production of endogenous ligands for PPAR-γ. More work is required to determine if SREBP-1-dependent production of PPAR-γ ligands plays a significant role in the activation of PPAR-γ by mTORC1. Lipin1, a phosphatidic acid phosphatase, was shown to play key role in adipogenesis by promoting triglyceride synthesis and by serving as a coactivator for PPAR-γ. Lipin1 is phosphorylated in response to insulin and amino acids in a rapamycin-sensitive fashion. Because rapamycin can inhibit mTORC2 activity in some cell types, it is unclear if lipin1 is a direct substrate of mTORC1 or mTORC2. The biological meaning of mTOR-mediated lipin1 phosphorylation remains to be characterized.
It is interesting to note that mTORC1 may also regulate adipogenesis through additional and complementary mechanisms. As discussed in the previous section, mTORC1 promotes lipid synthesis by increasing SREBP-1 cleavage and activation [10]. SREBP-1 activation triggers the production of endogenous ligands for PPAR-γ and this promotes the transactivation activity of this nuclear receptor [35]. Interestingly, Kim et al. observed that the synthetic PPAR-γ ligand troglitazone can overcome the inhibitory effect of rapamycin on adipogenesis, suggesting that mTORC1 inhibition may block adipogenesis by reducing the production of endogenous PPAR-γ ligands [24]. However, it is possible that troglitazone, as an unnatural ligand, may override the normal requirements for PPAR-γ activation and thus counters rapamycin inhibition unspecifically. The fact that conditioned media obtained from normally differentiated adipocytes cannot correct the adipogenic defect of adipocytes treated with rapamycin indicates that this possibility may be true [24]. Additional work is needed to clarify this point.
Lipin1 is a phosphatidic acid phosphatase that promotes triglyceride synthesis by converting phosphatidic acid to diacylglycerol. A null mutation in the lipin1 gene leads to lipodystrophy whereas lipin1 overexpression causes obesity (reviewed in [36]). In addition to directly promoting triglyceride synthesis through its phosphatidic acid phosphatase activity, lipin1 acts as a transcriptional coactivator for many transcription factors including PPAR-γ. Lipin1 physically interacts with PPAR-γ to induce the expression of many genes playing key roles in adipogenesis [37]. Accordingly, studies with lipin1 null embryonic fibroblasts revealed that lipin1 is required for the expression of key adipogenic markers, such as PPAR-γ and C/EBP-α, and for the attainment of mature adipocyte functions, including lipogenesis and lipid accumulation [38]. In consequence, lipin1 is though to affect adipogenesis and adipocyte maintenance by serving as a direct promoter of triglyceride synthesis and as an amplifier of the transcriptional activity of PPAR-γ. Lipin1 activity is regulated through many mechanisms, including by phosphorylation. In adipocytes, lipin1 is phosphorylated in response to insulin and amino acids in a rapamycin-sensitive fashion, thus suggesting that mTOR signaling may directly regulate adipogenesis/lipogenesis through the control of lipin1 activity [39]. More work is need to characterize the biological link between mTOR and lipin1 since the exact consequences of lipin1 phosphorylation on lipid synthesis and gene expression have not been characterized so far.
As described throughout this section, the link between mTORC1 and PPAR-γ in the control of adipogenesis and adipocyte maintenance has been well established in vitro. In order to determine the role of mTORC1 in adipocytes in vivo, Polak et al. ablated mTORC1 functions in adipose tissue by crossing raptor floxed mice with mice expressing a cre-recombinase under the control of the fatty acid binding protein-4 (FABP4)/ap2 promoter [28]. Because this promoter is expressed after the critical steps required for the induction of adipogenesis, this study mainly focused on the role of mTORC1 in adipocyte maintenance. In line with the in vitro studies, raptor deletion in fat results in lean mice with a reduction in adipocyte size and number. Unexpectedly, the expression of many genes involved in lipid production and storage, including PPAR-γ and C/EBP-α, is not reduced in adipose tissue of these animals. Polak et al. observed that mTORC1 inactivation reduces fat accumulation in adipose tissue by promoting energy expenditure through an induction of uncoupled respiration. Interestingly, similar increase in energy expenditure is observed in many animal models deficient for protein playing key roles in lipogenesis [40-42]. This raises the possibility that the reduction in fat mass and the induction of energy expenditure in adipose-specific raptor null animals could be linked to a defect in lipogenesis, defect that is likely independent of PPAR-γ-induced lipogenic gene expression. The potential implication of lipin1 or SREBP-1 in this effect has to be considered.
Obesity, defined as an excess amount of body fat relative to lean body mass, is a major health concern in the United States and an increasing problem in the developing world. Recent advances regarding the biology of adipose tissue have demonstrated that the white adipose tissue plays a central role in the control of energy balance and acts as a powerful endocrine organ that mediates numerous physiological and pathological processes (reviewed in [43]). It was observed that mTORC1 is highly active in adipose tissue of obese rodents [34]. The existence of a link between mTORC1 activation and adipogenesis/lipogenesis suggests that mTORC1 may play a significant role in the accumulation of fat when energy intake exceeds energy expenses. Understanding the role of mTORC1 signaling in the development and the maintenance of adipose tissue and in the control of its endocrine function may promote the development of new pharmacologic tools to treat metabolic diseases associated with obesity such as insulin resistance, type 2 diabetes and coronary-vascular diseases.
Possible implication of mTORC2 in lipid biosynthesis – lessons from lower organisms
Two recent reports indicate that impairment of TORC2 function affects fat accumulation in C.elegans [44, 45]. Despite developmental delay and reduced body size, rictor null worms show increased body fat accumulation, thus suggesting that TORC2 functions as a negative regulator of lipid deposition in this model. Jones et al. found that the induction of lipid storage in rictor null C.elegans depends on the TORC2 substrate SGK1 but not Akt, as only SGK1 loss can mimic the metabolic phenotype associated with rictor deletion [44]. The precise mechanism linking the TORC2/SGK1 axis to lipid metabolism was not described in this study. In the other report, Soukas et al. observed that the high-fat phenotype associated with rictor loss is associated with a reduction in oxygen consumption and is dependent on SGK1, but also Akt1 and Akt2 [45]. Soukas et al. also show that the site of action of rictor in the regulation of fat mass is the intestine, which is the key site for fat storage in C.elegans. Although the conclusions drawn in these reports do not perfectly match, these studies demonstrate that TORC2 plays a significant role in regulating fat accumulation in C.elegans and that SGK1 is required in this process. An interesting question arising from these observations is whether or not a similar link between mTORC2 and lipid synthesis exists in mammalian cells. The fact that adipose-specific rictor null mice [31] and whole-body SGKs deficient mice models (reviewed in [46]) do not show any defect in lipid metabolism suggests that mTORC2 may have a more subtle role to play in the control of lipid metabolism in mammals. Whether or not this is a consequence of the higher genetic redundancy of mammalian systems remains to be determined.
Although the implication of mTORC2 in the control of lipogenesis/adipogenesis appears to be limited in mammals, recent observations in yeast suggest that this complex may regulate other facets of lipid metabolism. Aranova et al. recently showed that the synthesis of minor and major ceramides species is impaired in yeast cells deficient in TORC2 activity [47]. In the same report, it was observed that TORC2 controls de novo sphingolipid synthesis by regulating the activity of ceramide synthase through a mechanism dependent on Ypk2, the yeast homologue of SGK1. Although the exact mechanism linking Ypk2 to ceramide synthase has not been characterized, these results indicate that TORC2 plays a role in transmitting growth signals to sphingolipid synthesis in yeast. Because the early steps in de novo sphingolipid synthesis are well conserved throughout evolution (reviewed in [48]), it is reasonable to believe that mTORC2 could control this pathway in mammals. Sphingolipids, which include sphigosine and ceramides, have been implicated in a variety of physiological functions including differentiation, cell migration and adhesion, cell growth arrest, senescence and apoptosis. These lipids are believed to play important roles in many pathological settings including cancer, heart disease, diabetes, microbial infection, neurological disorders and immune dysfunctions. The possible implication of mTORC2 in the regulation of ceramide levels indicates that mTORC2 may play important and yet undefined roles in these diseases and may also represent an interesting target for the development of new therapeutic avenues. Additional studies are required to determine if mTORC2 plays a significant role in controlling de novo shingolipid synthesis in mammalian cells.
Concluding remarks
Lipid biosynthesis is essential for the maintenance of cellular homeostasis and defects in lipid synthesis or processing contribute to the development of many diseases, including obesity, insulin resistance, type 2 diabetes, non-alcoholic fatty liver disease, and cancer. Over the last decade, many groups have shown that mTOR signaling regulates lipid synthesis through various effectors, suggesting that this pathway could be targeted for the development of new therapeutic tools for the treatment of many diseases. Despite outstanding advances, our understanding of the mechanisms by which mTOR signaling affect lipid synthesis in mammals is incomplete and important questions remain to be answered. For example, how does mTORC1 promote SREBP-1 cleavage and nuclear localization? What is the exact mechanism by which mTORC1 regulates the activity of PPAR-γ? How does mTORC1 control lipid synthesis in adipose tissue in vivo? What is the biological function associated with lipin1 phosphorylation by mTOR? What is the exact role of mTORC2 in the regulation of lipogenesis? Does mTORC2 regulate sphingolipid synthesis in mammals? Finding answers to these questions will improve our understanding of cell biology and may help to improve the way we treat many diseases.
References
- 1.Guertin DA, Sabatini DM. Defining the Role of mTOR in Cancer. Cancer cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:e27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- 3.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2:282–286. doi: 10.1093/embo-reports/kve071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porstmann T, Santos CR, Lewis C, Griffiths B, Schulze A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochem Soc Trans. 2009;37:278–283. doi: 10.1042/BST0370278. [DOI] [PubMed] [Google Scholar]
- 6.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Hormone research. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 8.Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 9.Hegarty BD, Bobard A, Hainault I, Ferre P, Bossard P, Foufelle F. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc Natl Acad Sci U S A. 2005;102:791–796. doi: 10.1073/pnas.0405067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown NF, Stefanovic-Racic M, Sipula IJ, Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism. 2007;56:1500–1507. doi: 10.1016/j.metabol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauvoisin D, Rocque G, Arfa O, Radenne A, Boissier P, Mounier C. Role of the PI3-kinase/mTor pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. Journal of cell communication and signaling. 2007;1:113–125. doi: 10.1007/s12079-007-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 15.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mTOR inhibitor reveals rapamycin-insensitive functions of mTORC1. J Biol Chem. 2009 doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-Site Inhibitors of mTOR Target Rapamycin-Resistant Outputs of mTORC1 and mTORC2. PLoS biology. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J. 2009 doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology. 2005;146:1328–1337. doi: 10.1210/en.2004-0777. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 23.Cho HJ, Park J, Lee HW, Lee YS, Kim JB. Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem Biophys Res Commun. 2004;321:942–948. doi: 10.1016/j.bbrc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 24.Kim JE, Chen J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53:2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 25.Bell A, Grunder L, Sorisky A. Rapamycin inhibits human adipocyte differentiation in primary culture. Obes Res. 2000;8:249–254. doi: 10.1038/oby.2000.29. [DOI] [PubMed] [Google Scholar]
- 26.Gagnon A, Lau S, Sorisky A. Rapamycin-sensitive phase of 3T3-L1 preadipocyte differentiation after clonal expansion. Journal of cellular physiology. 2001;189:14–22. doi: 10.1002/jcp.1132. [DOI] [PubMed] [Google Scholar]
- 27.El-Chaar D, Gagnon A, Sorisky A. Inhibition of insulin signaling and adipogenesis by rapamycin: effect on phosphorylation of p70 S6 kinase vs eIF4E-BP1. Int J Obes Relat Metab Disord. 2004;28:191–198. doi: 10.1038/sj.ijo.0802554. [DOI] [PubMed] [Google Scholar]
- 28.Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Yeh WC, Bierer BE, McKnight SL. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc Natl Acad Sci U S A. 1995;92:11086–11090. doi: 10.1073/pnas.92.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Cybulski N, Polak P, Auwerx J, Ruegg MA, Hall MN. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0811321106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS ONE. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest. 2007;117:387–396. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 35.Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998;95:4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reue K, Brindley DN. Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J Lipid Res. 2008;49:2493–2503. doi: 10.1194/jlr.R800019-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh YK, Lee MY, Kim JW, Kim M, Moon JS, Lee YJ, Ahn YH, Kim KS. Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma 2. J Biol Chem. 2008;283:34896–34906. doi: 10.1074/jbc.M804007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phan J, Peterfy M, Reue K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J Biol Chem. 2004;279:29558–29564. doi: 10.1074/jbc.M403506200. [DOI] [PubMed] [Google Scholar]
- 39.Huffman TA, Mothe-Satney I, Lawrence JC., Jr. Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Natl Acad Sci U S A. 2002;99:1047–1052. doi: 10.1073/pnas.022634399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A. 2003;100:10207–10212. doi: 10.1073/pnas.1733877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, et al. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS ONE. 2008;3:e2890. doi: 10.1371/journal.pone.0002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 44.Jones KT, Greer ER, Pearce D, Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS biology. 2009;7:e60. doi: 10.1371/journal.pbio.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 47.Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, Powers T. Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab. 2008;7:148–158. doi: 10.1016/j.cmet.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickson RC. Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. J Lipid Res. 2008;49:909–921. doi: 10.1194/jlr.R800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]