Abstract
Upregulation of cytoprotective enzymes by therapeutic agents to prevent damage by reactive oxygen species and xenobiotic electrophiles is a strategy for cancer chemoprevention. The Kelch-like ECH-associated protein 1 (Keap1) and its binding partner, transcription factor NF-E2-related factor-2 (Nrf2), are chemoprevention targets because of their role in regulating the antioxidant response element (ARE) in response to oxidative stress and exposure to electrophiles. Modification of the sensor protein Keap1 by electrophiles such as the isothiocyanate sulforaphane can direct Nrf2 accumulation in the nucleus and subsequent ARE activation. Since our previous matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF MS)-based screening method to discover natural products that modify Keap1 does not detect covalent modification of Keap1 by some highly reversible agents such as sulforaphane, a more sensitive screening assay was developed. In this new assay, electrophiles that have reversibly modified Keap1 can be released, trapped and detected as β-mercaptoethanol adducts by mass spectrometry. Isoliquiritigenin and sulforaphane, known ARE activators that target Keap1, were used to validate the assay. To determine the ability of the assay to identify electrophiles in complex matrixes that modify Keap1, sulforaphane was spiked into a cocoa extract, and LC-MS/MS using high resolution mass spectrometry with accurate mass measurement was used to identify β-mercaptoethanol adducts of sulforaphane that had been released from Keap1. This screening assay permits identification of potential chemoprevention agents in complex natural product mixtures that reversibly modify Keap1 but cannot be detected using MALDI-TOF MS.
Introduction
Induction of phase II detoxification enzymes such as NAD(P)H-quinone oxidoreductase 1 (NQO1) and glutathione S-transferases (GSTs), and endogenous antioxidant enzymes such as heme oxygenase-1 (HO-1) by natural chemoprevention agents is a promising strategy for reducing the risk of cancer and other chronic diseases (1). Activation of the Kelch-like ECH-associated protein 1 (Keap1) NF-E2-related factor-2 (NRF2) signaling pathway regulates the antioxidant response element (ARE), a cis-acting element in the promoter region of multiple cytoprotective genes. Upregulation of these genes is an adaptive response to reactive oxygen species and xenobiotic electrophiles. Under basal conditions, Nrf2 is bound to a Keap1 dimer in the cytosol. Keap1 serves as an adaptor between the Cullin3 (Cul3)-based E3-ligase ubiquitination complex and Nrf2, and thus directs proteasomal degradation of Nrf2 (2). Upon exposure of cells to ARE inducers, Nrf2 ubiquitination becomes inhibited, and Nrf2 levels increase in the nucleus where it binds with a Maf protein, then to AREs and stimulates cytoprotective gene transcription (3).
Many ARE activators have been identified, some of which are abundant in edible plants including sulforaphane in broccoli sprouts (4), xanthohumol in hops (5), isoliquiritigenin in licorice (6), and quercetin in green and black tea (7). Although there is no common structure among these inducers, Talalay and coworkers (8–10) have categorized them into 10 distinct classes and found that most are electrophiles with reactivity toward sulfhydryl groups. Modification of Keap1 cysteines by electrophiles appears to modulate the Keap1-Nrf2 signaling pathway and ARE activation (11–13). Because of the significant role of Keap1 in chemoprevention, there is keen interest in discovering new compounds that modify this protein and ultimately activate the ARE.
Previously, we reported a screening method to discover natural products, either individual compounds or constituents of complex mixtures, that covalently modify Keap1 (14). This assay begins with MALDI-TOF MS measurement to determine whether any of the test compounds covalently bind to Keap1 and increase its mass. If the active compound is a constituent of a complex mixture such as a botanical extract, the mixture is incubated with glutathione (GSH), and precursor ion tandem mass spectrometry is used to detect GSH conjugates followed by product ion tandem mass spectrometry to obtain structural information. However, some ARE activators such as sulforaphane are not detected using this method. Although incubation of Keap1 with sulforaphane at a molar ratio of 1:15 resulted in an average of 9 molecules of sulforaphane attached per molecule of Keap1 (based on absorbance spectrophotometry) (15), MALDI-TOF MS did not detect any modification of Keap1. The most probable explanation for this observation is that sulforaphane dissociated from Keap1 during MALDI.
Therefore, a new mass spectrometry-based screening method was developed to enable the detection of compounds that reversibly modify Keap1 like sulforaphane. This new method is based on reaction of electrophiles with Keap1 followed by exchange with β-mercaptoethanol (BME) and then analysis using high resolution accurate mass measurement. This approach was inspired by Freeman and coworkers (16), who used a similar method for detection and quantification of reversibly adducted electrophiles in plasma, organelles, cells, and tissue homogenates (17). This strategy is effective for the detection and identification of compounds that form reversible adducts with Keap1 including those that cannot be detected using MALDI-TOF MS.
Experimental Procedures
Materials
Recombinant human Keap1 protein was expressed and purified as described previously (15). The protein (100 µM) was stored in 50 mM Tris-HCl buffer (pH 8.0), 2 mM tris[2-carboxyethyl]phosphine hydrochloride (TCEP) (Thermo Fisher Scientific; Rockford, IL), 250 mM sodium chloride, and 20% glycerol (v/v). Isoliquiritigenin, naringenin, Tris-HCl, sodium chloride, glycerol, formic acid, dimethyl sulfoxide, and β-mercaptoethanol (BME) were purchased from Sigma-Aldrich (St. Louis, MO). Sulforaphane was purchased from ICN Biomedical (Costa Mesa, CA), and micro Bio-Spin 6 columns were purchased from Bio-Rad (Hercules, CA). Deionized water was prepared using a Milli-Q purification system (Millipore, Bedford, MA). Methanol (HPLC grade) was purchased from Thermo Fisher (Hanover Park, IL), and cocoa nibs were provided by Hershey Foods (Hershey, PA).
Modification of Keap1 and BME exchange
A 10 mM stock solution of isoliquiritigenin was prepared in dimethyl sulfoxide. Keap1 (10 µM) was incubated with isoliquiritigenin at molar ratios from 1:0.5 to 1:5 (Keap1/isoliquiritigenin) in 100 µL 20 mM Tris-HCl buffer (pH 8.0). The reaction was carried out at room temperature for 2 h. In control experiments, 20 mM Tris-HCl buffer (pH 8.0) was substituted for human Keap1. Modified Keap1 was separated from free isoliquiritigenin using a micro Bio-Spin 6 size exclusion column. The fraction containing modified Keap1 was incubated for 1 h with 0.2 M BME at room temperature to trap isoliquiritigenin as it dissociated from Keap1. The incubation mixture was evaporated to dryness and reconstituted in 10% aqueous methanol containing 250 nM naringenin as an internal standard prior to analysis using LC-MS-MS with selected reaction monitoring.
To evaluate the selectivity of the screening assay, a methanolic extract of cocoa nibs was prepared as described previously (18). The extract (10 mg/mL) was spiked with sulforaphane (20 µM final concentration) and incubated with Keap1 (10 µM) or 100 µL 20 mM Tris-HCl buffer (pH 8.0) for 2 h. Size exclusion chromatography and exchange with BME was carried out as described for isoliquiritigenin. Since it was unknown if the cocoa extract contained compounds in addition to sulforaphane that could reversibly modify Keap1, a Shimadzu high resolution LC-IT-TOF mass spectrometer was used to screen for all possible BME adducts. Positive and negative ion electrospray mass spectra were acquired over the mass range of m/z 100–800.
LC-MS chromatograms of the experiment (with Keap1) and control (without Keap1) were compared using Shimadzu MetID Solution software to identify peaks corresponding to BME adducts. Peaks enhanced in the chromatogram of the Keap1 incubation but not in the control incubation were expected to be BME adducts. Identification of BME adducts was carried out using LC-MS/MS product ion analysis with accurate mass measurement and comparison with standards. A sulforaphane-BME standard was prepared by incubating sulforaphane and BME at a ratio of 1:10 (sulforaphane/BME).
MALDI-TOF mass spectrometry
Positive ion MALDI-TOF mass spectra of intact Keap1 were acquired using an Applied Biosystems (Foster, CA) Voyager DE Pro mass spectrometer. A 1 µL aliquot of the Keap1 solution was mixed with 1 µL matrix solution which contained sinapinic acid (10 mg/mL) in acetonitrile/water (1:1, v/v) acidified with 0.1% (v/v) trifluoroacetic acid. A 1 µL aliquot of the mixture was then spotted on a MALDI-TOF sample stage and air dried before analysis. For each sample, mass spectra from 300 laser shots were acquired in linear mode and signal averaged over the range m/z 65,000 to m/z 80,000.
LC-MS-MS analysis of BME-isoliquiritigenin adducts
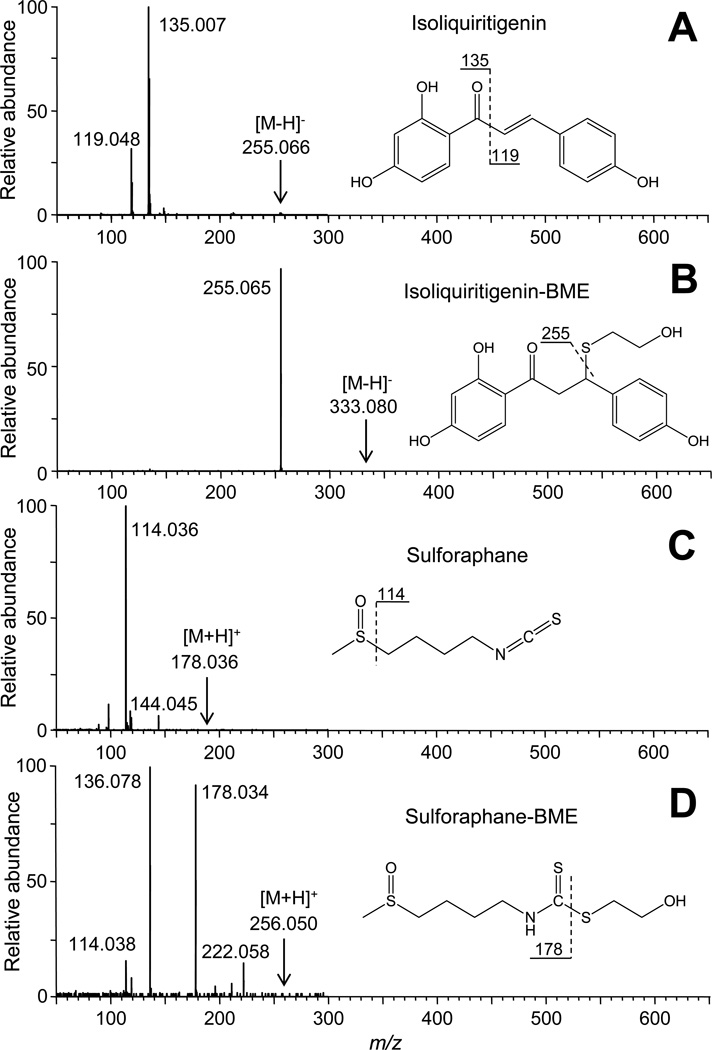
Levels of isoliquiritigenin and isoliquiritigenin-BME were analyzed using negative ion electrospray on a Thermo (San Jose, CA) Surveyor HPLC system interfaced to a Thermo TSQ Quantum triple quadrupole mass spectrometer. Isoliquiritigenin and its BME adduct were separated using a YMC C18 reverse phase column (2.0 mm × 50 mm, 5 µm, 120 Å) using a 6 min linear gradient from 10% to 100% methanol with a counter solvent of 0.1 % formic acid in water at a flow rate of 0.2 mL/min. Argon was used as the collision gas for collision-induced dissociation at 23 eV for isoliquiritigenin, 20 eV for its BME adduct, and 21 eV for naringenin (internal standard). Selected reaction monitoring (SRM) was used to monitor the transition from the deprotonated molecule of each analyte to its most abundant product ion. Specifically, isoliquiritigenin and naringenin were measured using the SRM transitions of m/z 255 to m/z 119 and m/z 271 to m/z 150.5, respectively. The SRM transition of m/z 333 to m/z 255, corresponding to elimination of BME from the deprotonated molecule, was used to measure BME-isoliquiritigenin. Product ion tandem mass spectra of isoliquiritigenin and sulforaphane and their BME adducts are shown in Figure 1. These tandem mass spectra were used for the selection of product ions for SRM and for confirmation of chemical structures.
Figure 1.
Chemical structures and electrospray product ion tandem mass spectra of the natural chemoprevention agents used in this study and their corresponding adducts with β-mercaptoethanol (BME). A) isoliquiritigenin; B) isoliquiritigenin-BME adduct; C) sulforaphane; and D) sulforaphane-BME adduct.
LC-IT-TOF MS analysis of BME-sulforaphane in an extract of cocoa nibs
After incubation of modified Keap1 and control with BME, samples were analyzed using a high mass accuracy Shimadzu (Kyoto, Japan) IT-TOF hybrid mass spectrometer. The same HPLC separation conditions were used as for isoliquiritigenin, except that a 15-min linear gradient was used from 10% to 100% methanol using a Shimadzu Prominence HPLC system. Positive and negative ion electrospray mass spectra were acquired using polarity switching over the mass range of m/z 100 to m/z 800.
The LC-MS chromatograms of both experiment and control were processed using Shimadzu MetID Solution software to identify peaks corresponding to adducts with BME. Background subtraction was allowed, and peak integration was performed on the peaks with at least 10 sec width and 500/min slope. Ion retention time within 0.1 min, and peak area difference within 50% between experiment and control were set to be determined as the same peak. New or enhanced peaks due to Keap1 modification in the experiment data file compared with control were identified based on automated data processing and confirmation by manual inspection. The elemental compositions of BME-adducts (within 5 ppm) were determined using a formula predictor based on high resolution accurate mass measurements.
Results and Discussion
Comparison of screening methods
Isoliquiritigenin (2,4,4’-trihydroxychalcone) (Figure 1A) is an example of a chemoprevention agent containing a Michael acceptor which is common to many ARE inducers (19). The α,β-unsaturated carbonyl group in isoliquiritigenin has been shown to modify specific cysteine residues of Keap1 in vitro (20). Since Michael addition reactions are reversible, the equilibrium of their reaction with Keap1 cysteines can be shifted by addition of another thiol nucleophile at a high concentration to form a new thioether adduct with isoliquiritigenin (16). Although several thiol-based nucleophiles should be suitable such as BME, dithiothreitol (DTT) and TCEP, BME was selected for this study due to its small size and therefore minimal steric hindrance during reaction with electrophiles like isoliquiritigenin.
The first step was to establish the feasibility of using BME to trap reversibly-bound electrophiles as they dissociated from Keap1 and to detect these electrophile-BME adducts by using LC-MS-MS. Keap1 was incubated with isoliquiritigenin, passed over a size exclusion column to remove free isoliquiritigenin, and the recovered covalently modified protein was treated with BME. Levels of isoliquiritigenin and isoliquiritigenin-BME were monitored by using LC-MS-MS. Initially, LC-MS-MS with constant neutral loss scanning of the neutral molecule BME from the deprotonated molecules was used as a general approach to detect thioether adducts of BME (21). Since constant neutral loss scanning was not sensitive enough for measuring low levels of isoliquiritigenin-BME (data not shown), this approach was not pursued. Instead, LC-MS-MS with collision-induced dissociation and SRM was used to measure isoliquiritigenin-BME to establish feasibility.
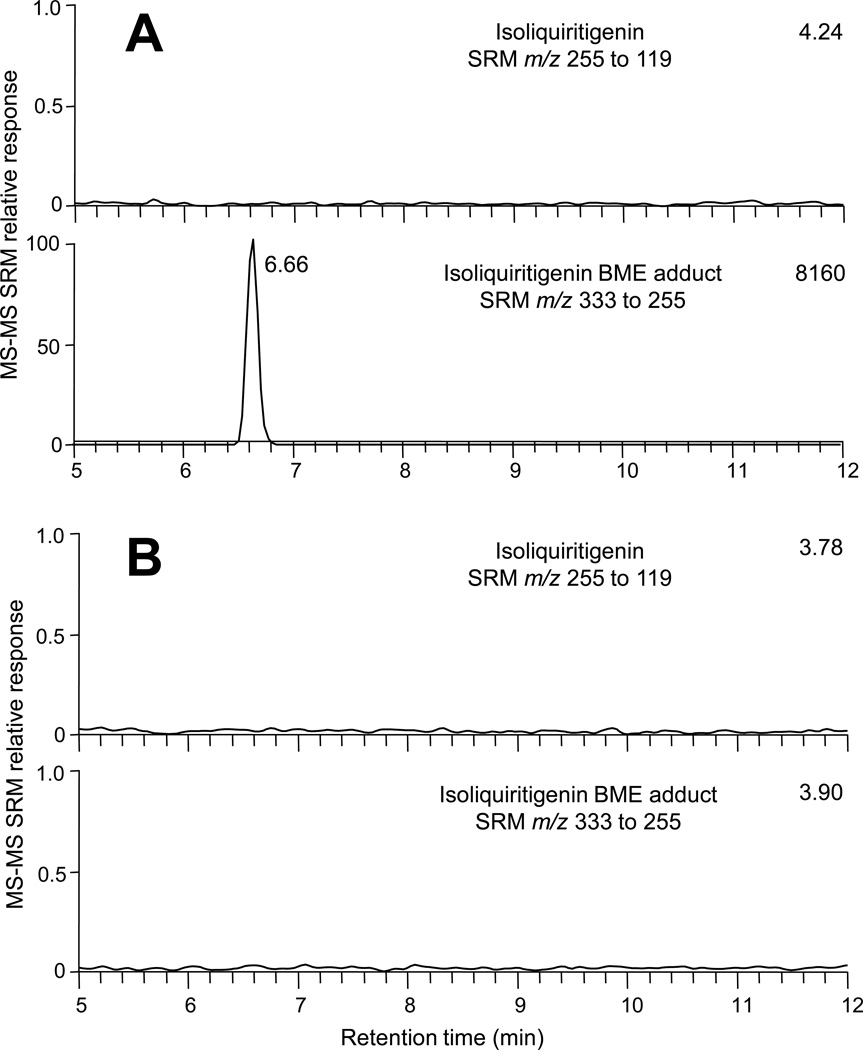
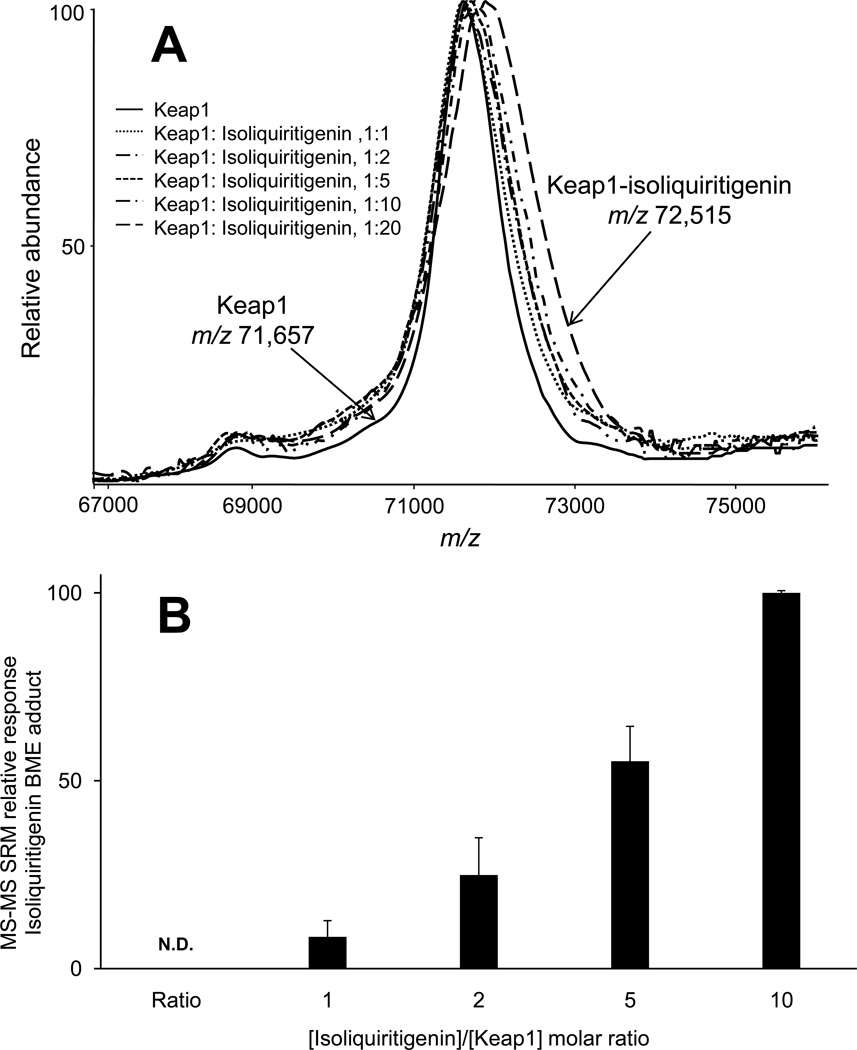
An isoliquiritigenin-BME adduct was detected after Keap1 was incubated with isoliquiritigenin followed by BME (Figure 2). As expected, no isoliquiritigenin or its BME adduct were detected in control incubations from which Keap1 had been omitted. This indicated that the isoliquiritigenin-BME adduct detected using LC-MS-MS (Figure 2A) was formed by reaction of BME with isoliquiritigenin that had been released from Keap1. These results establish a proof-of-concept that BME may be used to capture electrophiles released from Keap1. There was no free isoliquiritigenin carried over from the Keap1 (or buffer control) incubation during gel filtration. In addition, the amount of BME-isoliquiritigenin adduct increased in proportion to increasing molar ratios of [isoliquiritigenin]/[Keap1] (Figure 3A). This is consistent with past observations that the number of Keap1 cysteines modified by isoliquiritigenin is related to the concentration of isoliquiritigenin in the incubation (20).
Figure 2.
Computer-reconstructed mass chromatograms for negative ion electrospray LC-MS-MS analysis of isoliquiritigenin-BME adducts formed from isoliquiritigenin that had modified Keap1 and was then exchanged by incubation with BME. Selected reaction monitoring (SRM) of m/z 255 to m/z 119 was used to monitor isoliquiritigenin, and SRM of m/z 333 to m/z 255 was used to monitor isoliquiritigenin-BME. A) Isoliquiritigenin was incubated with Keap1 at a 2:1 molar ratio followed by gel filtration and BME exchange. B) Control incubation using Tris-HCl buffer but no Keap1. The relative MS-MS SRM responses are shown on the y-axis (normalized to the peak at 6.66 min in part A, and the absolute MS-MS responses (in arbitrary units) are shown in the upper right of each chromatogram.
Figure 3.
Detection of Keap1 modification by isoliquiritigenin. A) Positive ion MALDI TOF mass spectra of Keap1 after incubation with isoliquiritigenin at molar ratios of 1:1, 1:2, 1:5, 1:10, and 1:20 [Keap1/isoliquiritigenin] or with Tris-HCl buffer as control (solid line). The mass of Keap1 increased due to covalent attachment of isoliquiritigenin molecules to Keap1 cysteine sulfhydryl groups. B) Negative ion electrospray LC-MS-MS with SRM of isoliquiritigenin-BME adducts. The concentration of isoliquiritigentin-BME adducts increased in proportion to the molar ratio of isoliquiritigenin/Keap1. No isoliquiritigenin-BME adduct was detected in the control experiment that contained no Keap1 (N.D. = none detected).
Our previous MALDI-TOF MS-based assay is most effective for the detection of compounds that cause a mass shift in a significant number of Keap1 molecules. For example, MALDI-TOF MS-based screening can detect a mass shift of Keap1 due to reaction with isoliquiritigenin, but at least a 20-fold excess of isoliquiritigenin over Keap1 is required so that at least three molecules of isoliquiritigenin become bound to Keap1 (Figure 3A). The 858 Da increase in the mass of Keap1 indicated that an average of 3.3 isoliquiritigenin molecules were covalently attached to each molecule of Keap1. Using the new approach of gel filtration followed by LC-MS-MS, isoliquiritigenin modification of Keap1 was detected sensitively and quantitatively at a molar ratio of 1:1. As long as a compound modifies Keap1 and can be trapped by BME, it can be detected by LC-MS-MS. This new approach, which is more sensitive and more quantitative than the previous MALDI TOF MS-based assay, is limited only by the detection limit of the LC-MS-MS system. In this application, the BME exchange method was approximately 20-fold more sensitive than the MALDI TOF-MS screening method.
Screening of complex mixtures for Keap1 modifiers using BME and LC-MS
To investigate the potential of the new Keap1/BME assay for screening mixtures such as botanical extracts for compounds that react to form covalent adducts with Keap1, sulforaphane (R-1-isothiocyanato-4-methylsulfinylbutane) (Figure 1C) was selected as a model compound, and a cocoa extract was used as the test matrix. Sulforaphane is a potent ARE activator from cruciferous vegetables (22) that is being evaluated for safety and efficacy in clinical trials (23–25). Since modification of Keap1 cysteines by sulforaphane could not be detected in our previous MALDI-TOF MS assay, the utility of the new approach using BME trapping and LC-MS analysis was investigated by testing a cocoa extract spiked with sulforaphane (0.2% of total weight).
Instead of using LC-MS-MS with SRM transitions for detection of isoliquiritigenin-BME, LC-MS with a high resolution IT-TOF mass spectrometer was used in developing the general screening procedure. Unlike scanning instruments like quadrupole mass spectrometers that typically record up to a few mass spectra per second, the TOF analyzer can record thousands of mass spectra per second. Therefore, TOF-based mass spectrometers can be used to obtain high quality LC-MS chromatograms while recording entire mass spectra. Comparing experiment and control LC-MS data containing thousands of mass spectra to find BME adducts in incubations with Keap1 can be labor intensive if each pair of chromatograms is searched manually, one m/z value at a time. Therefore, this task was automated using software originally designed to find drug metabolites (Shimadzu Met-ID Solution software). The software automatically searched all possible computer-reconstructed mass chromatograms to find peaks enhanced in the experiment compared with the control.
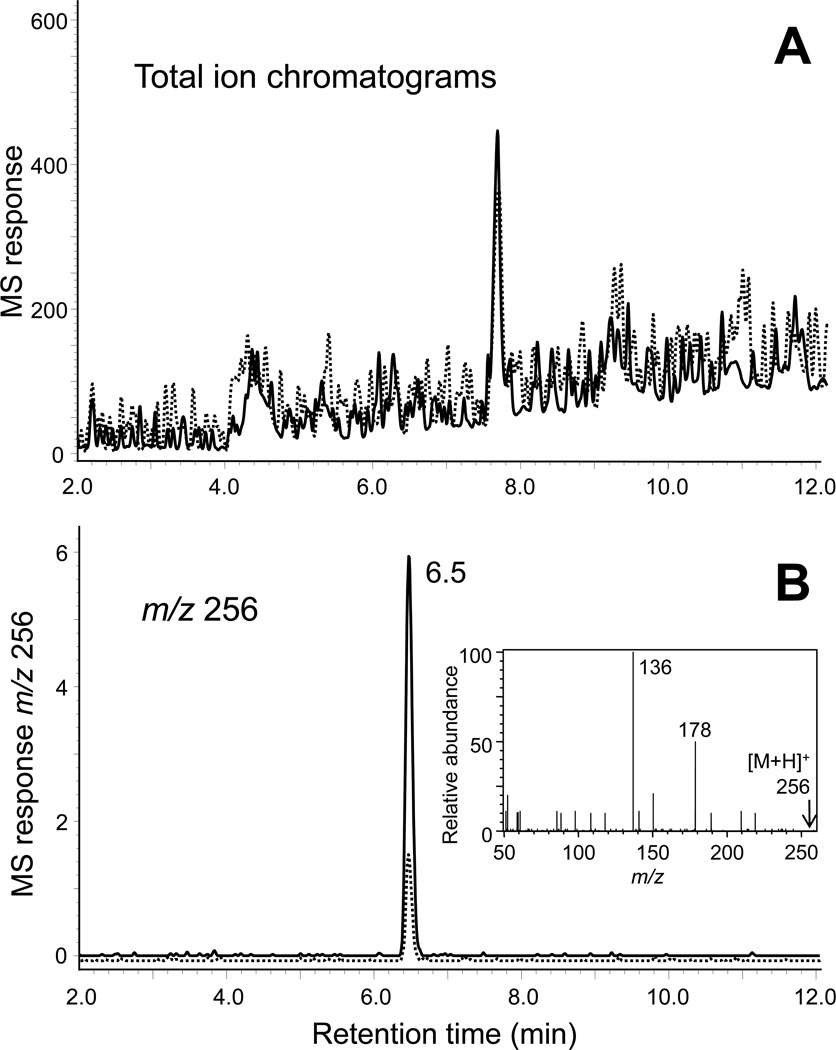
Positive ion electrospray and negative ion electrospray LC-MS data files of the incubations of the cocoa extract containing sulforaphane with and without Keap1 were compared, and peaks that were enhanced in one chromatogram relative to the other were identified. Only one enhanced peak was detected in the cocoa/sulforaphane experiment during positive ion electrospray LC-MS. The peak was observed at a retention time of 6.5 min and was enhanced 4-fold in the experiment relative to the control (Figure 4). Accurate mass measurement indicated that this peak corresponded to a protonated molecule of m/z 256.0498. There are four formulae within 5 ppm of this measured mass (Table 1), and the elemental composition of C8H17NO2S3 (ΔM 1.56 ppm) is the only formula that contains at least one sulfur, one oxygen and at least two carbon atoms contributed by the BME moiety. Therefore, this formula probably corresponded to an adduct between BME and sulforaphane (Figure 1D). Observation of a fragment ion of m/z 178 corresponding to loss of BME and a base peak of m/z 136 during positive ion electrospray tandem mass spectrometry (Figure 4B, inset) were also consistent with a BME adduct. Finally, the adduct was identified as sulforaphane-BME by comparing the retention time and fragmentation patterns with an authentic standard (Figure 1).
Figure 4.
Electrospray LC-IT-TOF MS analysis of a cocoa nibs methanolic extract spiked with sulforaphane (0.2% by weight) and incubated with Keap1 (solid line) or buffer (dashed line), followed by gel filtration to isolate the protein and then treatment with BME to trap reversibly bound electrophiles such as sulforaphane. A) Total ion chromatograms of experiment (solid line) and control (dashed line). B) Computer-reconstructed mass chromatograms showing the detection of a sulforaphane-BME adduct of m/z 256 eluting at 6.5 min that was enhanced in the experiment (solid line) compared with the control (dashed line). The positive ion tandem mass spectrum shown in the insert identifies this peak as sulforaphane-BME (see standard in Figure 1).
Table 1.
Elemental compositions within 5 ppm of the measured mass of m/z 256.0498 for the peak eluting at 6.5 min in the Keap1/BME LC-MS screening experiment shown in Figure 4.
| Rank | Score | Formula | Ion | Meas. m/z |
Pred. m/z |
Diff (ppm) |
Iso Score |
DBE |
|---|---|---|---|---|---|---|---|---|
| 1 | 61.45 | C8H9N5O3S | [M+H]+ | 256.0498 | 256.0499 | −0.39 | 61.45 | 7.0 |
| 2 | 61.19 | CH9N11OS2 | [M+H]+ | 256.0498 | 256.0506 | −3.12 | 64.61 | 3.0 |
| 3 | 50.86 | C8H17NO2S3 | [M+H]+ | 256.0498 | 256.0494 | 1.56 | 51.58 | 1.0 |
| 4 | 44.78 | C16H5N3O | [M+H]+ | 256.0498 | 256.0505 | −2.73 | 46.81 | 16.0 |
Since the Keap1 modification experiment shown in Figure 4 used cocoa spiked with sulforaphane, detection and identification of the sulforaphane-BME adduct was a simple process. In case an unknown adduct is detected as an enhanced peak in the experiment compared with the control by automatic software analysis, a dereplication strategy would be used. Positive and negative ion MS2 and MS3 analyses would be used to provide structural information regarding the electrophilic molecule that had reacted with BME and Keap1. The elemental composition, retention time and fragmentation patterns would be compared with standards prepared by reaction of BME with known constituents of the sample or literature values for these compounds obtained using natural products databases such as the NAPRALERT database (26).
This new assay is designed to detect electrophiles that reversibly bind to Keap1 such as the natural products sulforaphane and isoliquiritigenin but is not appropriate for compounds that form irreversible adducts with Keap1 such as the synthetic electrophile N-iodoacetyl-N-biotinylhexylenediamine (IAB). However, reversible Keap1 modifiers might be superior chemotherapeutic agents, since they tend to display less cytotoxicity than irreversible modifiers, which might also irreversibly bind to DNA and other important biological molecules (27). Also, reversible binding to Keap1 might produce an appropriate chemoprevention signaling response (instead of hyperactivation) while allowing Keap1 to be reused within the cell instead of requiring degradation and energy-consuming re-synthesis (21).
Summary
A new screening assay has been developed to screen compounds and complex mixtures such as botanical extracts for potential chemoprevention agents that form covalent but reversible adducts with Keap1. This assay is based on the detection of adducts of BME with electrophiles that were reversibly bound to Keap1 using LC-MS with automated peak detection software and high resolution accurate mass measurement. Compounds such as sulforaphane can be detected using this new screening approach that are missed by our MALDI MS-based screening assay due to its facile dissociation from Keap1. In addition, this strategy combines identification and characterization of chemoprevention agents in a single mass spectrometry step, whereas our previous assay used MALDI MS for detection followed by LC-MS/MS characterization and identification.
Acknowledgments
This research was supported by NIH grants P50 AT00155 and P01 CA48112. The authors would like to thank Dr. Shunyan Mo for assistance with tandem mass spectrometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicol. Lett. 1995;82–83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Kim DC, Choi SY, Kim SH, Yun BS, Yoo ID, Reddy NR, Yoon HS, Kim KT. Isoliquiritigenin selectively inhibits H(2) histamine receptor signaling. Mol. Pharmacol. 2006;70:493–500. doi: 10.1124/mol.106.023226. [DOI] [PubMed] [Google Scholar]
- 7.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad Sci. U. S. A. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggler AL, Small E, Hannink M, Mesecar AD. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. Biochem. J. 2009;422:171–180. doi: 10.1042/BJ20090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Eggler AL, Dietz BM, Mesecar AD, Bolton JL, Pezzuto JM, van Breemen RB. Screening method for the discovery of potential cancer chemoprevention agents based on mass spectrometric detection of alkylated Keap1. Anal. Chem. 2005;77:6407–6414. doi: 10.1021/ac050892r. [DOI] [PubMed] [Google Scholar]
- 15.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schopfer FJ, Batthyany C, Baker PR, Bonacci G, Cole MP, Rudolph V, Groeger AL, Rudolph TK, Nadtochiy S, Brookes PS, Freeman BA. Detection and quantification of protein adduction by electrophilic fatty acids: mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic. Biol. Med. 2009;46:1250–1259. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schopfer FJ, Batthyany C, Baker PR, Bonacci G, Cole MP, Rudolph V, Groeger AL, Rudolph TK, Nadtochiy S, Brookes PS, Freeman BA. Detection and quantification of protein adduction by electrophilic fatty acids: mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic Biol Med. 2009;46:1250–1259. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderon AI, Wright BJ, Hurst WJ, van Breemen RB. Screening antioxidants using LC-MS: case study with cocoa. Journal of agricultural and food chemistry. 2009;57:5693–5699. doi: 10.1021/jf9014203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuendet M, Oteham CP, Moon RC, Pezzuto JM. Quinone reductase induction as a biomarker for cancer chemoprevention. J. Nat. Prod. 2006;69:460–463. doi: 10.1021/np050362q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Eggler AL, Liu D, Liu G, Mesecar AD, van Breemen RB. Sites of alkylation of human Keap1 by natural chemoprevention agents. J. Am. Soc. Mass Spectrom. 2007;18:2226–2232. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, Kerns ML, Talalay P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol. Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 24.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol. Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 25.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loub WD, Farnsworth NR, Soejarto DD, Quinn ML. NAPRALERT: computer handling of natural product research data. J Chem Inf Comput Sci. 1985;25:99–103. doi: 10.1021/ci00046a009. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]